Interpreting ABGs Suneel Kumar MD Arterial Blood Gases
Interpreting ABGs Suneel Kumar MD
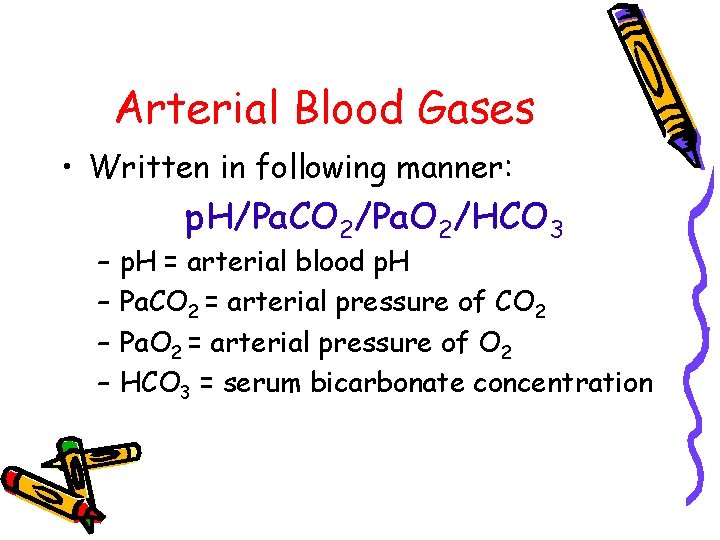
Arterial Blood Gases • Written in following manner: – – p. H/Pa. CO 2/Pa. O 2/HCO 3 p. H = arterial blood p. H Pa. CO 2 = arterial pressure of CO 2 Pa. O 2 = arterial pressure of O 2 HCO 3 = serum bicarbonate concentration
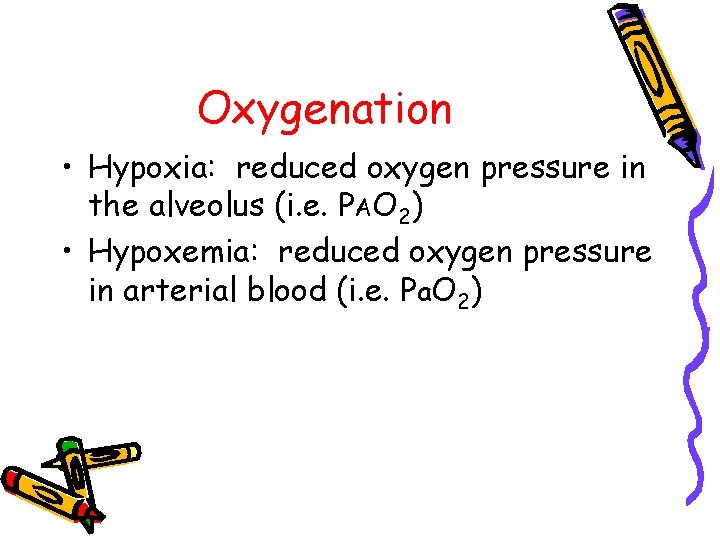
Oxygenation • Hypoxia: reduced oxygen pressure in the alveolus (i. e. PAO 2) • Hypoxemia: reduced oxygen pressure in arterial blood (i. e. Pa. O 2)

Hypoxia with Low Pa. O 2 • Alveolar diffusion impairment • Decreased alveolar PO 2 – Decreased Fi. O 2 – Hypoventilation – High altitude • R L shunt • V/Q mismatch
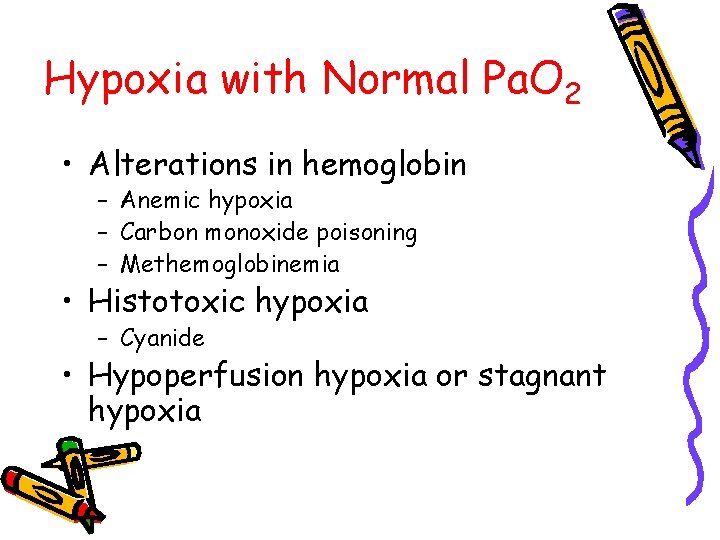
Hypoxia with Normal Pa. O 2 • Alterations in hemoglobin – Anemic hypoxia – Carbon monoxide poisoning – Methemoglobinemia • Histotoxic hypoxia – Cyanide • Hypoperfusion hypoxia or stagnant hypoxia

