Interpretation of Pharmacovigilance Guidance Regulations July 2020 Trans








- Slides: 15

Interpretation of Pharmacovigilance Guidance & Regulations July 2020

Trans. Celerate: A Not-for-Profit Entity Created to Foster Collaboration Our Shared Vision: To improve the health of people around the world by accelerating and simplifying the research and development of innovative new therapies. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 2

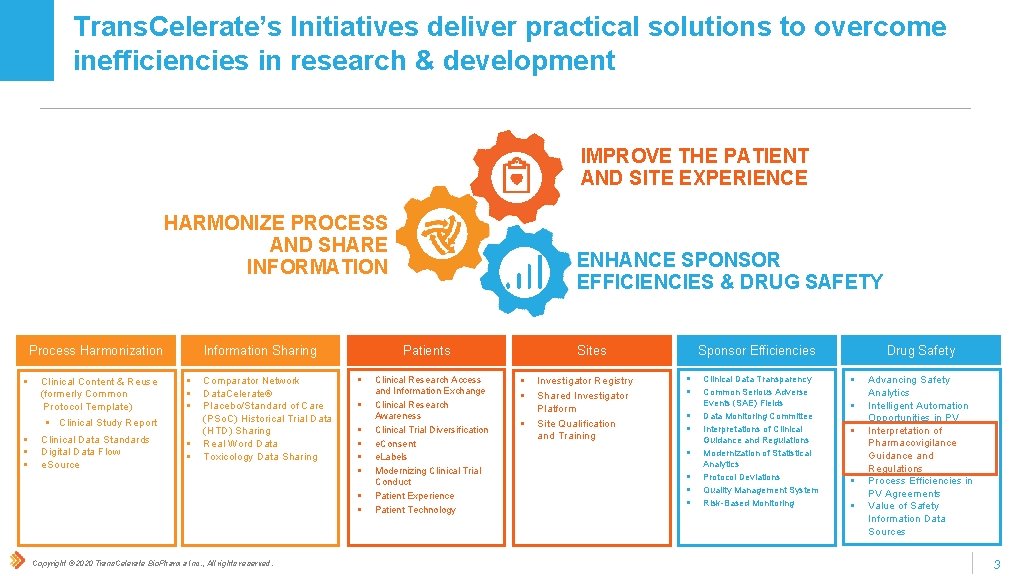
Trans. Celerate’s Initiatives deliver practical solutions to overcome inefficiencies in research & development IMPROVE THE PATIENT AND SITE EXPERIENCE HARMONIZE PROCESS AND SHARE INFORMATION Process Harmonization § Clinical Content & Reuse (formerly Common Protocol Template) § § Clinical Study Report Clinical Data Standards Digital Data Flow e. Source Information Sharing § § § Comparator Network Data. Celerate® Placebo/Standard of Care (PSo. C) Historical Trial Data (HTD) Sharing Real Word Data Toxicology Data Sharing Patients § § § § Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. ENHANCE SPONSOR EFFICIENCIES & DRUG SAFETY Clinical Research Access and Information Exchange Clinical Research Awareness Clinical Trial Diversification e. Consent e. Labels Modernizing Clinical Trial Conduct Patient Experience Patient Technology Sites § § § Investigator Registry Shared Investigator Platform Site Qualification and Training Drug Safety Sponsor Efficiencies § § § § Clinical Data Transparency Common Serious Adverse Events (SAE) Fields Data Monitoring Committee Interpretations of Clinical Guidance and Regulations Modernization of Statistical Analytics Protocol Deviations Quality Management System Risk-Based Monitoring § § § Advancing Safety Analytics Intelligent Automation Opportunities in PV Interpretation of Pharmacovigilance Guidance and Regulations Process Efficiencies in PV Agreements Value of Safety Information Data Sources 3

