Interstellar ion chemistry More than a dozen ions






- Slides: 56

Interstellar ion chemistry More than a dozen ions hitherto identified in the interstellar medium. Interstellar chemistry once thought to be dominated by ion chemistry. Ions found in interstellar clouds, shock waves, ionospheres, etc. The “Horsehead nebula” (Ori) The “Cat’s eye” (Draco) Aurora over Alaska

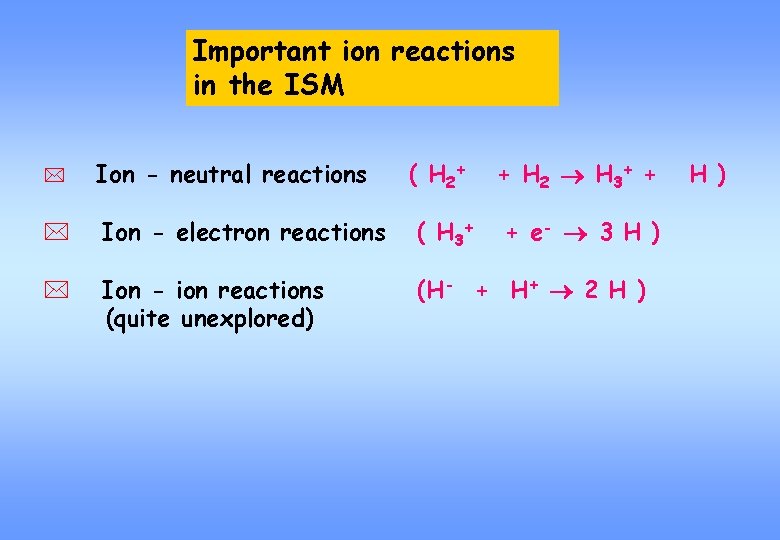
Important ion reactions in the ISM * Ion - neutral reactions ( H 2+ + H 2 H 3+ + * Ion - electron reactions ( H 3+ * Ion - ion reactions (quite unexplored) (H- + H+ 2 H ) + e- 3 H )

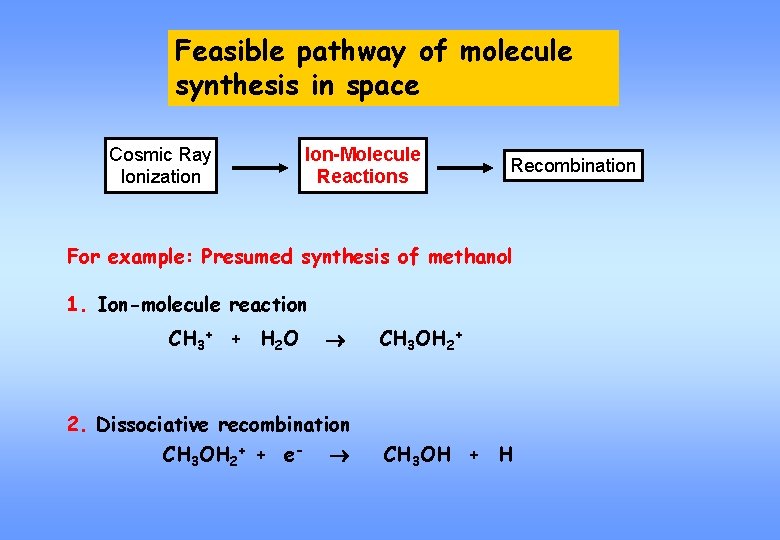
Feasible pathway of molecule synthesis in space Cosmic Ray Ionization Ion-Molecule Reactions Recombination For example: Presumed synthesis of methanol 1. Ion-molecule reaction CH 3+ + H 2 O CH 3 OH 2+ 2. Dissociative recombination CH 3 OH 2+ + e- CH 3 OH + H

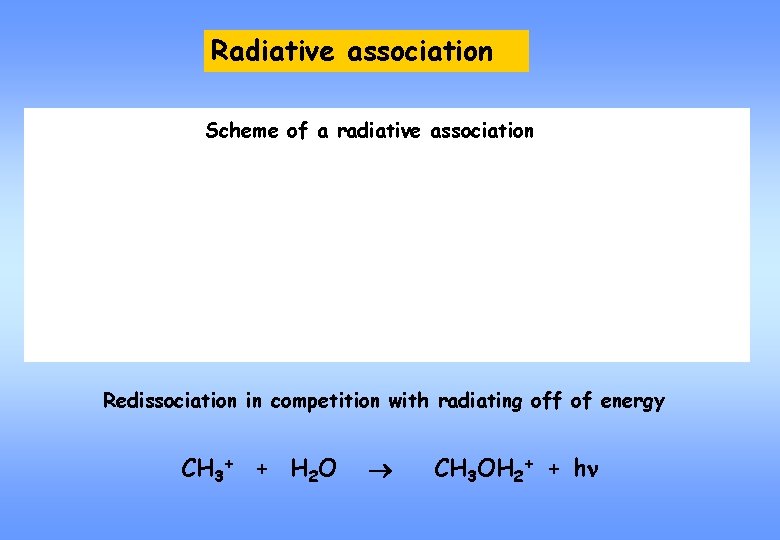
Radiative association Scheme of a radiative association Redissociation in competition with radiating off of energy CH 3+ + H 2 O CH 3 OH 2+ + hn

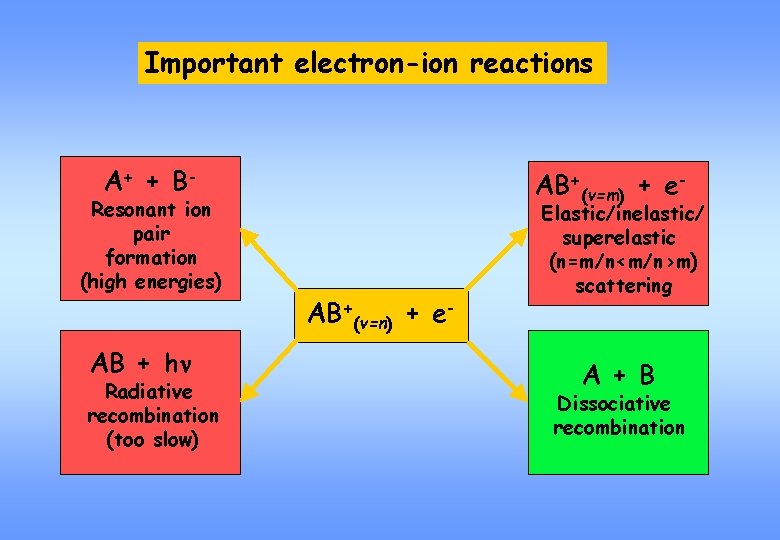
Important electron-ion reactions A+ + B - Resonant ion pair formation (high energies) AB + hn Radiative recombination (too slow) AB+(v=m) + e- AB+(v=n) + e- Elastic/inelastic/ superelastic (n=m/n<m/n>m) scattering A + B Dissociative recombination

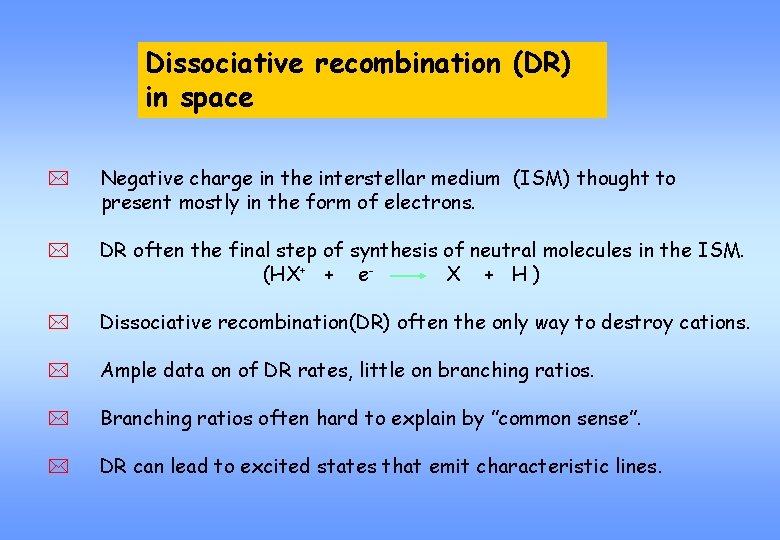
Dissociative recombination (DR) Dissociative recombination in spacein interstellar chemistry * Negative charge in the interstellar medium (ISM) thought to present mostly in the form of electrons. * DR often the final step of synthesis of neutral molecules in the ISM. (HX+ + e. X + H) * Dissociative recombination(DR) often the only way to destroy cations. * Ample data on of DR rates, little on branching ratios. * Branching ratios often hard to explain by ”common sense”. * DR can lead to excited states that emit characteristic lines.