Alveolar—Arterial Gradient • Indirect measurement of V/Q abnormalities • Normal A-a gradient is 10 mm. Hg • Rises with age • Rises by 5 -7 mm. Hg for every 0. 10 rise in Fi. O 2, from loss of hypoxic vasoconstriction in the lungs
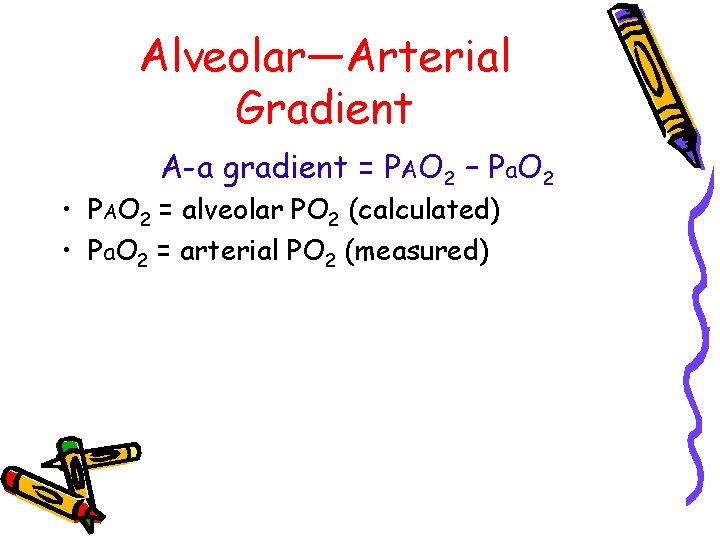
Alveolar—Arterial Gradient A-a gradient = PAO 2 – Pa. O 2 • PAO 2 = alveolar PO 2 (calculated) • Pa. O 2 = arterial PO 2 (measured)
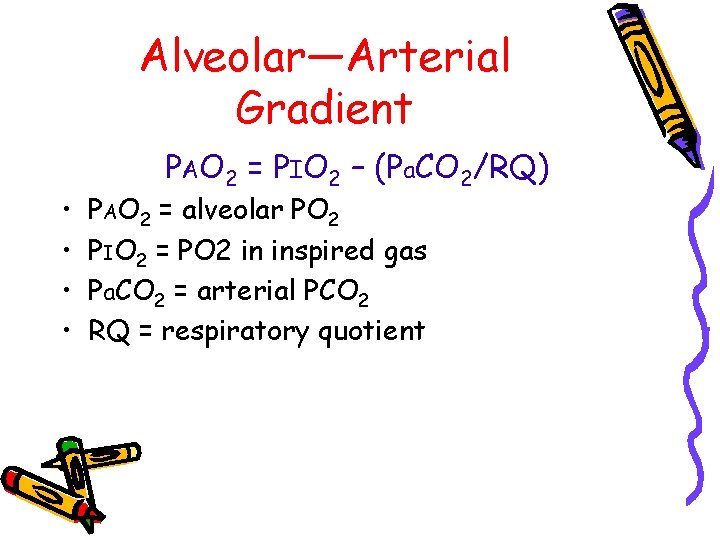
Alveolar—Arterial Gradient • • PAO 2 = PIO 2 – (Pa. CO 2/RQ) PAO 2 = alveolar PO 2 PIO 2 = PO 2 in inspired gas Pa. CO 2 = arterial PCO 2 RQ = respiratory quotient
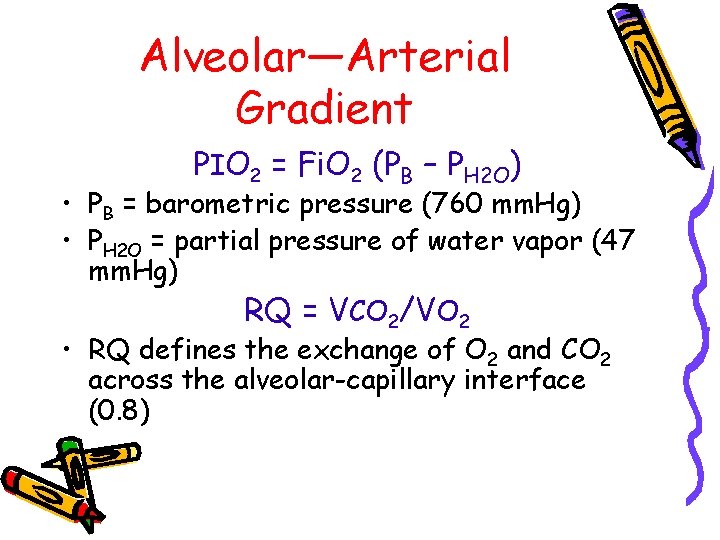
Alveolar—Arterial Gradient PIO 2 = Fi. O 2 (PB – PH 2 O) • PB = barometric pressure (760 mm. Hg) • PH 2 O = partial pressure of water vapor (47 mm. Hg) RQ = VCO 2/VO 2 • RQ defines the exchange of O 2 and CO 2 across the alveolar-capillary interface (0. 8)
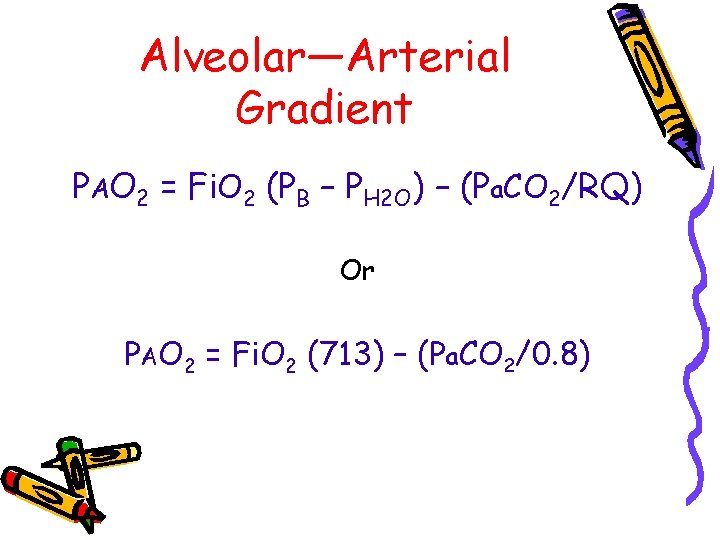
Alveolar—Arterial Gradient PAO 2 = Fi. O 2 (PB – PH 2 O) – (Pa. CO 2/RQ) Or PAO 2 = Fi. O 2 (713) – (Pa. CO 2/0. 8)
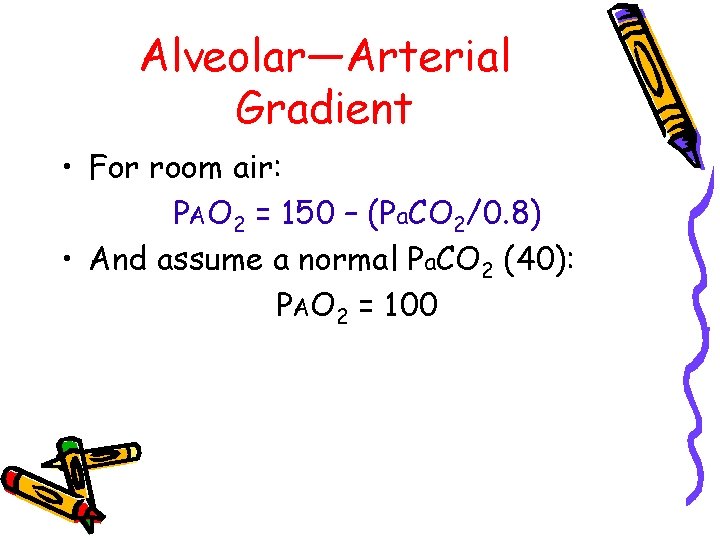
Alveolar—Arterial Gradient • For room air: PAO 2 = 150 – (Pa. CO 2/0. 8) • And assume a normal Pa. CO 2 (40): PAO 2 = 100

Acid-Base • Acidosis or alkalosis: any disorder that causes an alteration in p. H • Acidemia or alkalemia: alteration in blood p. H; may be result of one or more disorders.
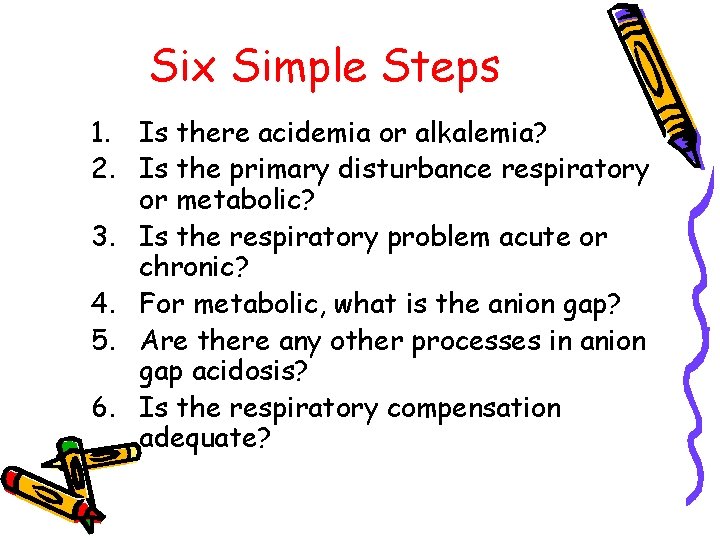
Six Simple Steps 1. Is there acidemia or alkalemia? 2. Is the primary disturbance respiratory or metabolic? 3. Is the respiratory problem acute or chronic? 4. For metabolic, what is the anion gap? 5. Are there any other processes in anion gap acidosis? 6. Is the respiratory compensation adequate?
![Henderson-Hasselbach Equation p. H = p. K + log [HCO 3/Pa. CO 2] x Henderson-Hasselbach Equation p. H = p. K + log [HCO 3/Pa. CO 2] x](http://slidetodoc.com/presentation_image_h/d8f1b3cc157c163b5e7d0cf8764df274/image-14.jpg)
Henderson-Hasselbach Equation p. H = p. K + log [HCO 3/Pa. CO 2] x K (K = dissociation constant of CO 2) Or [H+] = 24 x Pa. CO 2/HCO 3
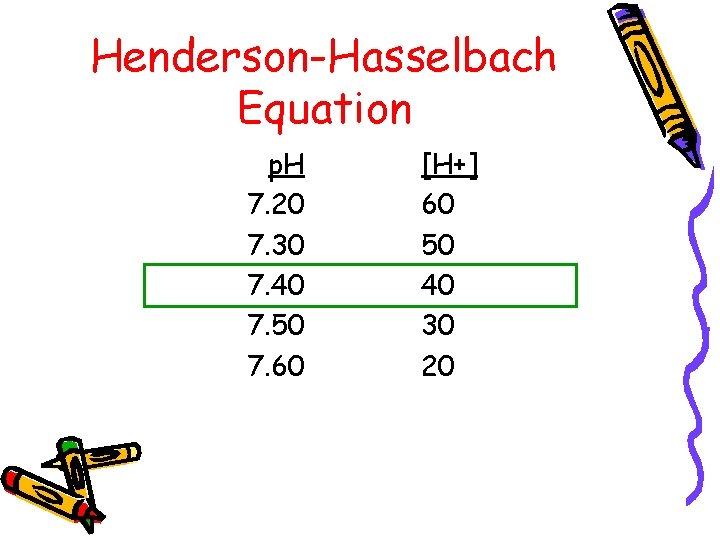
Henderson-Hasselbach Equation p. H 7. 20 7. 30 7. 40 7. 50 7. 60 [H+] 60 50 40 30 20
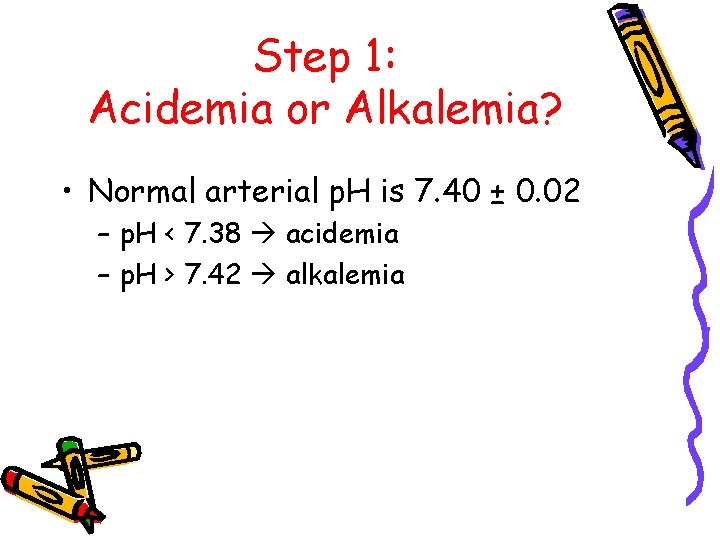
Step 1: Acidemia or Alkalemia? • Normal arterial p. H is 7. 40 ± 0. 02 – p. H < 7. 38 acidemia – p. H > 7. 42 alkalemia