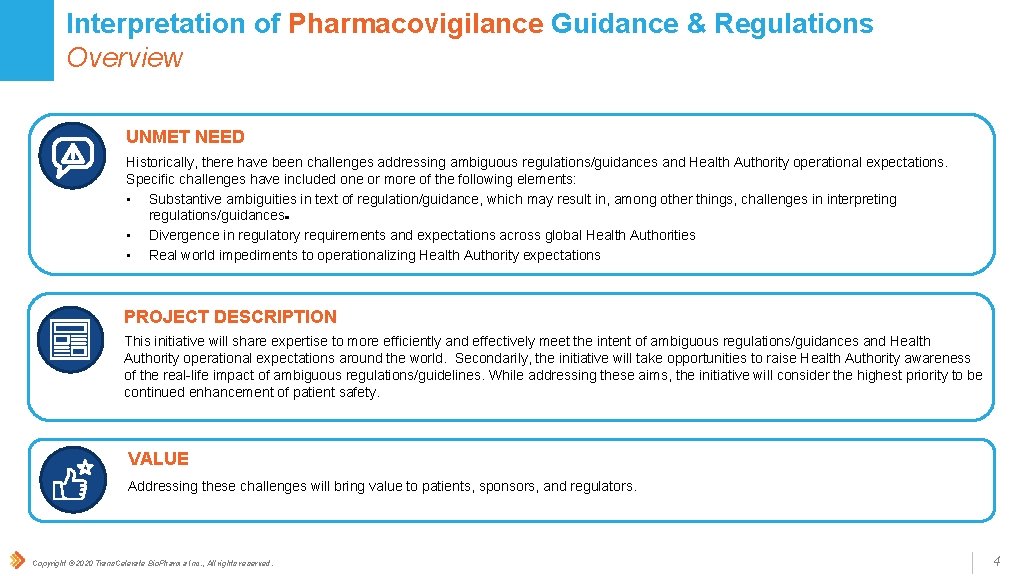
Interpretation of Pharmacovigilance Guidance & Regulations Overview UNMET NEED Historically, there have been challenges addressing ambiguous regulations/guidances and Health Authority operational expectations. Specific challenges have included one or more of the following elements: • Substantive ambiguities in text of regulation/guidance, which may result in, among other things, challenges in interpreting regulations/guidances • Divergence in regulatory requirements and expectations across global Health Authorities • Real world impediments to operationalizing Health Authority expectations PROJECT DESCRIPTION This initiative will share expertise to more efficiently and effectively meet the intent of ambiguous regulations/guidances and Health Authority operational expectations around the world. Secondarily, the initiative will take opportunities to raise Health Authority awareness of the real-life impact of ambiguous regulations/guidelines. While addressing these aims, the initiative will consider the highest priority to be continued enhancement of patient safety. VALUE Addressing these challenges will bring value to patients, sponsors, and regulators. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 4

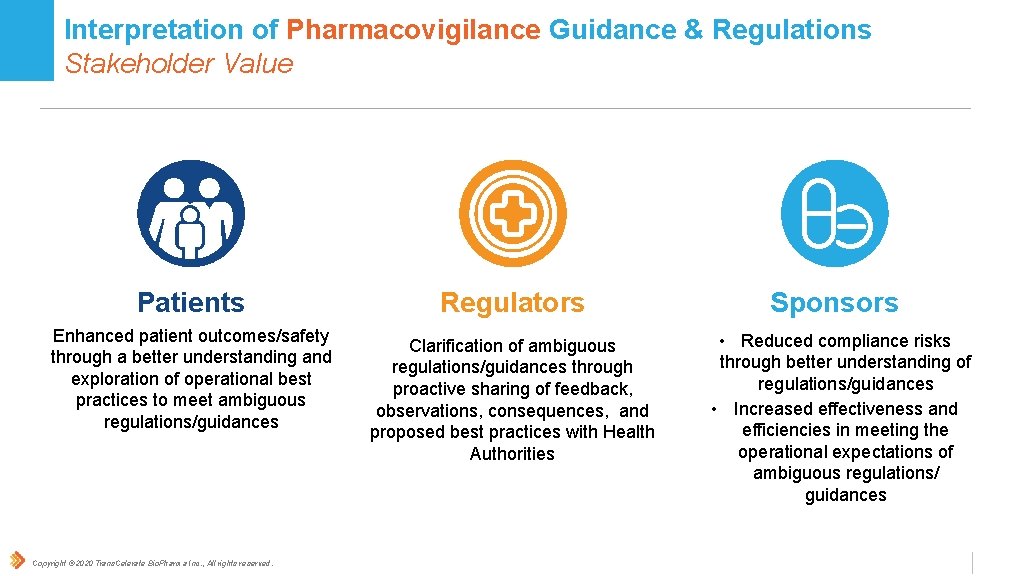
Interpretation of Pharmacovigilance Guidance & Regulations Stakeholder Value Patients Enhanced patient outcomes/safety through a better understanding and exploration of operational best practices to meet ambiguous regulations/guidances Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. Regulators Clarification of ambiguous regulations/guidances through proactive sharing of feedback, observations, consequences, and proposed best practices with Health Authorities Sponsors • Reduced compliance risks through better understanding of regulations/guidances • Increased effectiveness and efficiencies in meeting the operational expectations of ambiguous regulations/ guidances