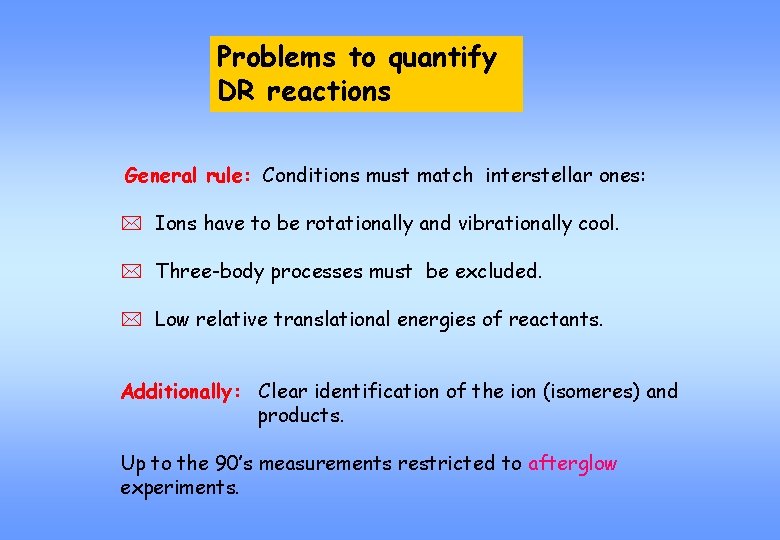
Problems to quantify DR reactions General rule: Conditions must match interstellar ones: * Ions have to be rotationally and vibrationally cool. * Three-body processes must be excluded. * Low relative translational energies of reactants. Additionally: Clear identification of the ion (isomeres) and products. Up to the 90’s measurements restricted to afterglow experiments.

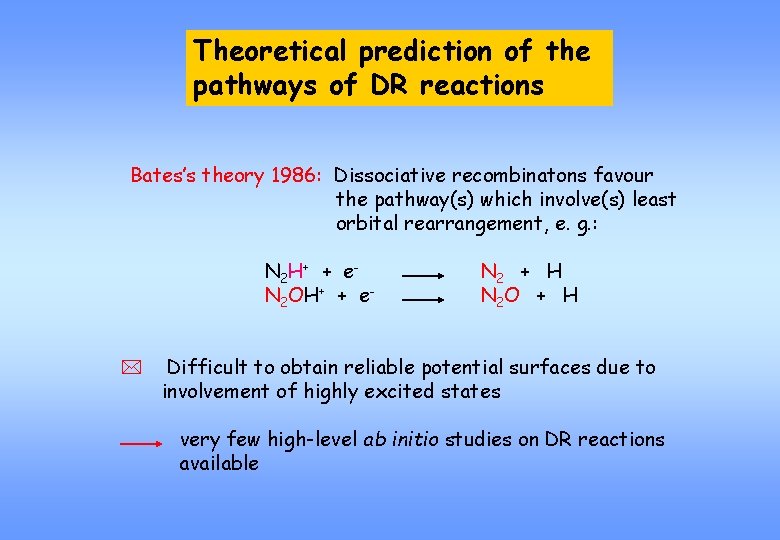
Theoretical prediction of the pathways of DR reactions Bates’s theory 1986: Dissociative recombinatons favour the pathway(s) which involve(s) least orbital rearrangement, e. g. : N 2 H+ + e. N 2 OH+ + e- * N 2 + H N 2 O + H Difficult to obtain reliable potential surfaces due to involvement of highly excited states very few high-level ab initio studies on DR reactions available

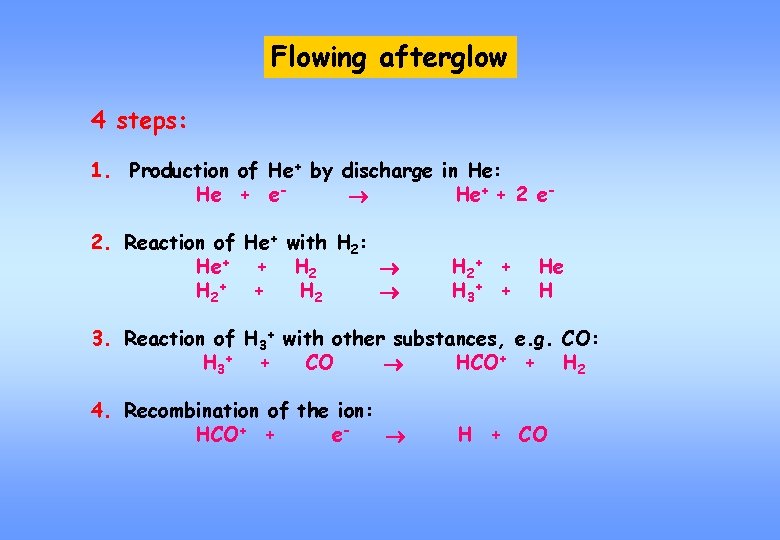
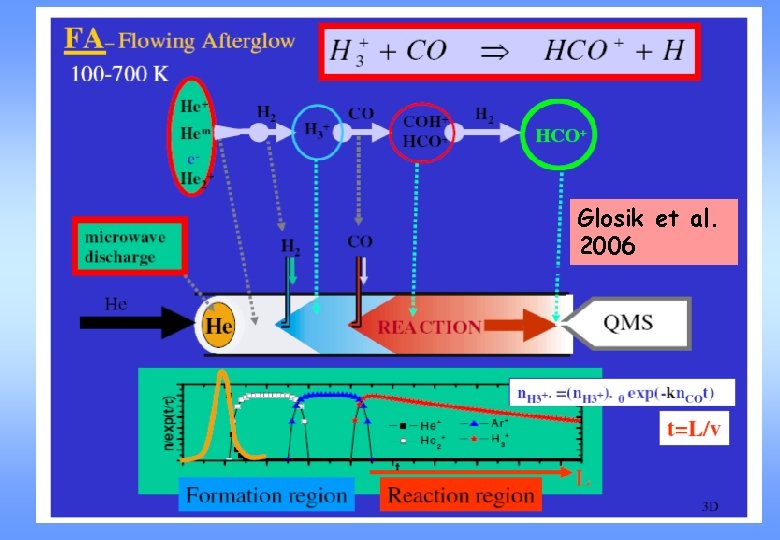
Flowing afterglow 4 steps: 1. Production of He+ by discharge in He: He + e He+ + 2 e 2. Reaction of He+ with H 2: He+ + H 2+ + H 3+ + He H 3. Reaction of H 3+ with other substances, e. g. CO: H 3+ + CO HCO+ + H 2 4. Recombination of the ion: HCO+ + e H + CO

Glosik et al. 2006

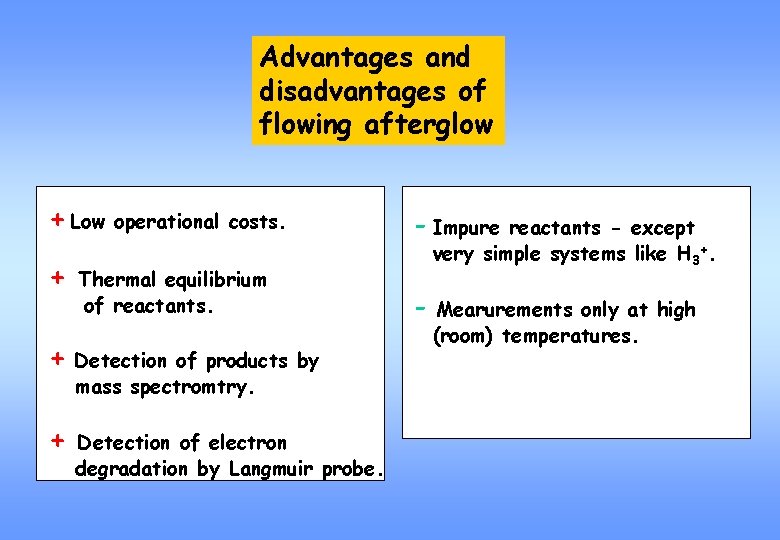
Advantages and disadvantages of flowing afterglow + Low operational costs. + Thermal equilibrium of reactants. + Detection of products by mass spectromtry. + Detection of electron degradation by Langmuir probe. - Impure reactants - except very simple systems like H 3+. - Mearurements only at high (room) temperatures.