Step 2: Primary Disturbance • Anything that alters HCO 3 is a metabolic process • Anything that alters Pa. CO 2 is a respiratory process

Step 2: Primary Disturbance • If 6 p. H, there is either 5 Pa. CO 2 or 6 HCO 3 • If 5 p. H, there is either 6 Pa. CO 2 or 5 HCO 3

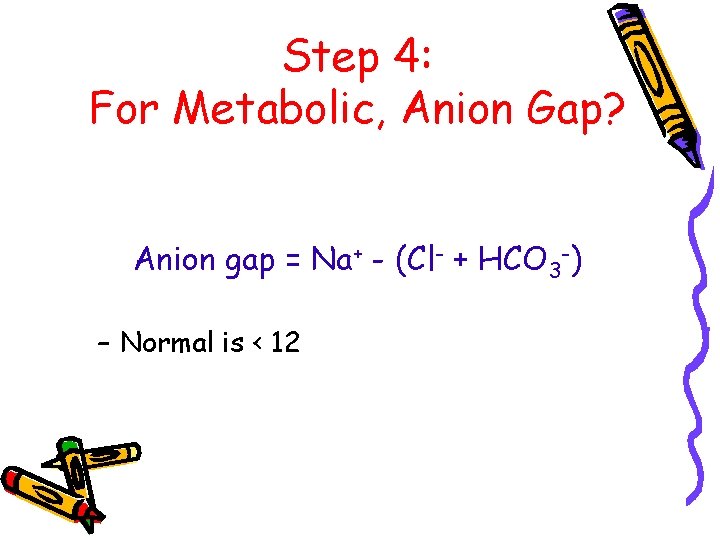
Step 4: For Metabolic, Anion Gap? Anion gap = Na+ - (Cl- + HCO 3 -) – Normal is < 12

Increased Anion Gap • Ingestion of drugs or toxins – – – – Ethanol Methanol Ethylene glycol Paraldehyde Toluene Ammonium chloride Salicylates

Increased Anion Gap • Ketoacidosis – DKA – Alcoholic – Starvation • Lactic acidosis • Renal failure

Step 4: For Metabolic, Anion Gap? • If + AG, calculate Osm gap: Calc Osm = (2 x Na+) + (glucose/18) + (BUN/2. 8) + (Et. OH/4. 6) Osm gap = measured Osm – calc Osm Normal < 10 m. Osm/kg
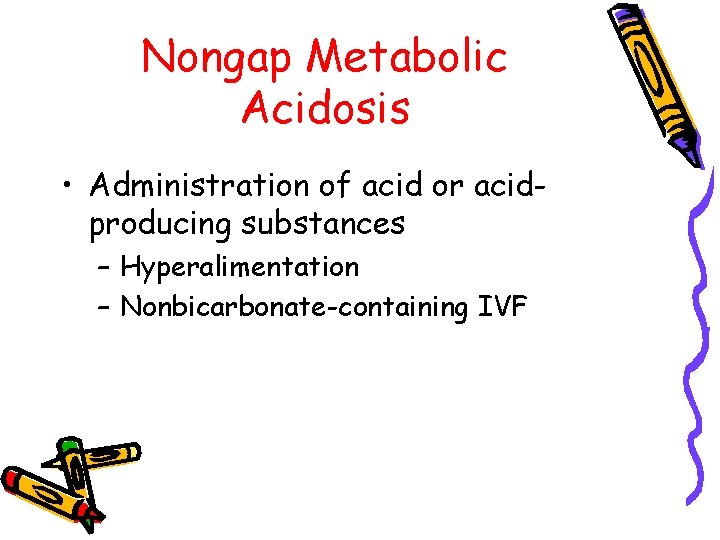
Nongap Metabolic Acidosis • Administration of acid or acidproducing substances – Hyperalimentation – Nonbicarbonate-containing IVF
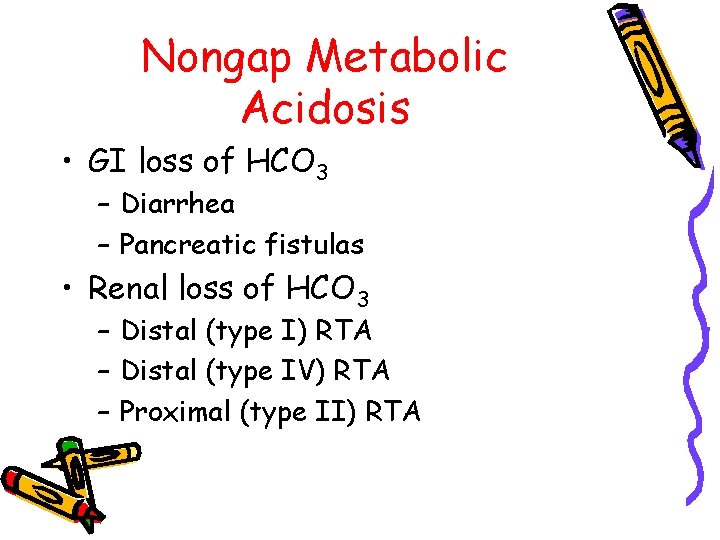
Nongap Metabolic Acidosis • GI loss of HCO 3 – Diarrhea – Pancreatic fistulas • Renal loss of HCO 3 – Distal (type I) RTA – Distal (type IV) RTA – Proximal (type II) RTA
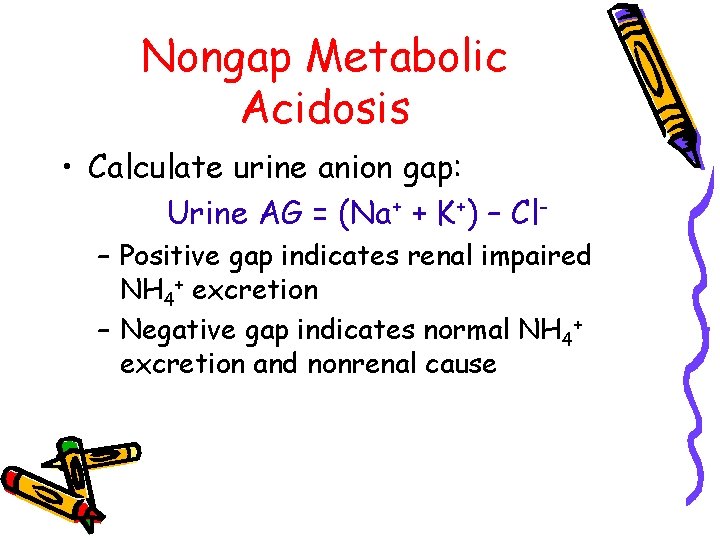
Nongap Metabolic Acidosis • Calculate urine anion gap: Urine AG = (Na+ + K+) – Cl– Positive gap indicates renal impaired NH 4+ excretion – Negative gap indicates normal NH 4+ excretion and nonrenal cause
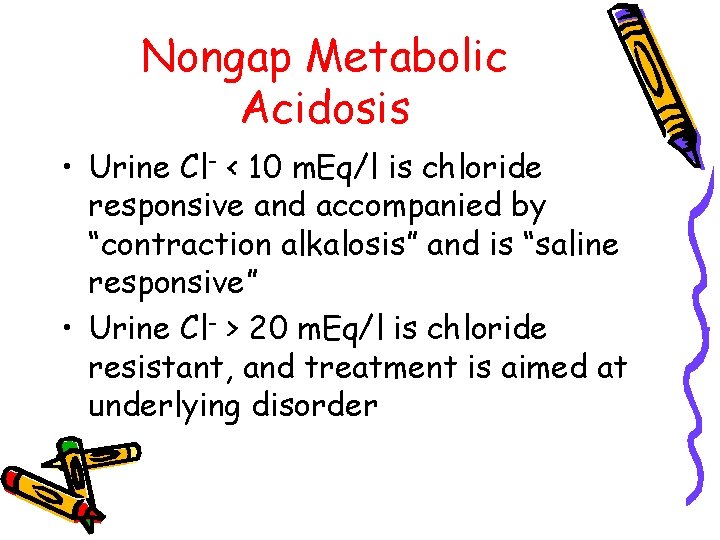
Nongap Metabolic Acidosis • Urine Cl- < 10 m. Eq/l is chloride responsive and accompanied by “contraction alkalosis” and is “saline responsive” • Urine Cl- > 20 m. Eq/l is chloride resistant, and treatment is aimed at underlying disorder