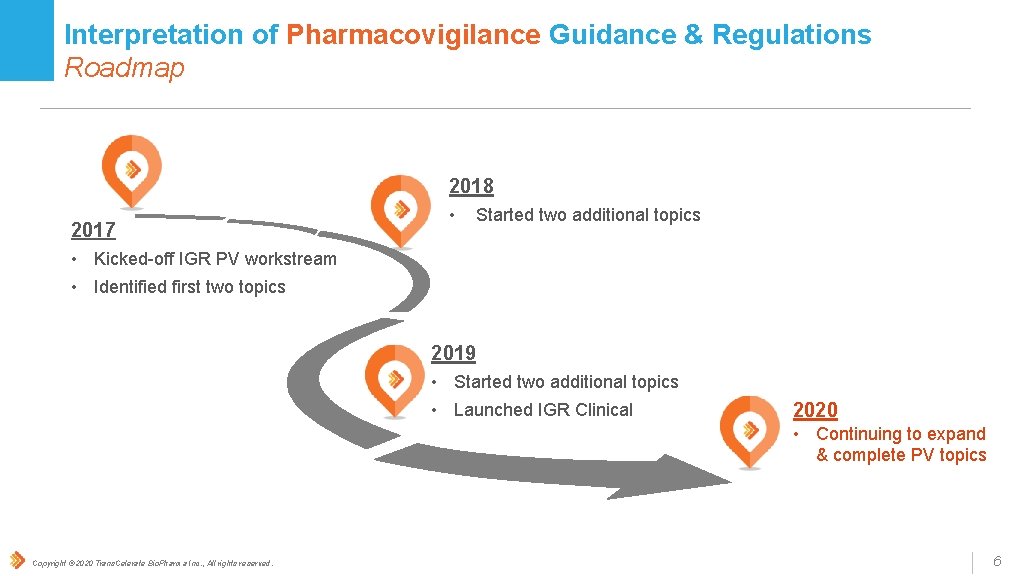
Interpretation of Pharmacovigilance Guidance & Regulations Roadmap 2018 2017 • Started two additional topics • Kicked-off IGR PV workstream • Identified first two topics 2019 • Started two additional topics • Launched IGR Clinical 2020 • Continuing to expand & complete PV topics Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 6

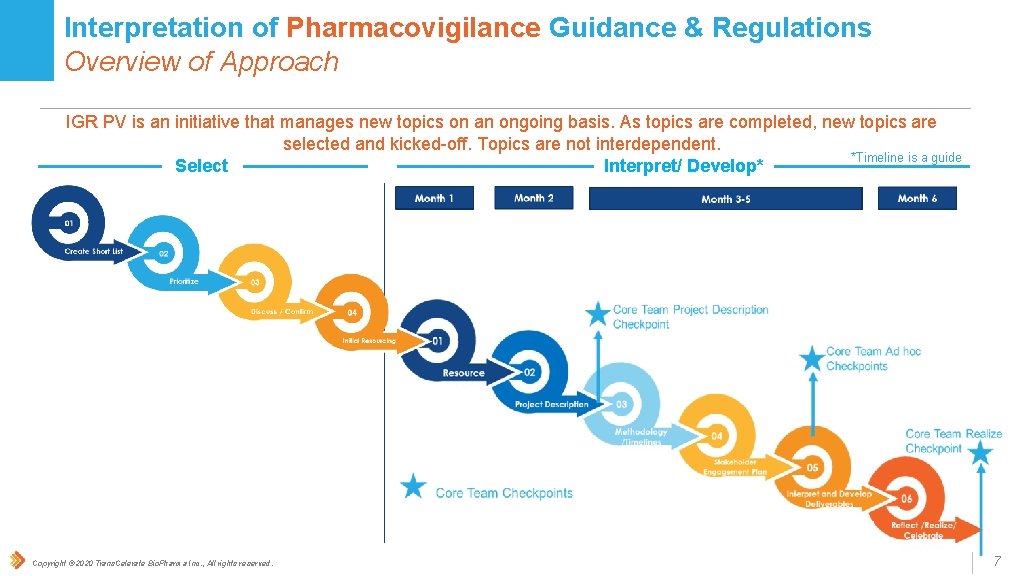
Interpretation of Pharmacovigilance Guidance & Regulations Overview of Approach IGR PV is an initiative that manages new topics on an ongoing basis. As topics are completed, new topics are selected and kicked-off. Topics are not interdependent. *Timeline is a guide Interpret/ Develop* Select Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. 7