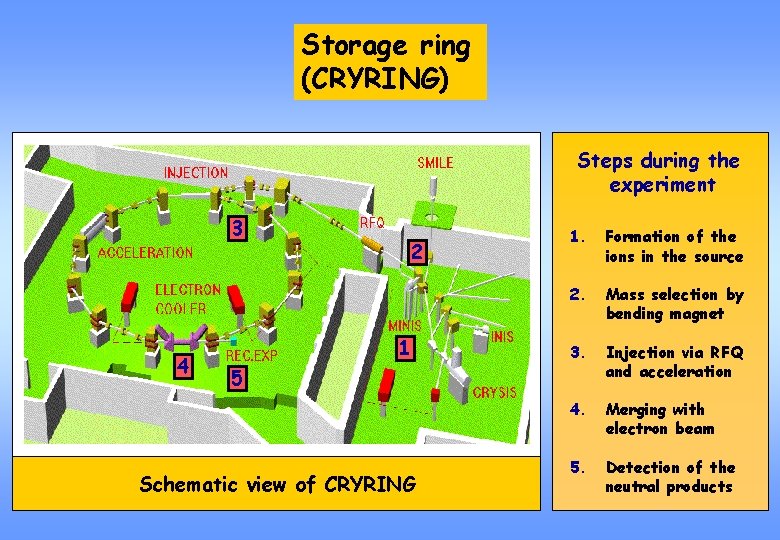
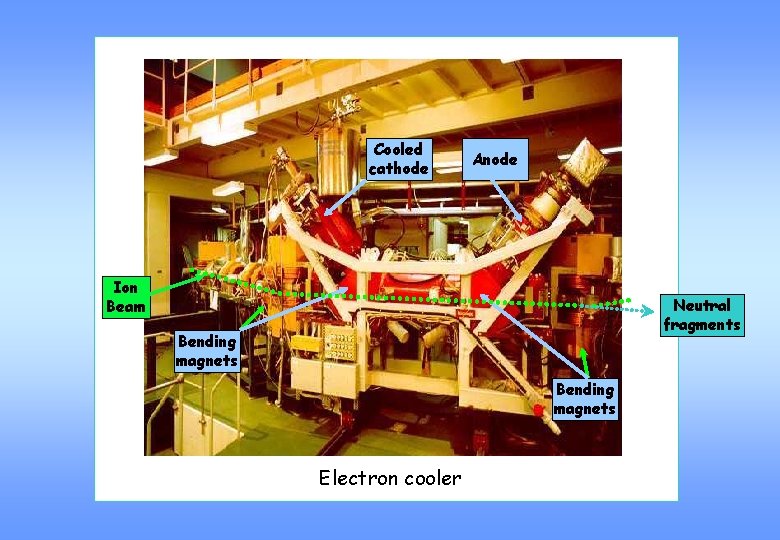
Storage ring (CRYRING) Steps during the experiment 3 4 2 1 1. Formation of the ions in the source 2. Mass selection by bending magnet 3. Injection via RFQ and acceleration 4. Merging with electron beam 5. Detection of the neutral products 5 Schematic view of CRYRING

Cooled cathode Anode Ion Beam Neutral fragments Bending magnets Electron cooler

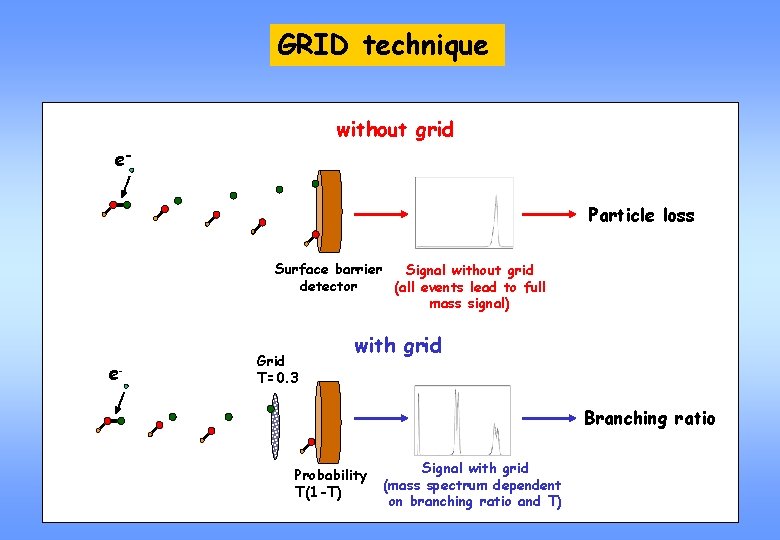
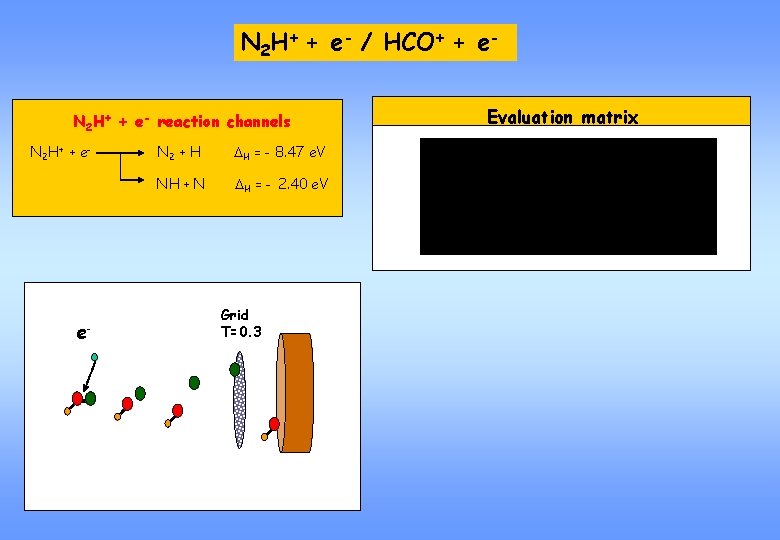
GRID technique without grid e. Particle loss Surface barrier Signal without grid detector (all events lead to full mass signal) e- Grid T=0. 3 with grid Branching ratio Probability T(1 -T) Signal with grid (mass spectrum dependent on branching ratio and T)

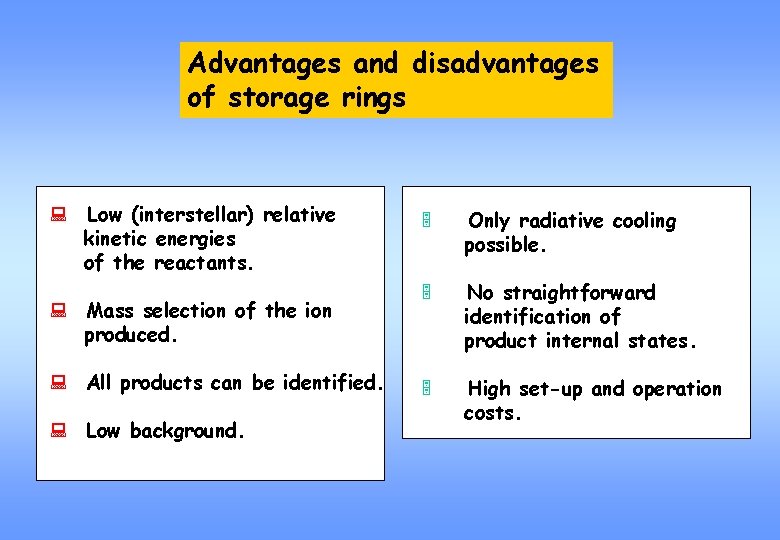
Advantages and disadvantages of storage rings : Low (interstellar) relative kinetic energies of the reactants. : Mass selection of the ion produced. : All products can be identified. : Low background. 5 Only radiative cooling possible. 5 No straightforward identification of product internal states. 5 High set-up and operation costs.