Step 5: Any other process with elevated AG? • Calculate rgap, or “gap-gap”: r. Gap = Measured AG – Normal AG (12)

Step 5: Any other process with elevated AG? • Add rgap to measured HCO 3 – If normal (22 -26), no other metabolic problems – If < 22, then concomitant metabolic acidosis – If > 26, then concomitant metabolic alkalosis

Step 6: Adequate respiratory compensation? Winter’s Formula Expected Pa. CO 2 = (1. 5 x HCO 3) + 8 ± 2 – If measured Pa. CO 2 is higher, then concomitant respiratory acidosis – If measured Pa. CO 2 is lower, then concomitant respiratory alkalosis

Step 6: Adequate respiratory compensation? • In metabolic alkalosis, Winter’s formula does not predict the respiratory response – Pa. CO 2 will rise > 40 mm. Hg, but not exceed 50 -55 mm. Hg – For respiratory compensation, p. H will remain > 7. 42

Clues to a Mixed Disorder • Normal p. H with abnormal Pa. CO 2 or HCO 3 • Pa. CO 2 and HCO 3 move in opposite directions • p. H changes in opposite direction for a known primary disorder

Case 1 • A 24 year old student on the 6 year undergraduate plan is brought to the ER cyanotic and profoundly weak. His roommate has just returned from a semester in Africa. The patient had been observed admiring his roommate's authentic African blowgun and had scraped his finger on the tip of one of the poison darts (curare).
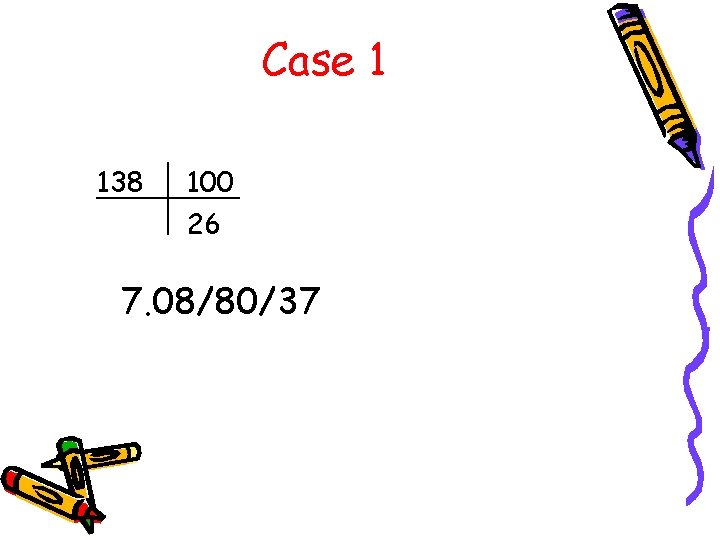
Case 1 138 100 26 7. 08/80/37

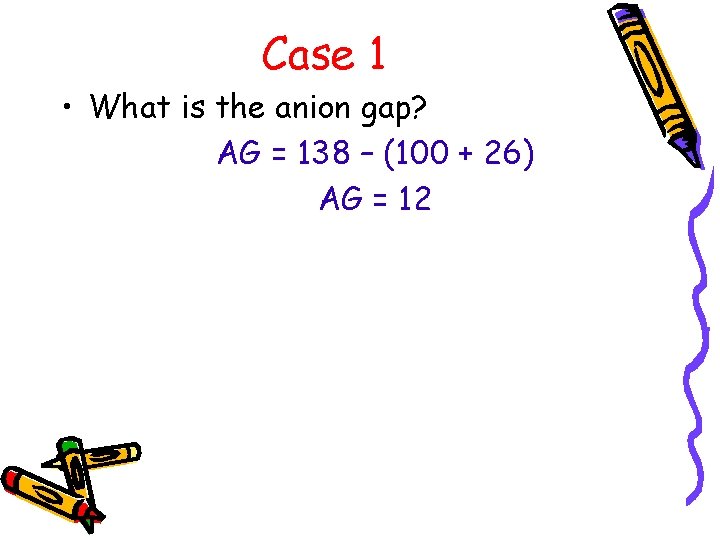
Case 1 • What is the anion gap? AG = 138 – (100 + 26) AG = 12

Case 1 • Acute respiratory acidosis

Case 2 • A 42 year old diabetic female who has been on insulin since the age of 13 presents with a 4 day history of dysuria which has progressed to severe right flank pain. She has a temperature of 38. 8ºC, a WBC of 14, 000, and is disoriented.

Case 2 135 4. 8 99 12 7. 23/25/113
![Case 2 • What is the A-a gradient? A-a = [150 – 25/0. 8] Case 2 • What is the A-a gradient? A-a = [150 – 25/0. 8]](http://slidetodoc.com/presentation_image_h/d8f1b3cc157c163b5e7d0cf8764df274/image-40.jpg)
Case 2 • What is the A-a gradient? A-a = [150 – 25/0. 8] – 113 = 6 • Acidemia or alkalemia? • Primary respiratory or metabolic? • What is the anion gap? AG = 135 – (99 + 12) = 24
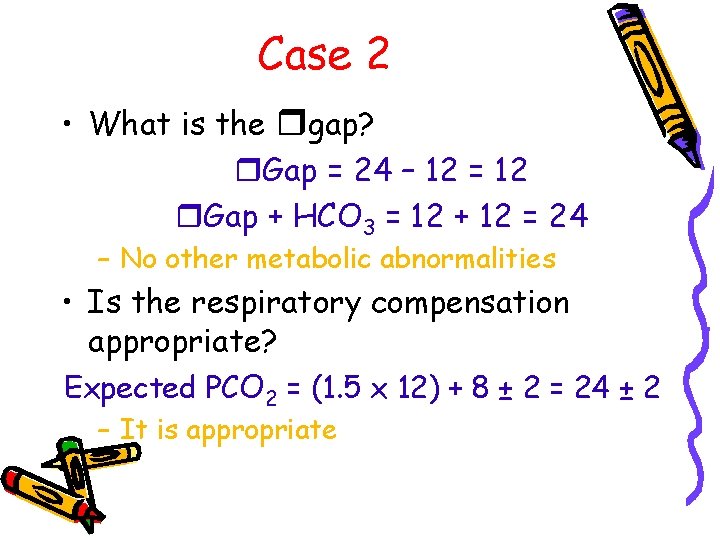
Case 2 • What is the rgap? r. Gap = 24 – 12 = 12 r. Gap + HCO 3 = 12 + 12 = 24 – No other metabolic abnormalities • Is the respiratory compensation appropriate? Expected PCO 2 = (1. 5 x 12) + 8 ± 2 = 24 ± 2 – It is appropriate
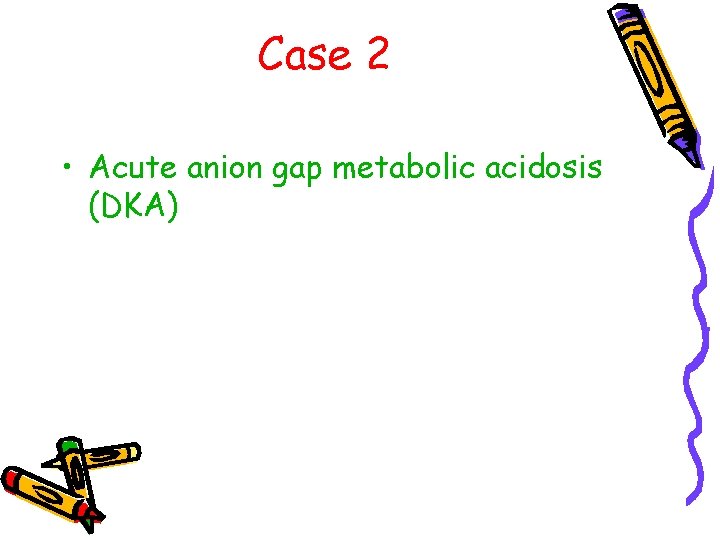
Case 2 • Acute anion gap metabolic acidosis (DKA)