Interpretation of Pharmacovigilance Guidance & Regulations Current Regulations & Guidances The initiative has or is addressing the following ambiguous Pharmacovigilance regulations and guidances Draft Guidance for Industry Safety Assessment for IND Safety Reporting CTFG Q&A document – Reference Safety Information (Draft Guidance Issue Date Dec 2015) (Q&A Issue Date Nov 2017) Complete Interpret ambiguous portions of draft guidance, engage with regulators, and share findings on implementation challenges and approaches Interpret ambiguous portions of RSI Guidance Q&A; engage with regulators, share findings on implementation challenges, approaches, and regulator feedback Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. PMSR for Combination Products Draft Guidance for Industry and FDA Staff (Draft Guidance Issue Date Mar 2018) Safety Issue Notification Guidances/ Regulations (Australia, Canada, Israel, New Zealand, Saudi Arabia, Switzerland, Turkey) Complete Interpret ambiguous portions of RSI Guidance Q&A; engage with regulators, share findings on implementation challenges, approaches, and regulator feedback Support patient safety commitments by identifying various approaches to meet Health Authority expectations for safety issue notifications in an effective and efficient manner (Draft Guidances Issue Date Oct 2019) Presentation of Risks Throughout the Lifecycle (Multiple Guidances) Complete Ongoing Provide a consolidated set of Trans. Celerate Member Company comments to FDA specific to the draft guidances on IND Safety Reporting to FAERS Map out differing regulatory requirements and operational considerations across the product lifecycle stages for sponsors to use preparing key regulatory and safety documents IND Safety Reporting to FAERS

Interpretation of Pharmacovigilance Guidance & Regulations IND Safety Reporting Description Guidance/Regulation Draft Guidance for Industry Safety Assessment for IND Safety Reporting (Dec 2015) AGENCY FDA GUIDANCE DATE December 2015 APPROACH 1. Reviewed 2015 Draft Guidance and identified areas that may need clarification 2. Conducted Member Company surveys to examine how companies interpreted recommendations in the 2015 Draft Guidance 3. Shared findings with FDA VALUE Understanding current approaches of Sponsors to Clinical Trial Safety Monitoring and Reporting and interpretation of Draft 2015 IND Guidance. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. Deliverables MANUSCRIPT A manuscript intended to provide industry experience and perspective on Clinical Trial Safety Reporting to the FDA (Link to Manuscript)

Interpretation of Pharmacovigilance Guidance & Regulations Reference Safety Information Description Guidance/Regulation CTFG Q&A document – Reference Safety Information (Nov 2017) AGENCY EMA GUIDANCE DATE November 2017 APPROACH 1. Review 2017 CTFG Q&A regarding RSI and identify areas needing clarification 2. Conduct Member Company survey to see how they implemented the new RSI guidelines in context of other global regulations VALUE • Raises industry and Health Authority awareness of diverging regulations/guidances through sharing key points in publications and conferences • Provides visibility into how sponsors are meeting the requirements for RSI Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. Deliverables MANUSCRIPT Examines how pharmaceutical companies are operationalizing the CTFG Q&A guidance on RSI and managing compliance with non-harmonized worldwide regulatory requirements. (Link to Manuscript) CALL FOR RE-HARMONIZATION A letter to the editor of Clinical Trials, noting a trend towards divergence of pharmacovigilance regulations. This letter is a call for re-harmonization to raise awareness of the issue and call for all stakeholders, in particular Health Authorities, to take efforts to re-harmonize. (Link to Letter to the Editor)

Interpretation of Pharmacovigilance Guidance & Regulations Postmarketing Safety Reporting for Combination Products Description Guidance/Regulation Postmarketing Safety Reporting for Combination Products Draft Guidance for Industry & FDA Staff AGENCY FDA GUIDANCE DATE March 2018 APPROACH 1. Reviewed Draft Guidance 2. Identified ambiguous areas to interpret and shared best practices through the implementation guide 3. Conducted industry-wide webinars sharing the implementation guide VALUE Facilitating adherence to unclear regulatory guidance calling for applicants to take a more holistic view of combination products to enhance patient safety. Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. Deliverables IMPLEMENTATION GUIDE An Implementation Guide to aide pharmaceutical manufacturers‘ understanding of ambiguous areas of the PMSR for Combination Products Draft Guidance for Industry and FDA Staff (March 2018). (Link to Implementation Guide) WEBINAR A webinar presentation where Trans. Celerate team members provide an overview of the FDA Post Marketing Safety Reporting for Drug-Device Combinations Implementation Guide. (Link to Webinar)