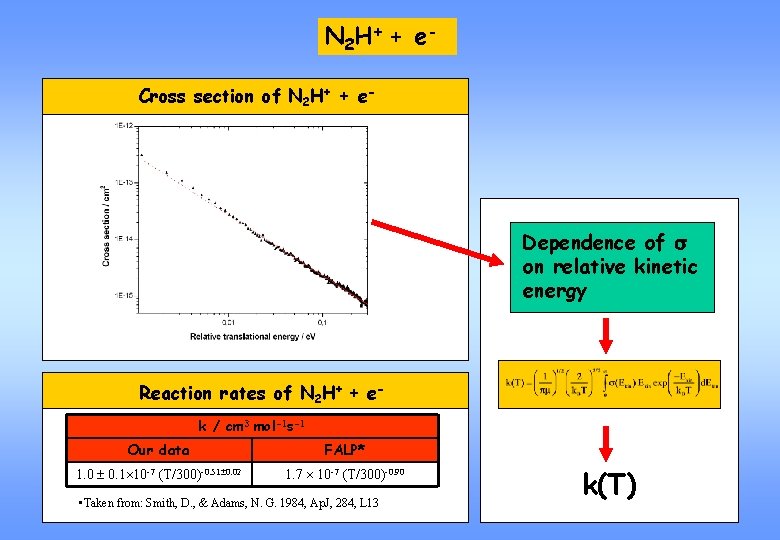
N 2 H+ + e One of the most prominent ions in dark interstellar clouds. N 2 lost through protonation might be fully recovered by DR of N 2 H+: N 2 + H 3 N 2 H+ + e- N 2 H+ + H 2 N 2 + H Most of interstellar nitrogen thought to be stored as N 2. Tracer for the unobservable N 2. Present in Titan’s ionosphere. Saturn’s satellite Titan

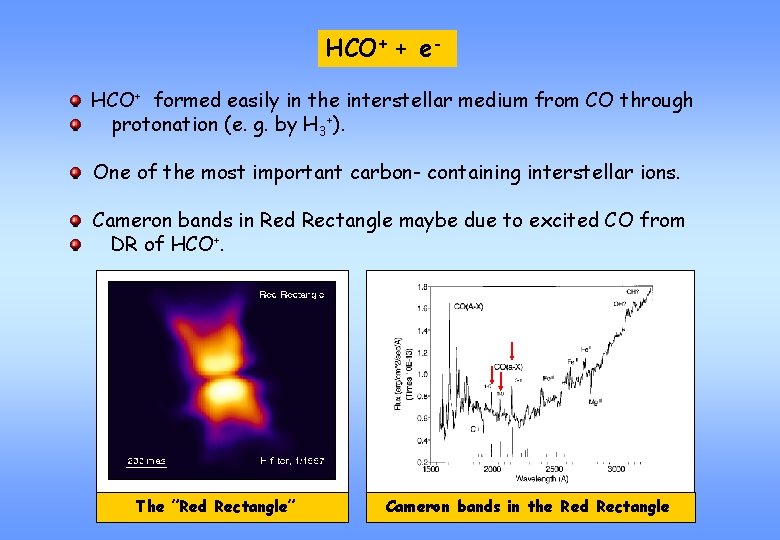
HCO+ + e. HCO+ formed easily in the interstellar medium from CO through protonation (e. g. by H 3+). One of the most important carbon- containing interstellar ions. Cameron bands in Red Rectangle maybe due to excited CO from DR of HCO+. The ”Red Rectangle” Cameron bands in the Red Rectangle

HCS+ + e. HCS+ is the most important sulfur-containing interstellar molecular ion. In dark clouds, a high HCS+/CS ratio is found. CS presumably formed by DR of HCS+. Very low rate of DR used in astrophysical models. How does the rate and branching ratio of the DR affect the HCS+/CS ratio ?

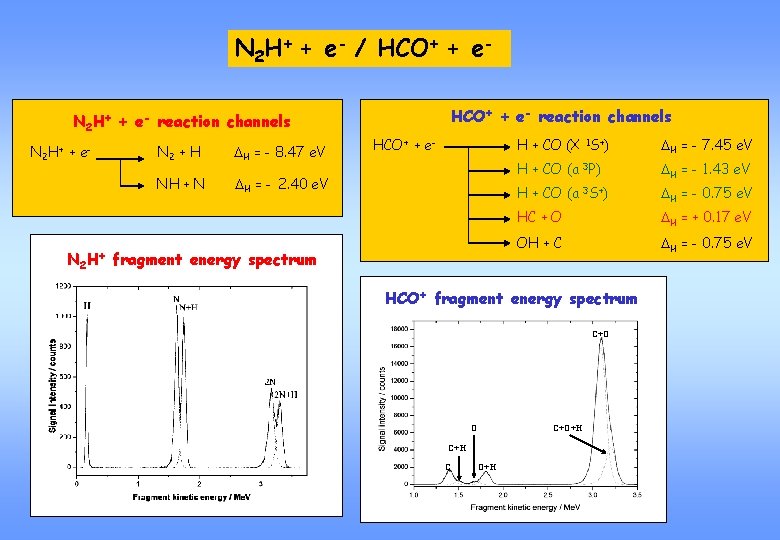
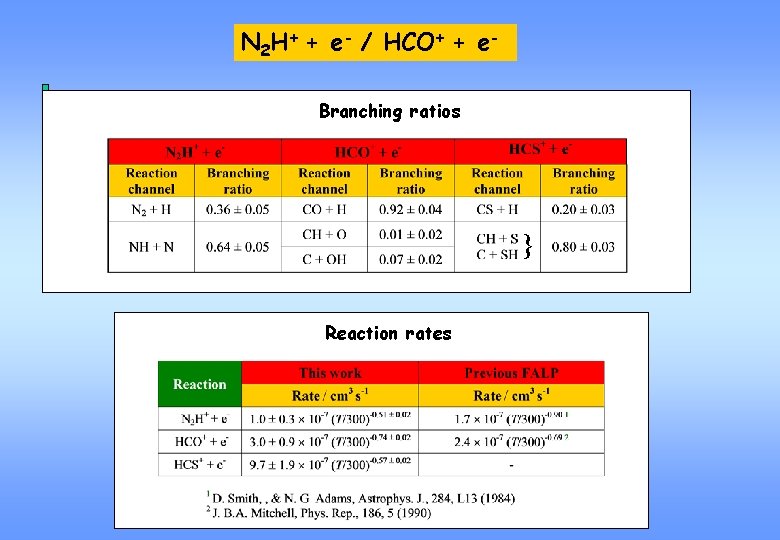
N 2 H+ + e- / HCO+ + e- reaction channels N 2 H+ + e- N 2 H+ N 2 + H DH = - 8. 47 e. V NH + N DH = - 2. 40 e. V HCO+ + e- fragment energy spectrum H + CO (X 1 S+) DH = - 7. 45 e. V H + CO (a 3 P) DH = - 1. 43 e. V H + CO (a 3 S+) DH = - 0. 75 e. V HC + O DH = + 0. 17 e. V OH + C DH = - 0. 75 e. V HCO+ fragment energy spectrum C+O O C+O+H C O+H

N 2 H+ + e- / HCO+ + e. N 2 H+ + e- reaction channels N 2 H+ + e- e- N 2 + H DH = - 8. 47 e. V NH + N DH = - 2. 40 e. V Grid T=0. 3 Evaluation matrix

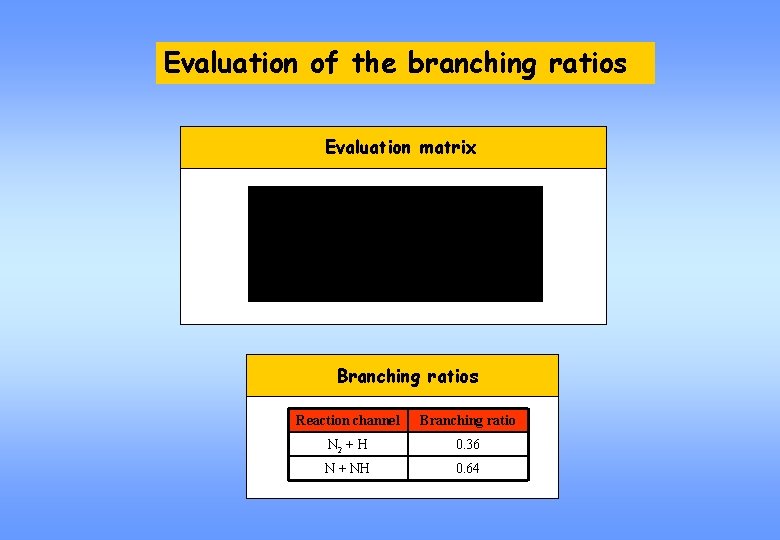
Evaluation of the branching ratios Evaluation matrix Branching ratios Reaction channel Branching ratio N 2 + H 0. 36 N + NH 0. 64

N 2 H+ + e Cross section of N 2 H+ + e- Dependence of s on relative kinetic energy Reaction rates of N 2 H+ + ek / cm 3 mol-1 s-1 Our data FALP* 1. 0 0. 1 10 -7 (T/300)-0. 51 0. 02 1. 7 10 -7 (T/300)-0. 90 • Taken from: Smith, D. , & Adams, N. G. 1984, Ap. J, 284, L 13 k(T)