Case 3 • A 71 year old male, retired machinist, is admitted to the ICU with a history of increasing dyspnea, cough, and sputum production. He has a 120 pack -year smoking history, and quit 5 years previously. On exam he is moving minimal air despite using his accessory muscles of respiration. He has acral cyanosis.
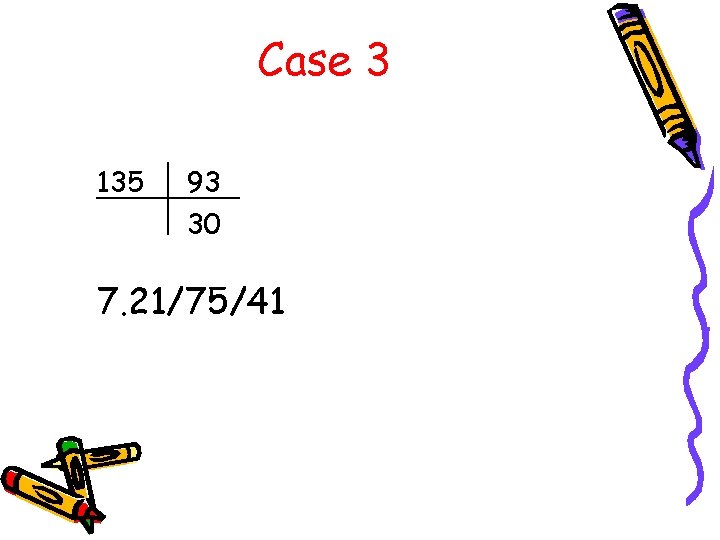
Case 3 135 93 30 7. 21/75/41
![Case 3 • What is the A-a gradient? A-a = [150 – 75/. 8] Case 3 • What is the A-a gradient? A-a = [150 – 75/. 8]](http://slidetodoc.com/presentation_image_h/d8f1b3cc157c163b5e7d0cf8764df274/image-45.jpg)
Case 3 • What is the A-a gradient? A-a = [150 – 75/. 8] – 41 = 15 • Acidemic or alkalemic? • Primary respiratory or metabolic? • Acute or chronic? – Acute 5 PCO 2 by 35 would 6 p. H by 0. 28 – Chronic 5 PCO 2 by 35 would 6 p. H by 0. 105 • Somewhere in between
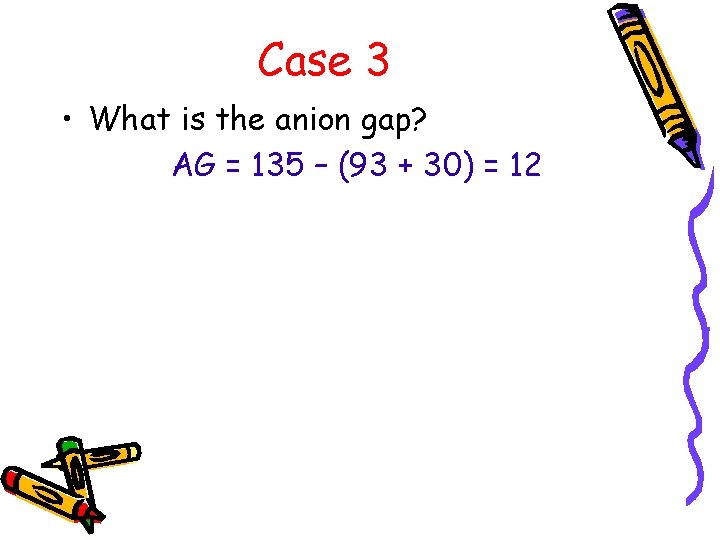
Case 3 • What is the anion gap? AG = 135 – (93 + 30) = 12

Case 3 • Acute on chronic respiratory acidosis (COPD)

Case 3 b • This same patient is intubated and mechanically ventilated. During the intubation he vomits and aspirates. He is ventilated with an Fi. O 2 of 50%, tidal volumes of 850 cc, PEEP of 5, rate of 10. One hour later his ABG is 7. 48/37/215.
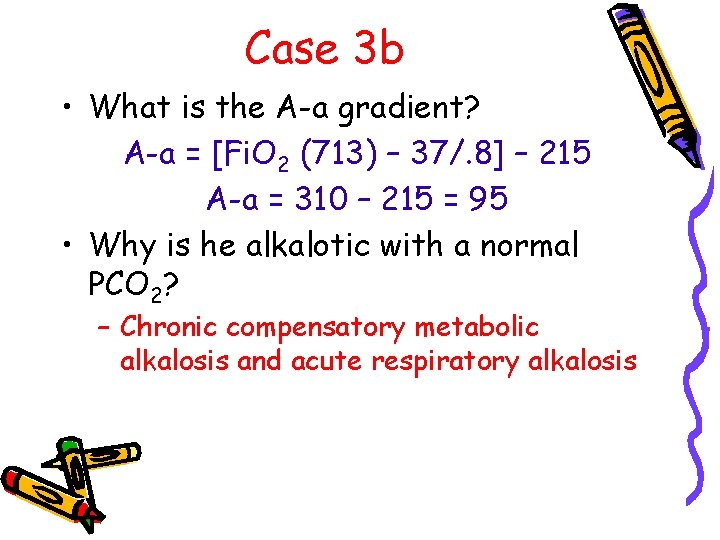
Case 3 b • What is the A-a gradient? A-a = [Fi. O 2 (713) – 37/. 8] – 215 A-a = 310 – 215 = 95 • Why is he alkalotic with a normal PCO 2? – Chronic compensatory metabolic alkalosis and acute respiratory alkalosis