Interpretation of Pharmacovigilance Guidance & Regulations Safety Issue Notification Guidances & Regulations Description Guidance/Regulation APPROACH 1. Reviewed applicable country guidances and proposed reference definitions 2. Gathered Marketing Authorization Holder MAH insights 3. Compiled findings and proposals into an implementation guide VALUE Supports patient safety commitments by identifying various approaches to meet Health Authority expectations for safety issue notifications in an effective and efficient manner Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. Deliverables SAFETY ISSUE NOTIFICATION GUIDE A guide to aide understanding of safety issue notification guidances and regulations for in-scope countries. (Link to Safety Issue Notification Guide) WEBINAR A webinar presentation where Trans. Celerate team members provide an overview of the Safety Issue Notification Guide. (Link to Webinar)

Interpretation of Pharmacovigilance Guidance & Regulations IND Safety Reporting to FAERS Description Guidance/Regulation Providing Regulatory Submissions in Electronic Format: IND Safety Reports - Draft Guidance for Industry (October 2019) Electronic Submission of IND Safety Reports - Technical Conformance Guide (October 2019) AGENCY FDA APPROACH 1. Reviewed 2019 draft guidance and technical conformance guide 2. Collated Member Company comments 3. Agreed upon set of consolidated comments to submit to the FDA docket GUIDANCE DATE October 2019 VALUE Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. • FDA found Member Company feedback informative • Allows Trans. Celerate comments to FDA to represent multiple Member Company perspectives Deliverables COMMENTS OF DRAFT GUIDANCE & TECHNICAL CONFORMANCE GUIDE Comments from Trans. Celerate Member Companies on the draft guidance and technical conformance guide were collated, and a consolidated set of comments was submitted to the FDA docket. (Comments submitted to FDA Docket)

Interpretation of Pharmacovigilance Guidance & Regulations Presentation of Risks Throughout the Lifecycle Description Guidance/Regulation Key guidances reviewed included: • Integrate Addendum to ICH E 6(R 1): Guideline for Good Clinical Practice E 6(R 2) • ICH Harmonised Tripartite Guideline Development Safety Update Report E 2 F • ICH Harmonised Tripartite Guideline Periodic Benefit-Risk Evaluation Report (PBRER) E 2 C(R 2) • Guideline on Good Pharmacovigilance Practices (GVP) Annex 1 - Definitions (Rev 3) • Guideline on Good Pharmacovigilance Practices (GVP) Module V - Risk Management Systems (Rev 2) • Guidance on the Format of the Risk Management Plan (RMP) in the EU – in Integrated Format • Guideline on Good Pharmacovigilance Practices (GVP) Module VII – Periodic Safety Update Report (Rev 1) • Explanatory Note to GVP Module VII • Revision of M 4 E Guideline on Enhancing the Format and Structure of Benefit-Risk Information in ICH Efficacy – M 4 E(R 2) Copyright © 2020 Trans. Celerate Bio. Pharma Inc. , All rights reserved. APPROACH 1. Reviewed definitions of risks in key guidances 2. Provided greater clarity on how to present risks throughout the lifecycle through a Framework VALUE Supports presentation of risk information in accordance with Health Authority expectations, through developing a Framework that maps out regulatory requirements and operational considerations for presenting risks at the various lifecycle stages. Deliverables A FRAMEWORK ON THE PRESENTATION OF RISKS THROUGHOUT THE PRODUCT LIFECYCLE A Framework including a comparison of requirements for presentation of risks across the guidelines and phases of the product lifecycle, along with operational considerations on how to present risks in key regulatory/safety documents. (Not yet available) FRAMEWORK SOLUTION LAUNCH VIDEO A video introducing A Framework on the Presentation of Risks Throughout the Product Lifecycle and its key points. (Not yet available)

For more information about Trans. Celerate, visit us: www. Trans. Celerate. Bio. Pharma. Inc. com Watch our “About Us” Video Sign up for our Newsletter, Accelerate to Innovate @Trans. Celerate Bio. Pharma Inc.