N 2 H+ + e- / HCO+ + e. Branching ratios } Reaction rates

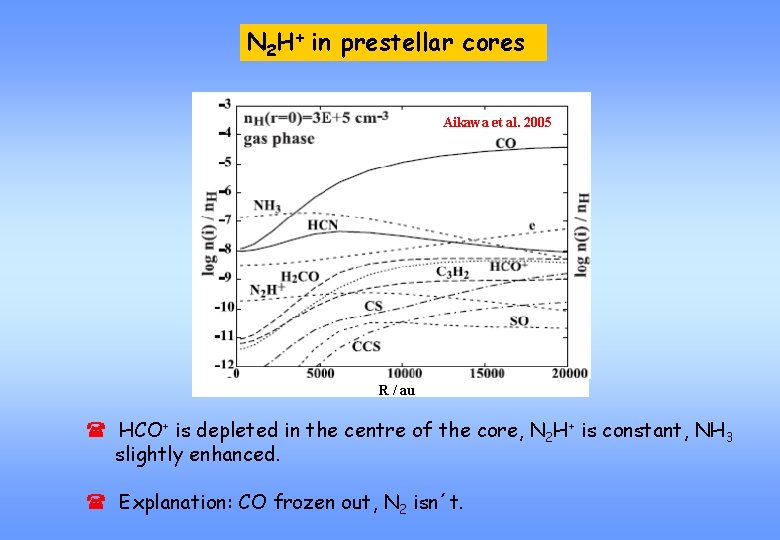
N 2 H+ in prestellar cores Aikawa et al. 2005 R / au ( HCO+ is depleted in the centre of the core, N 2 H+ is constant, NH 3 slightly enhanced. ( Explanation: CO frozen out, N 2 isn´t.

BUT ( Temperature desorption behaviour of N 2 and CO differs only slightly. (Schlemmer and co-workers 2006) ( No explanation for enhancement of ammonia near the core centre.

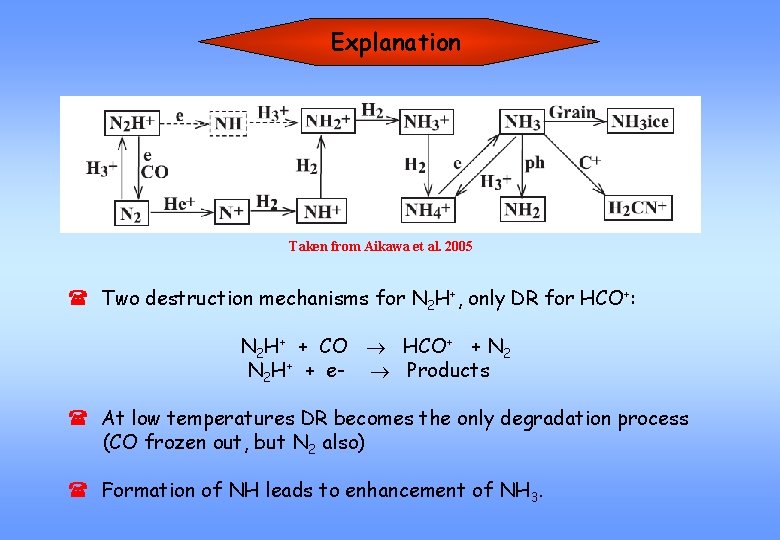
Explanation Taken from Aikawa et al. 2005 ( Two destruction mechanisms for N 2 H+, only DR for HCO+: N 2 H+ + CO HCO+ + N 2 H+ + e- Products ( At low temperatures DR becomes the only degradation process (CO frozen out, but N 2 also) ( Formation of NH leads to enhancement of NH 3.

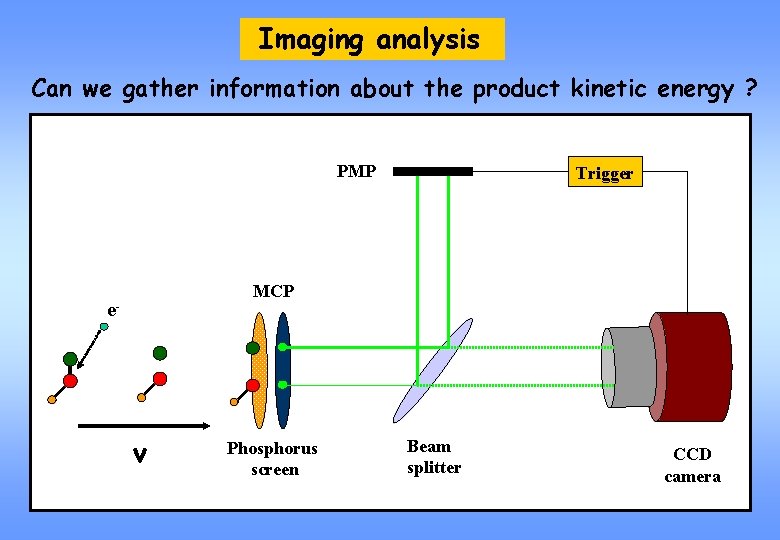
Imaging analysis Can we gather information about the product kinetic energy ? PMP Trigger MCP e- v Phosphorus screen Beam splitter CCD camera

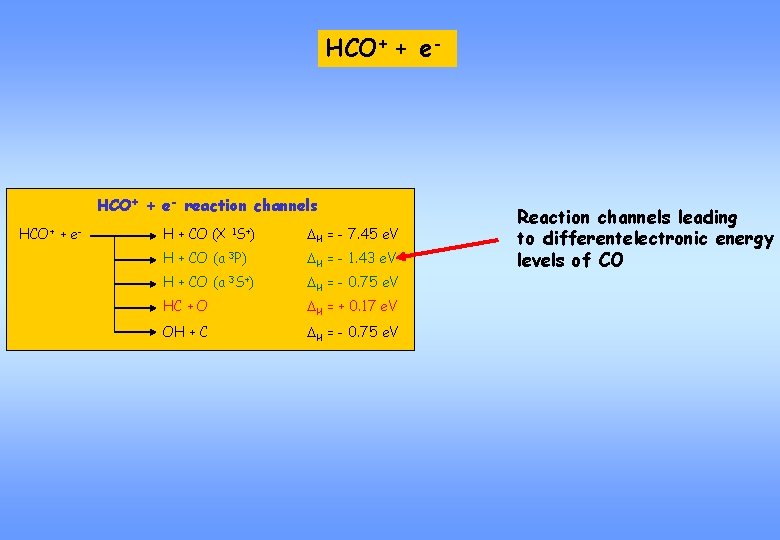
HCO+ + e- reaction channels HCO+ + e- H + CO (X 1 S+) DH = - 7. 45 e. V H + CO (a 3 P) DH = - 1. 43 e. V H + CO (a 3 S+) DH = - 0. 75 e. V HC + O DH = + 0. 17 e. V OH + C DH = - 0. 75 e. V Reaction channels leading to differentelectronic energy levels of CO

Imaging of DCO+ Fit of the different electronic state contributions

Conclusions N 2 H+/ HCO+/ HCS+ In the DR of N 2 H+, the break-up of the N-N bond dominates. In the DR of HCO+, the CO + H channel is preeminent. Recombination of HCO+ partly leads to CO in the 3 Pu state, which can explain the Cameron bands in the Red Rectangle. In the DR of HCS+, the break-up of the C-S bond is favoured. Reaction rate in the N 2 H+ and HCO+ DR reactions in agreement with previous FALP measurements.

New branching ratios in a model of TMC-1 *Ohishi, M. , Irvine, W. M. Kaifu, N. , Astronomy of Cosmic Phenomena, 171

Conclusions from model calculations Abundances of N-containing compounds predicted better assuming an older age of TMC-1. Some improvements for molecule densities that proved difficult to model (H 2 O, HCOOH). No big influence on models of circumstellar envelopes, planetary nebulae and diffuse clouds.