Case 4 • A 23 year old female presents to the Emergency Room complaining of chest tightness and light-headedness. Other symptoms include tingling and numbness in her fingertips and around her mouth. Her medications include Xanax and birth control pills, but she recently ran out of both.
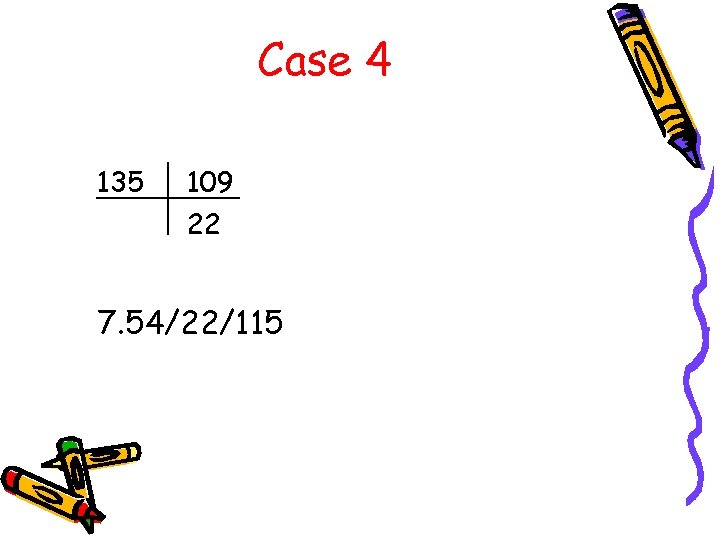
Case 4 135 109 22 7. 54/22/115
![Case 4 • What is the A-a gradient? A-a = [150 – 22/. 8] Case 4 • What is the A-a gradient? A-a = [150 – 22/. 8]](http://slidetodoc.com/presentation_image_h/d8f1b3cc157c163b5e7d0cf8764df274/image-52.jpg)
Case 4 • What is the A-a gradient? A-a = [150 – 22/. 8] – 115 = 8 • Acidemia or alkalemia? • Primary respiratory or metabolic? • Acute or chronic? – Acute 6 CO 2 by 18 would 5 p. H by 0. 144 • What is the anion gap? AG = 135 – (109 + 22) = 4
Case 4 • Acute respiratory alkalosis (panic attack)
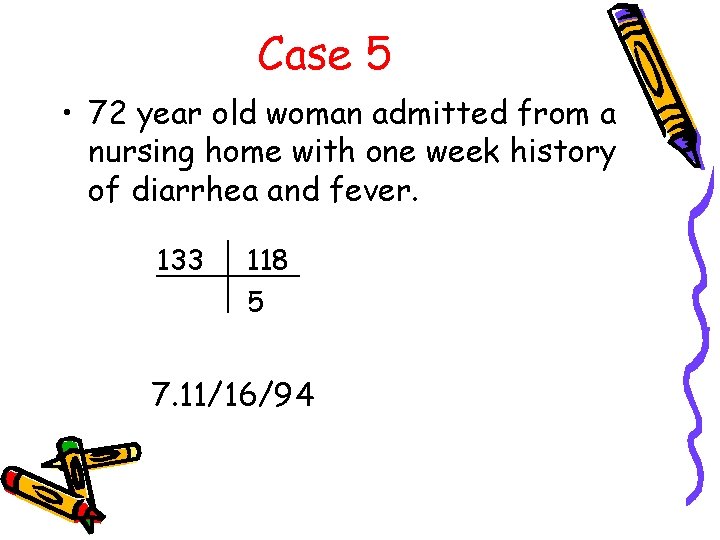
Case 5 • 72 year old woman admitted from a nursing home with one week history of diarrhea and fever. 133 118 5 7. 11/16/94
![Case 5 • What is the A-a gradient? A-a = [150 – 16/. 8] Case 5 • What is the A-a gradient? A-a = [150 – 16/. 8]](http://slidetodoc.com/presentation_image_h/d8f1b3cc157c163b5e7d0cf8764df274/image-55.jpg)
Case 5 • What is the A-a gradient? A-a = [150 – 16/. 8] – 94 = 36 • Acidemia or alkalemia? • Primary respiratory or metabolic? • What is the anion gap? AG = 133 – (118 + 5) = 10 • Is respiratory compensation adequate? PCO 2 = (1. 5 x 5) + 8 ± 2 = 16 ± 2

Case 5 • Non anion gap metabolic acidosis (diarrhea) • Compensatory respiratory alkalosis

Case 6 • A 27 year old pregnant alcoholic with IDDM is admitted one week after stopping insulin and beginning a drinking binge. She has experienced severe nausea and vomiting for several days.
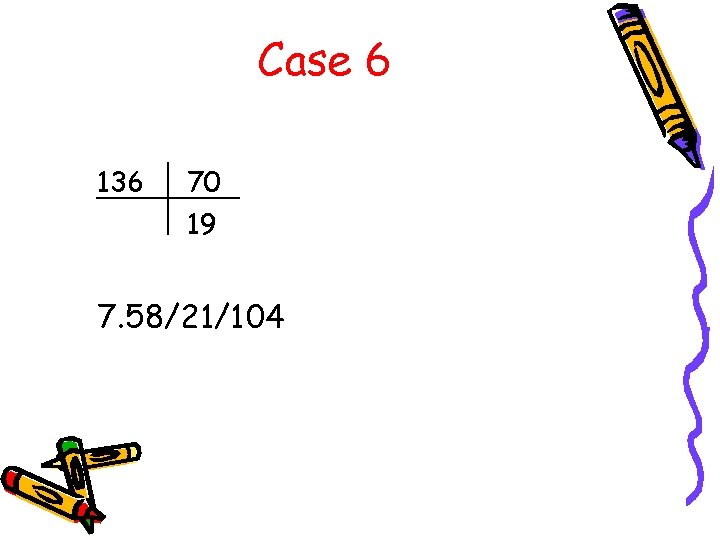
Case 6 136 70 19 7. 58/21/104
![Case 6 • What is the A-a gradient? A-a = [150 – 21/. 8] Case 6 • What is the A-a gradient? A-a = [150 – 21/. 8]](http://slidetodoc.com/presentation_image_h/d8f1b3cc157c163b5e7d0cf8764df274/image-59.jpg)
Case 6 • What is the A-a gradient? A-a = [150 – 21/. 8] – 104 = 20 • Acidemia or alkalemia? • Primary respiratory or metabolic? • What is the anion gap? AG = 136 – (70 + 19) = 47 • What is the rgap? r. Gap = 47 -12 = 35 r. Gap + HCO 3 = 54

Case 6 • Primary respiratory alkalosis (pregnancy) • Anion gap metabolic acidosos (ketoacidosis) • Metabolic alkalosis (vomiting)

Case 7 • 35 year old male presents to the ER unconscious. 145 70 23 7. 61/24/78 Creat 6. 1
![Case 7 • What is the A-a gradient? A-a = [150 – 24/. 8] Case 7 • What is the A-a gradient? A-a = [150 – 24/. 8]](http://slidetodoc.com/presentation_image_h/d8f1b3cc157c163b5e7d0cf8764df274/image-62.jpg)
Case 7 • What is the A-a gradient? A-a = [150 – 24/. 8] – 78 = 42 • Acidemia or alkalemia? • Primary respiratory or metabolic? • What is the anion gap? AG = 145 – (70 + 23) = 52
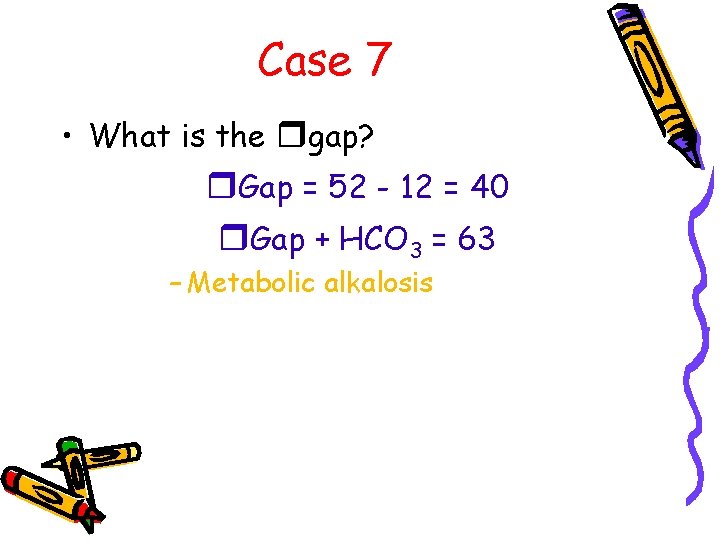
Case 7 • What is the rgap? r. Gap = 52 - 12 = 40 r. Gap + HCO 3 = 63 – Metabolic alkalosis
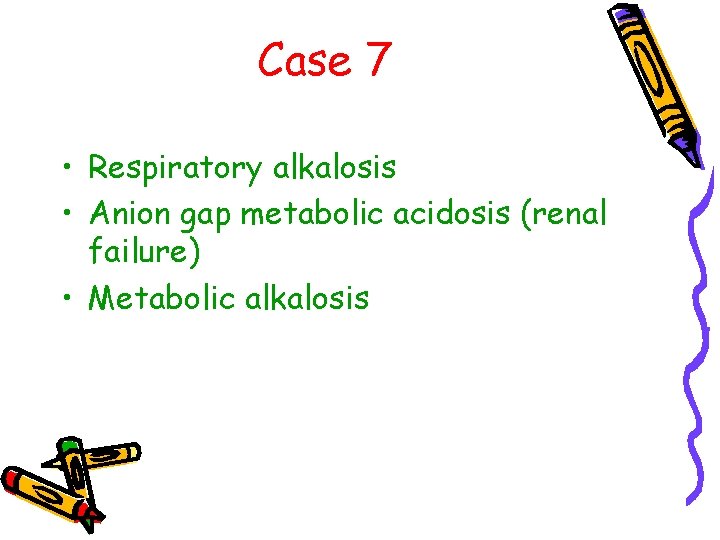
Case 7 • Respiratory alkalosis • Anion gap metabolic acidosis (renal failure) • Metabolic alkalosis

Bonus Case #1 • 51 year old man with polysubstance abuse, presented to ER with 3 -4 day h/o N/V and diffuse abdominal pain. Reports no Et. OH or cocaine in 2 weeks. He has been taking “a lot” of aspirin for pain. Denies dyspnea, but has been tachypneic since arrival.