SO 2+ + e. Influence on interstellar sulfur chemistry. SO 2 is found in atmospheres of planets (Venus) and satellites (Io). Important role of SO 2+ in the ionosphere of Io. Three-body break-up energetically allowed. Iupiter´s moon Io

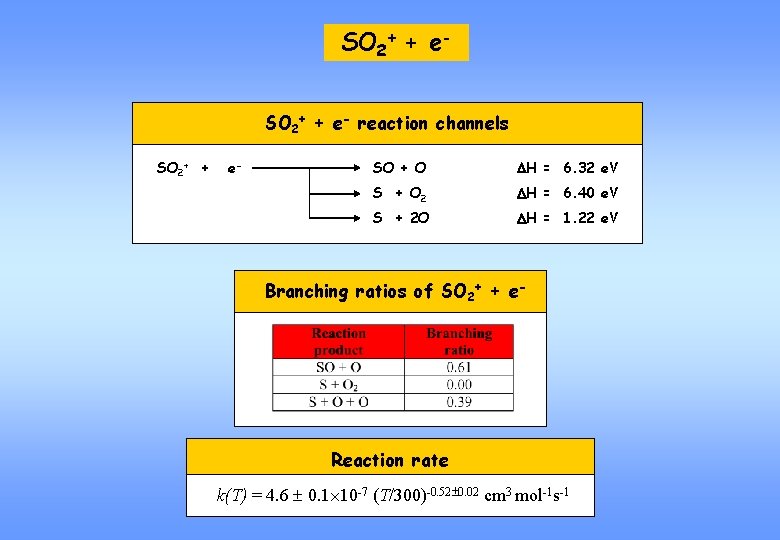
SO 2+ + e- reaction channels SO 2+ + e- SO + O DH = 6. 32 e. V S + O 2 DH = 6. 40 e. V S + 2 O DH = 1. 22 e. V Branching ratios of SO 2+ + e- Reaction rate k(T) = 4. 6 0. 1 10 -7 (T/300)-0. 52 0. 02 cm 3 mol-1 s-1

Consequences Decay of SO 2+ in Io’s ionosphere during eclipse probably caused by DR. Strong observed UV lines of O(I) and S(I) could be due to increased S- and O-atom production by three-body breakup in DR. Possible role in the ionosphere of Venus ?

Methanol in space Responsible for maser emission in star-forming regions. Evolution indicator in star-forming regions Used for determination of kinetic temperature and H 2 density simultaneously. From CH 3 OH 2+/CH 3 OH ratio electron temperature in cometary coma derived. The Bear Claw Nebula, where a strong methanol maser was detected

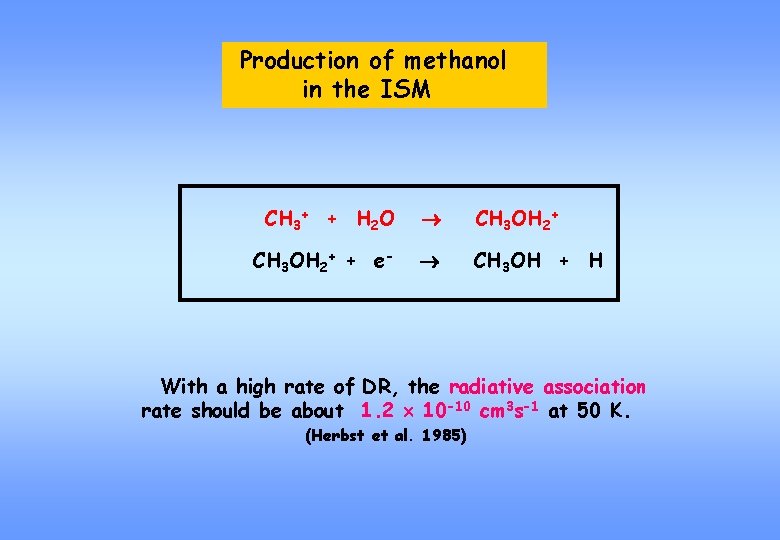
Production of methanol in the ISM CH 3+ + H 2 O CH 3 OH 2+ + e- CH 3 OH + H With a high rate of DR, the radiative association rate should be about 1. 2 10 -10 cm 3 s-1 at 50 K. (Herbst et al. 1985)

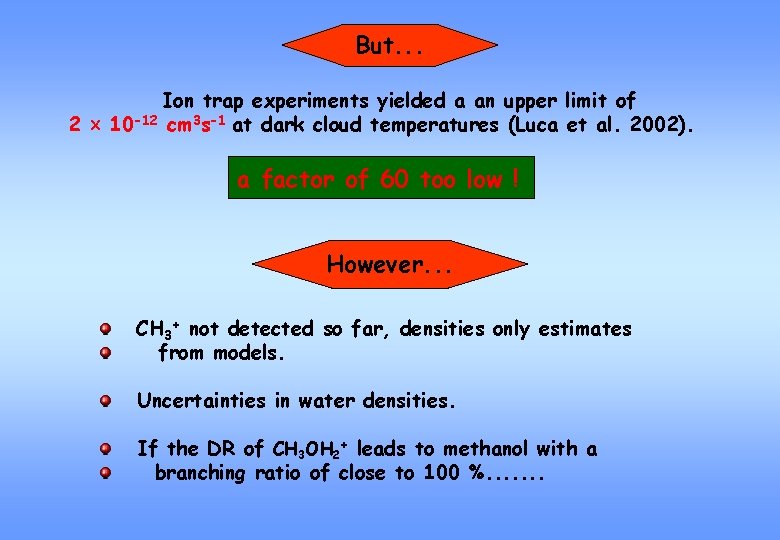
But. . . Ion trap experiments yielded a an upper limit of 2 10 -12 cm 3 s-1 at dark cloud temperatures (Luca et al. 2002). a factor of 60 too low ! However. . . CH 3+ not detected so far, densities only estimates from models. Uncertainties in water densities. If the DR of CH 3 OH 2+ leads to methanol with a branching ratio of close to 100 %. . . .

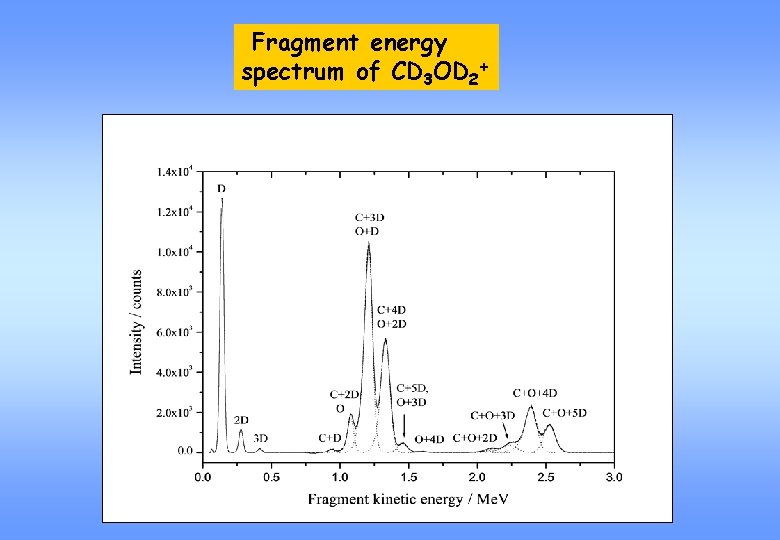
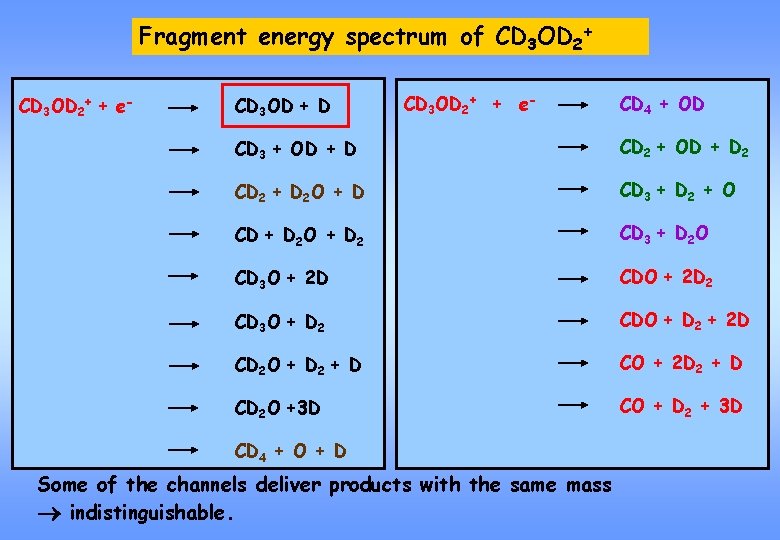
Fragment energy spectrum of CD 3 OD 2+

Fragment energy spectrum of CD 3 OD 2+ + e- CD 3 OD + D CD 3 OD 2+ + e- CD 4 + OD CD 3 + OD + D CD 2 + OD + D 2 CD 2 + D 2 O + D CD 3 + D 2 + O CD + D 2 O + D 2 CD 3 + D 2 O CD 3 O + 2 D CDO + 2 D 2 CD 3 O + D 2 CDO + D 2 + 2 D CD 2 O + D 2 + D CO + 2 D 2 + D CD 2 O +3 D CO + D 2 + 3 D CD 4 + O + D Some of the channels deliver products with the same mass indistinguishable.

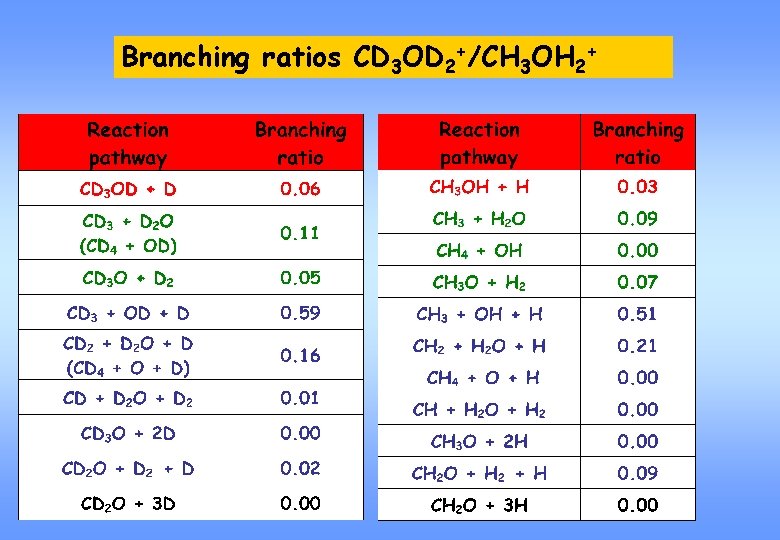
Branching ratios CD 3 OD 2+/CH 3 OH 2+

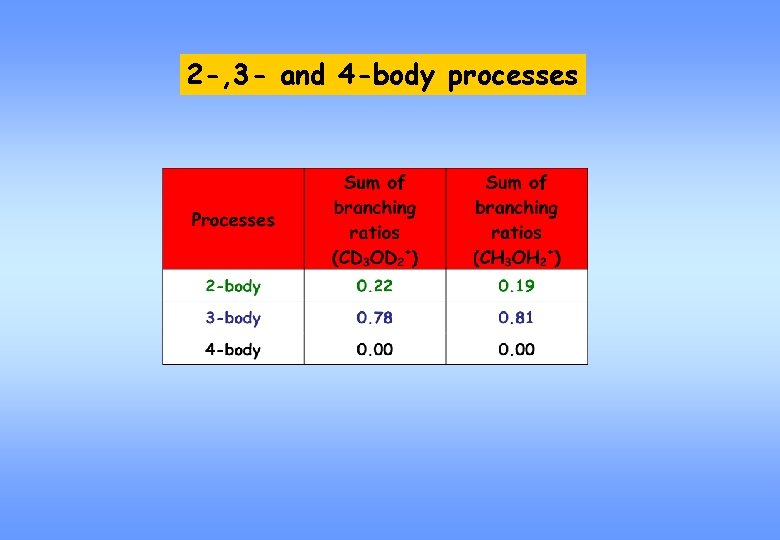
2 -, 3 - and 4 -body processes

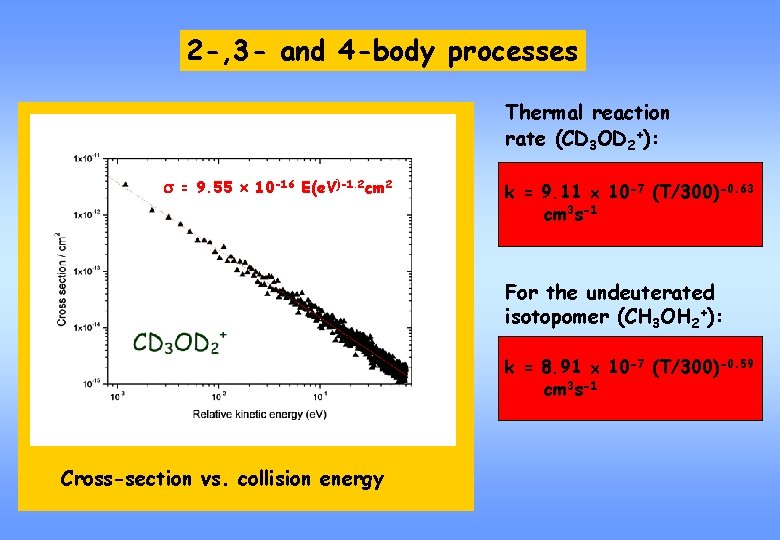
2 -, 3 - and 4 -body processes Thermal reaction rate (CD 3 OD 2+): s = 9. 55 10 -16 E(e. V)-1. 2 cm 2 k = 9. 11 10 -7 (T/300)-0. 63 cm 3 s-1 For the undeuterated isotopomer (CH 3 OH 2+): k = 8. 91 10 -7 (T/300)-0. 59 cm 3 s-1 Cross-section vs. collision energy

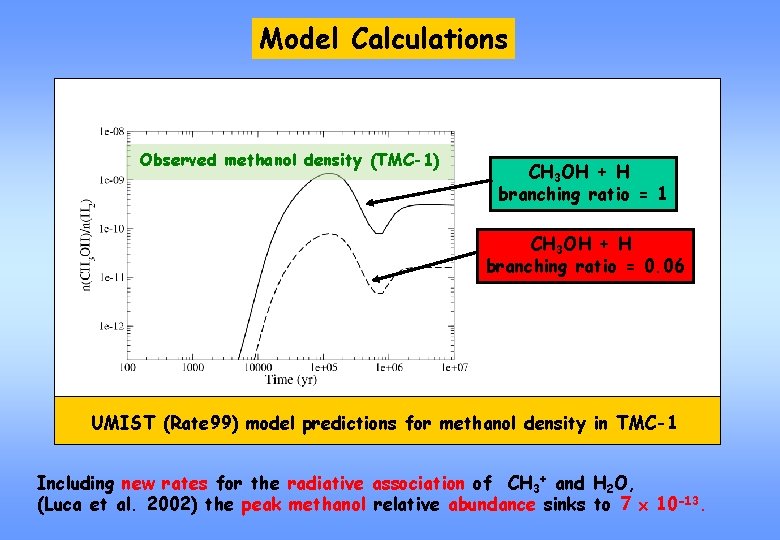
Model Calculations Observed methanol density (TMC-1) CH 3 OH + H branching ratio = 1 CH 3 OH + H branching ratio = 0. 06 UMIST (Rate 99) model predictions for methanol density in TMC-1 Including new rates for the radiative association of CH 3+ and H 2 O, (Luca et al. 2002) the peak methanol relative abundance sinks to 7 10 -13.

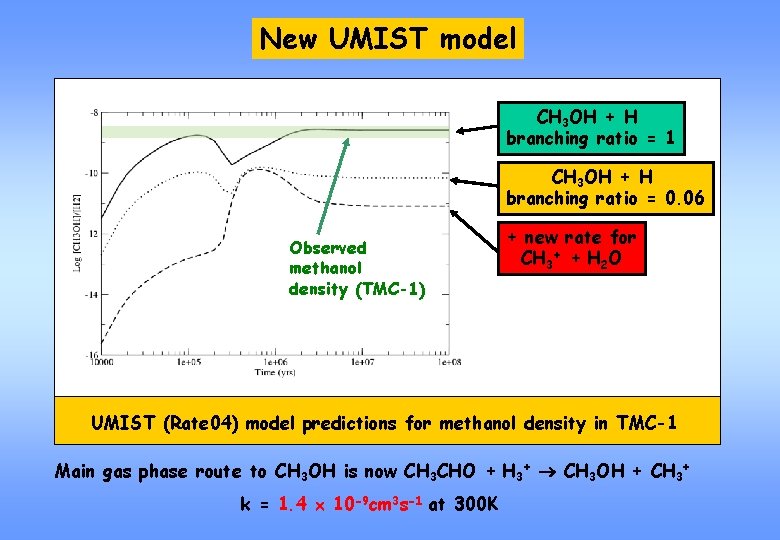
New UMIST model CH 3 OH + H branching ratio = 1 CH 3 OH + H branching ratio = 0. 06 Observed methanol density (TMC-1) + new rate for CH 3+ + H 2 O UMIST (Rate 04) model predictions for methanol density in TMC-1 Main gas phase route to CH 3 OH is now CH 3 CHO + H 3+ CH 3 OH + CH 3+ k = 1. 4 10 -9 cm 3 s-1 at 300 K

Conclusions Three-body break-ups dominate. Production of CH 3 OH only 3 % (CD 3 OD only 6 %). No big isotope effects Gas-phase mechanism for interstellar methanol very unlikely. In line with the following facts: Formation of methanol on CO ice surfaces possible at 10 K. (Watanabe et al. 2004) * Models including grain surface desorption reproduce methanol densities (Herbst 2006)

Can we close the books ? * Anticorrelation of CO and CH 3 OH in dense clouds. (Buckle, 2006) * No experimental evidence for surface desorption of freshly formed methanol