Bonus Case #1 • Afebrile, P 89, R 20, BP 142/57. Lethargic but arrousable, easily aggitated. Lungs clear, and abdomen is soft with mild tenderness in LUQ and LLQ.
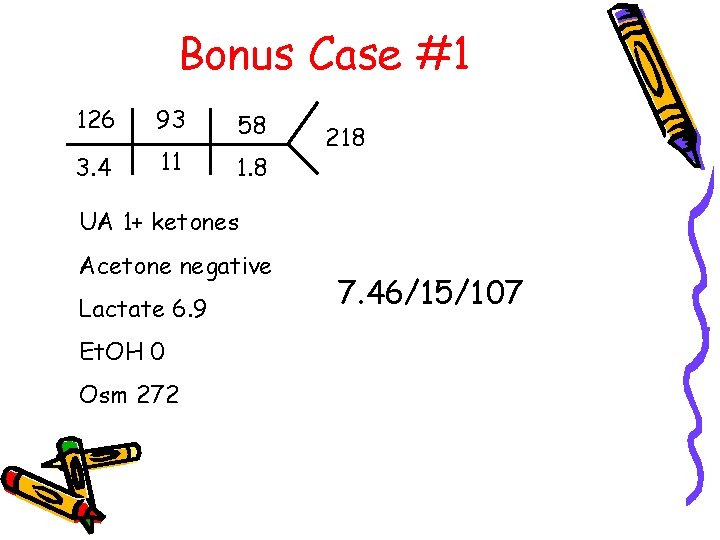
Bonus Case #1 126 93 58 3. 4 11 1. 8 218 UA 1+ ketones Acetone negative Lactate 6. 9 Et. OH 0 Osm 272 7. 46/15/107
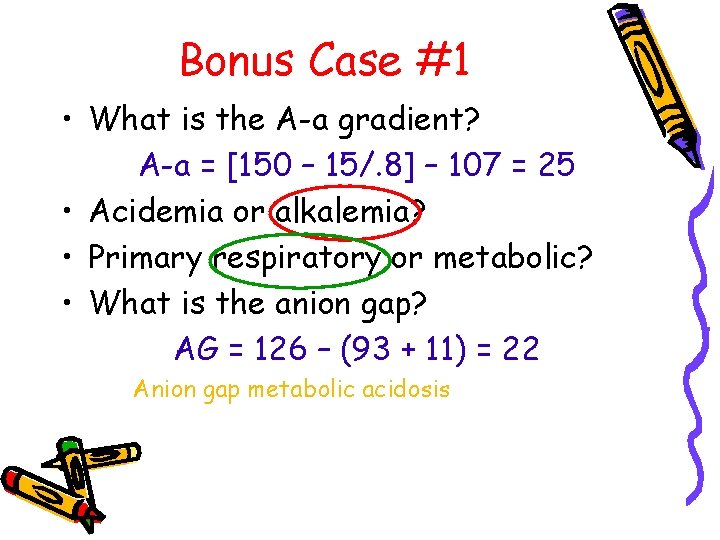
Bonus Case #1 • What is the A-a gradient? A-a = [150 – 15/. 8] – 107 = 25 • Acidemia or alkalemia? • Primary respiratory or metabolic? • What is the anion gap? AG = 126 – (93 + 11) = 22 Anion gap metabolic acidosis
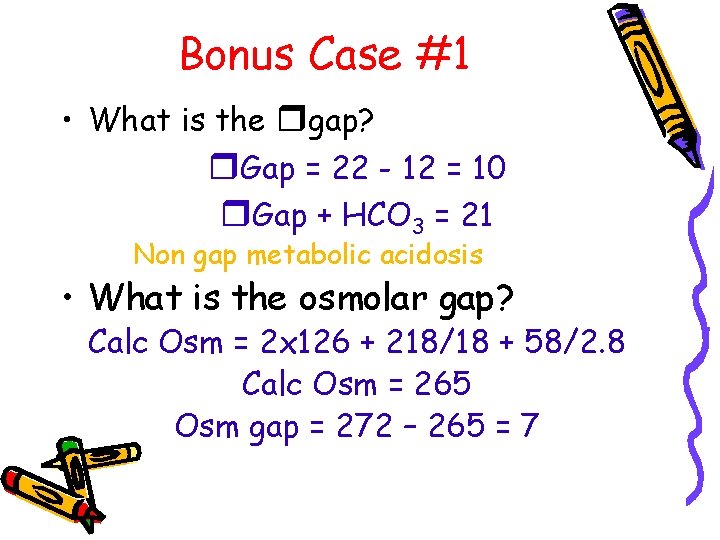
Bonus Case #1 • What is the rgap? r. Gap = 22 - 12 = 10 r. Gap + HCO 3 = 21 Non gap metabolic acidosis • What is the osmolar gap? Calc Osm = 2 x 126 + 218/18 + 58/2. 8 Calc Osm = 265 Osm gap = 272 – 265 = 7

Bonus Case #1 • Respiratory alkalosis (aspirin) • Anion gap metabolic acidosis (aspirin) • Non gap metabolic acidosis

Bonus Case # 2 • 20 year old college student brought to the ER by his fraternity brothers because they cannot wake him up. He had been in excellent health until the prior night.

Bonus Case #2 • Afebrile, P 118, R 32, BP 120/70. Anicteric sclerae, pupils 8 mm and poorly responsive to light. Fundoscopic exam with slight blurring of discs bilaterally and increased retinal sheen. Remainder of exam unremarkable.
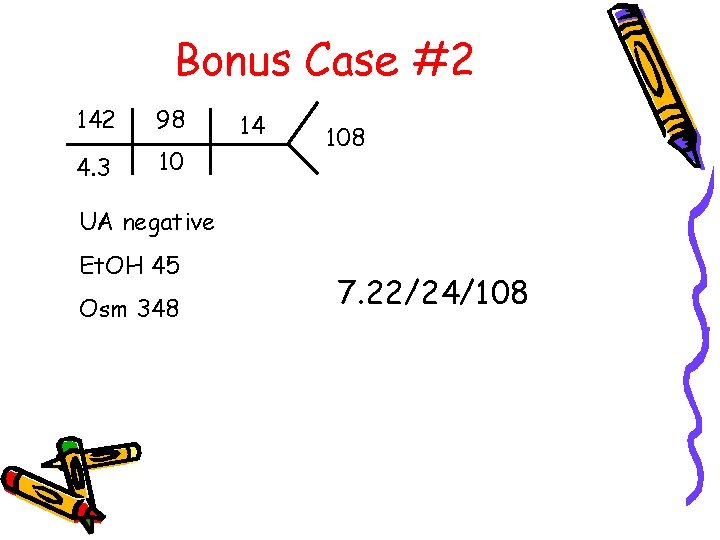
Bonus Case #2 142 98 4. 3 10 14 108 UA negative Et. OH 45 Osm 348 7. 22/24/108
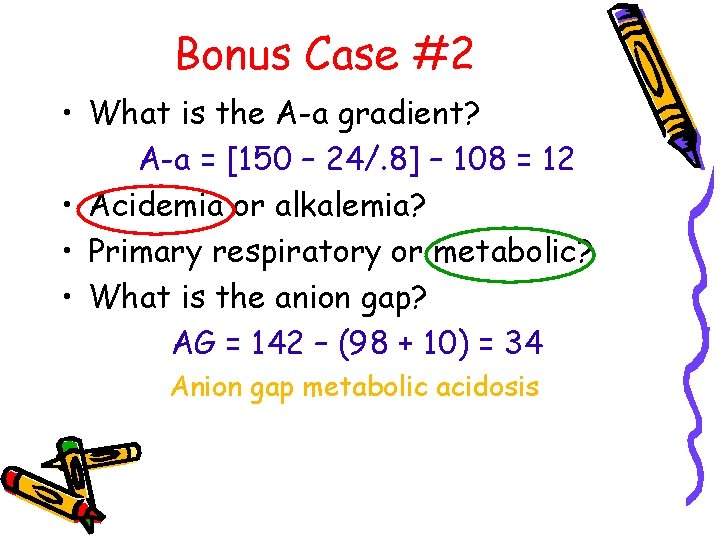
Bonus Case #2 • What is the A-a gradient? A-a = [150 – 24/. 8] – 108 = 12 • Acidemia or alkalemia? • Primary respiratory or metabolic? • What is the anion gap? AG = 142 – (98 + 10) = 34 Anion gap metabolic acidosis