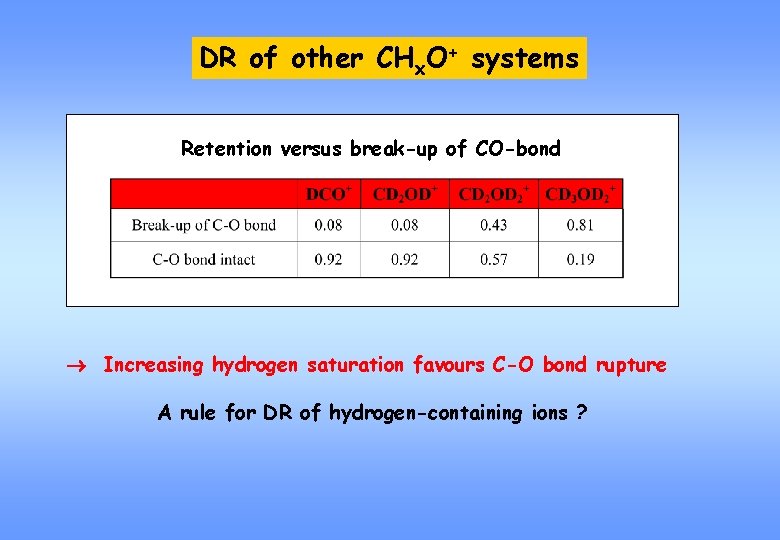
DR of other CHx. O+ systems Retention versus break-up of CO-bond Increasing hydrogen saturation favours C-O bond rupture A rule for DR of hydrogen-containing ions ?

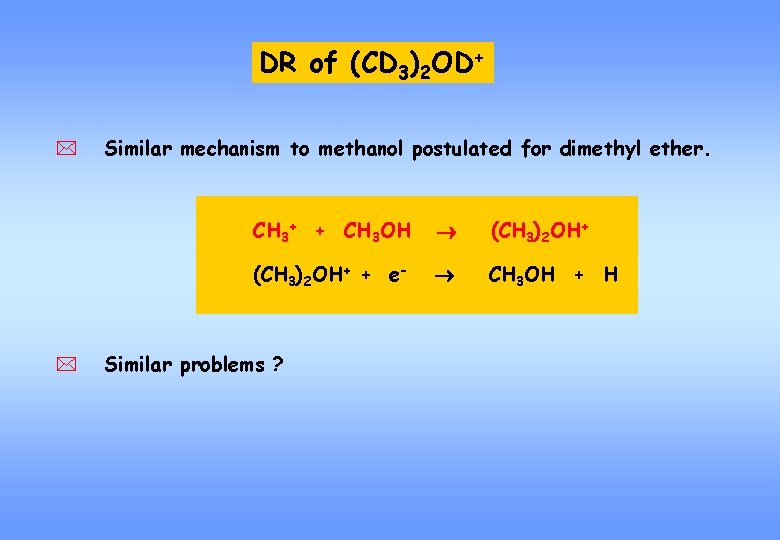
DR of (CD 3)2 OD+ * * Similar mechanism to methanol postulated for dimethyl ether. CH 3+ + CH 3 OH (CH 3)2 OH+ + e- CH 3 OH + H Similar problems ?

YES ! Production of (CD 3)2 O only 6 %) ! AND: Grain surface process formation of dimethyl ether unlikely (Ehrenfreund and co-workers, 2006)

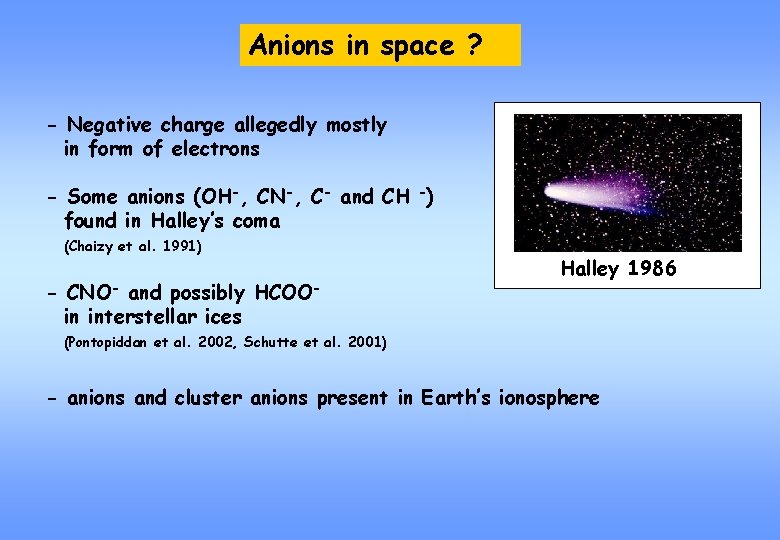
Anions in space ? - Negative charge allegedly mostly in form of electrons - Some anions (OH-, CN-, C- and CH -) found in Halley’s coma (Chaizy et al. 1991) - CNO- and possibly HCOOin interstellar ices Halley 1986 (Pontopiddan et al. 2002, Schutte et al. 2001) - anions and cluster anions present in Earth’s ionosphere

Possible importance of anions in space ? - Role of atomic anions in early universe H+ + H - H + H - Diffuse interstellar bands: possibly PAH anions and carbon-chain anions - CNO- and possibly HCOOin interstellar ices (Pontopiddan et al. 2002, Schutte et al. 2001) - High electron sticking coefficient of lage PAHs - Anion abundance constrains electron density

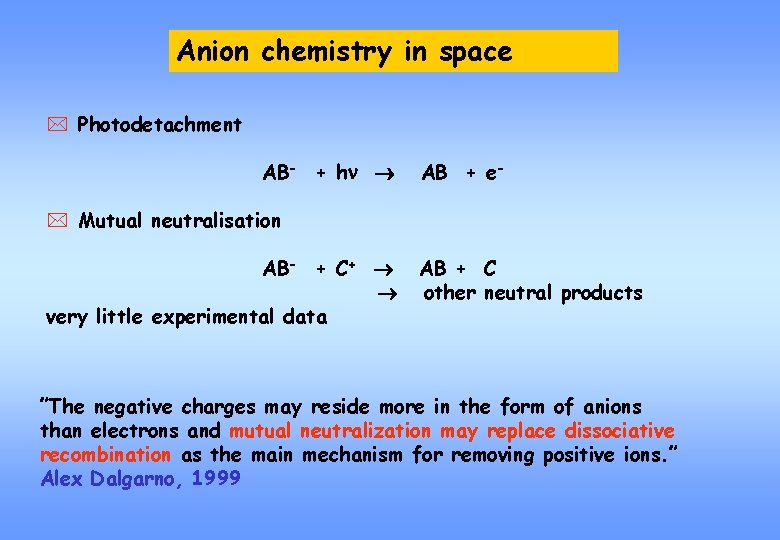
Anion chemistry in space * Photodetachment AB- + hn AB + e- * Mutual neutralisation AB- + C+ very little experimental data AB + C other neutral products ”The negative charges may reside more in the form of anions than electrons and mutual neutralization may replace dissociative recombination as the main mechanism for removing positive ions. ” Alex Dalgarno, 1999

DESIREE storage ring Double Electrostatic Ion Ring Experiment

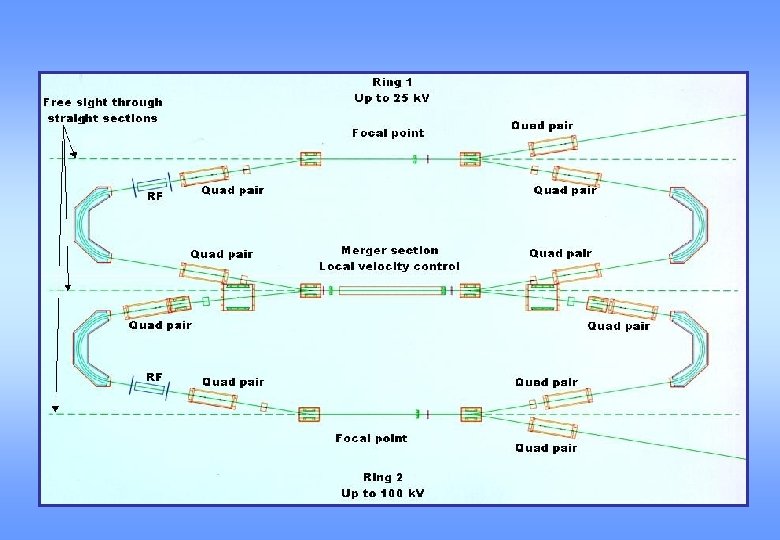
Features of DESIREE * Cryostat cooling down to at least 10 K * No restriction on ion mass * Electrospray ion source for large ions (PAHs) * Windows for laser spectroscopy