Bonus Case #2 • What is the rgap? r. Gap = 34 - 12 = 22 r. Gap + HCO 3 = 32 Metabolic alkalosis
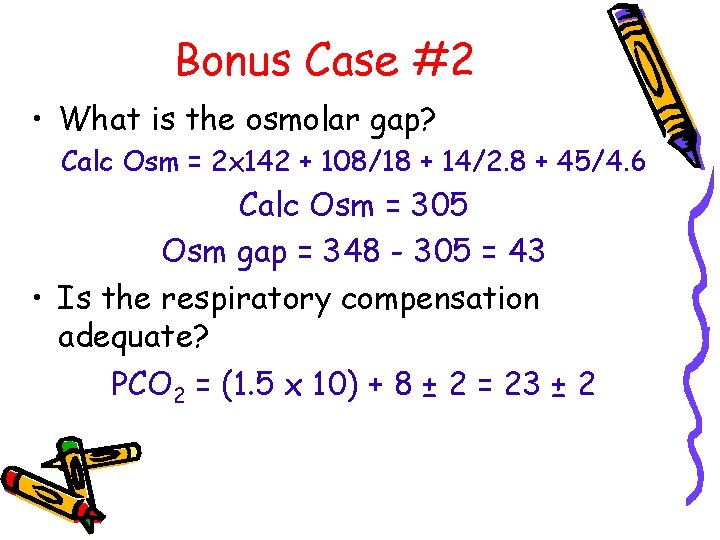
Bonus Case #2 • What is the osmolar gap? Calc Osm = 2 x 142 + 108/18 + 14/2. 8 + 45/4. 6 Calc Osm = 305 Osm gap = 348 - 305 = 43 • Is the respiratory compensation adequate? PCO 2 = (1. 5 x 10) + 8 ± 2 = 23 ± 2

Bonus Case #2 • Anion gap metabolic acidosis with elevated osmolar gap (methanol) • Metabolic alkalosis • Compensatory respiratory alkalosis

Bonus Case #3 • A 23 year old man presents with confusion. He has had diabetes since age 12, and has been suffering from an intestinal flu for the last 24 hours. He has not been eating much, has vague stomach pain, stopped taking his insulin, and has been vomiting. His glucose is high.
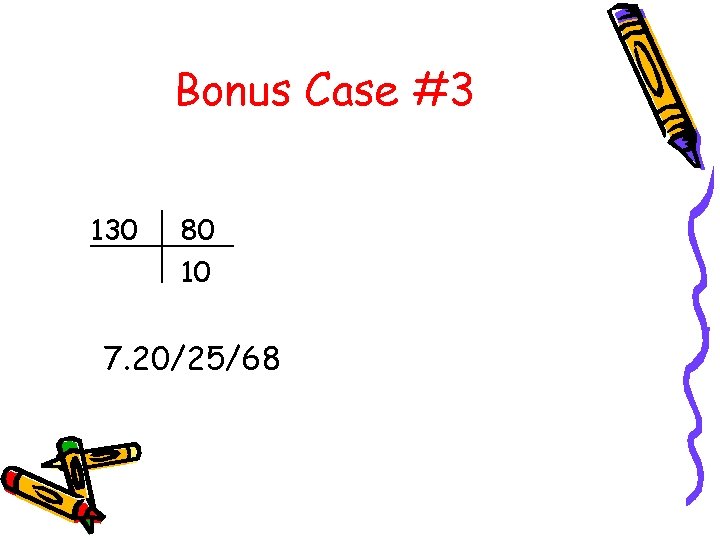
Bonus Case #3 130 80 10 7. 20/25/68
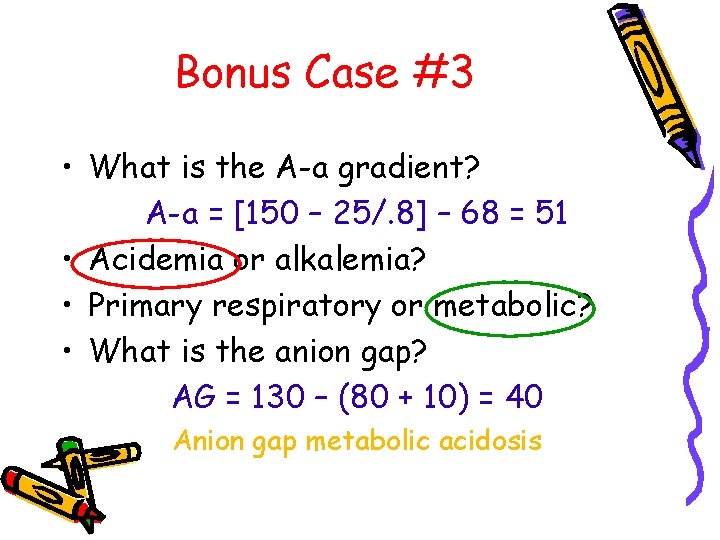
Bonus Case #3 • What is the A-a gradient? A-a = [150 – 25/. 8] – 68 = 51 • Acidemia or alkalemia? • Primary respiratory or metabolic? • What is the anion gap? AG = 130 – (80 + 10) = 40 Anion gap metabolic acidosis
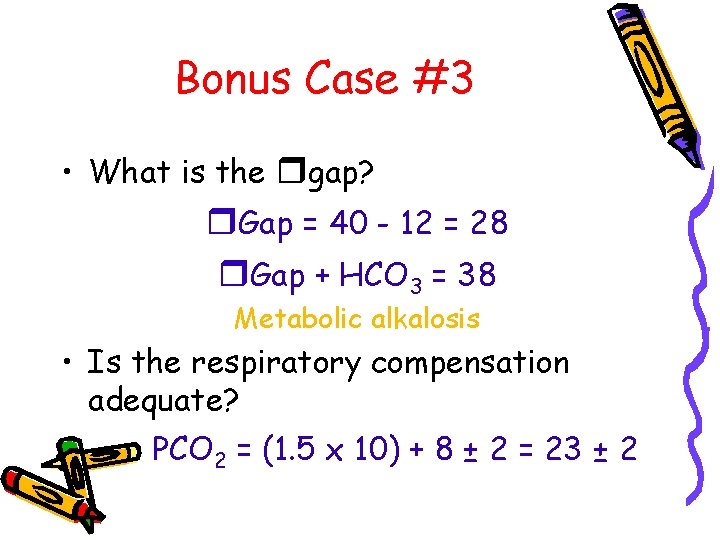
Bonus Case #3 • What is the rgap? r. Gap = 40 - 12 = 28 r. Gap + HCO 3 = 38 Metabolic alkalosis • Is the respiratory compensation adequate? PCO 2 = (1. 5 x 10) + 8 ± 2 = 23 ± 2

Bonus Case #3 • Anion gap metabolic acidosis (DKA) • Metabolic metabolic alkalosis (emesis) • Compensatory respiratory alkalosis

- Slides: 83