Interpretation of Hydrogen Emission Spectra White Light Spectrum


- Slides: 19

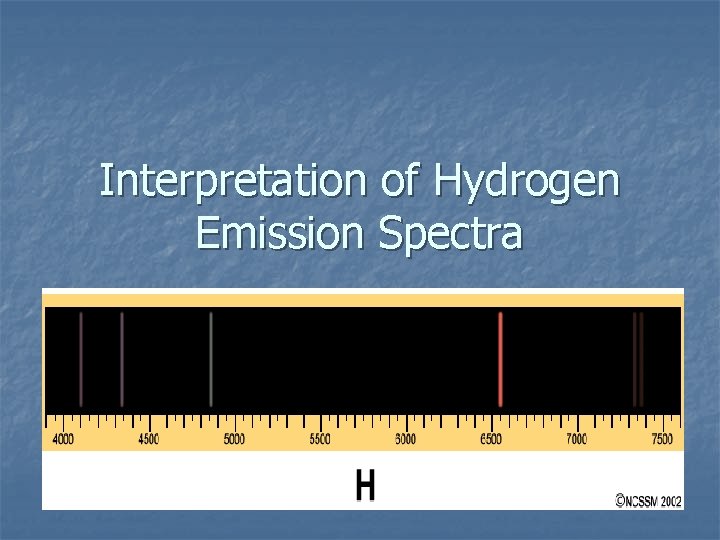
Interpretation of Hydrogen Emission Spectra

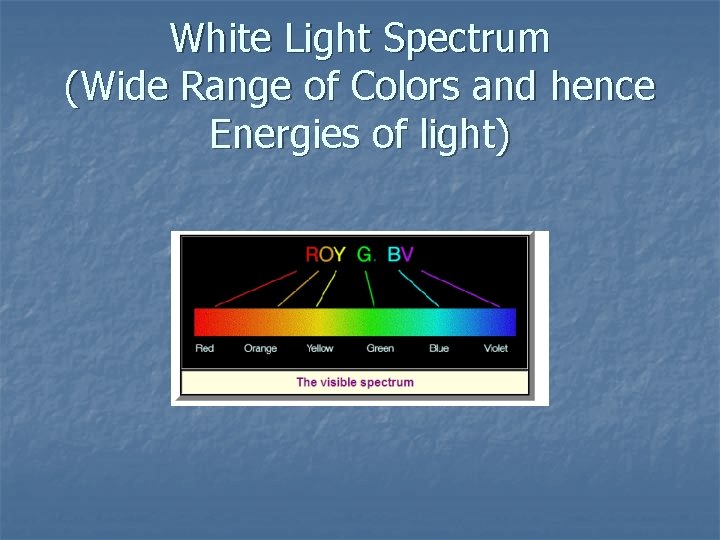
White Light Spectrum (Wide Range of Colors and hence Energies of light)

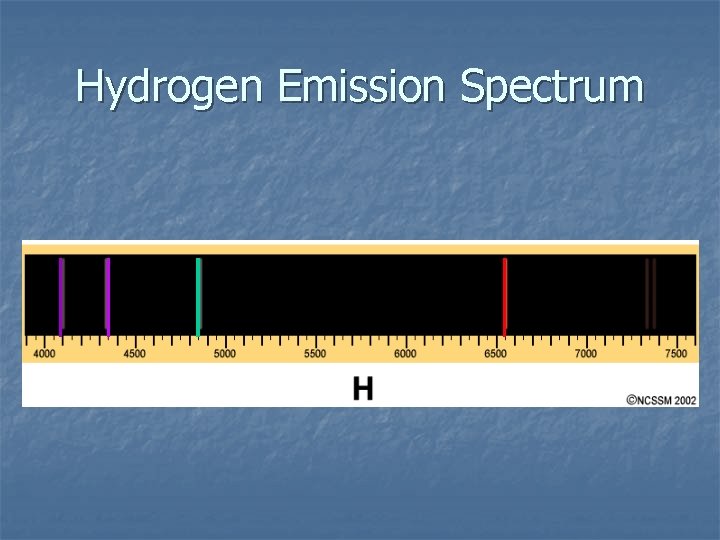
Hydrogen Emission Spectrum

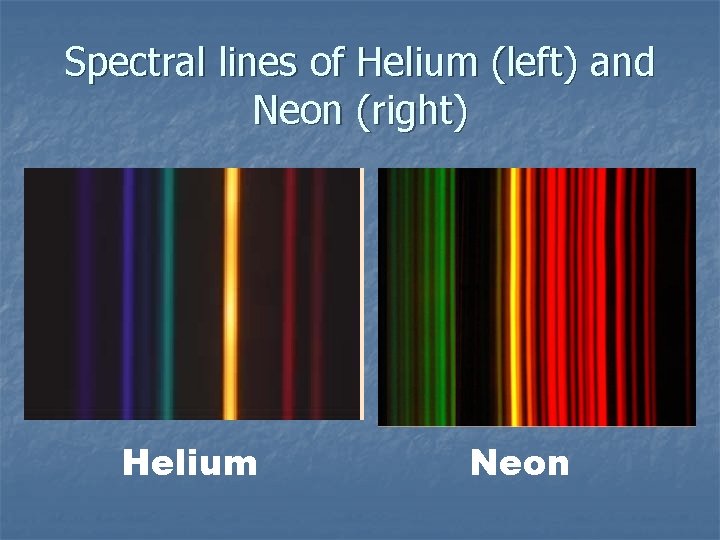
Spectral lines of Helium (left) and Neon (right) Helium Neon

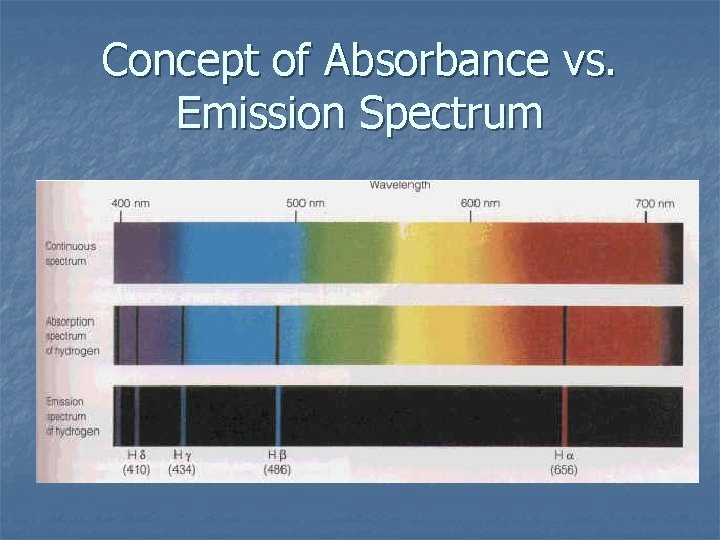
Concept of Absorbance vs. Emission Spectrum

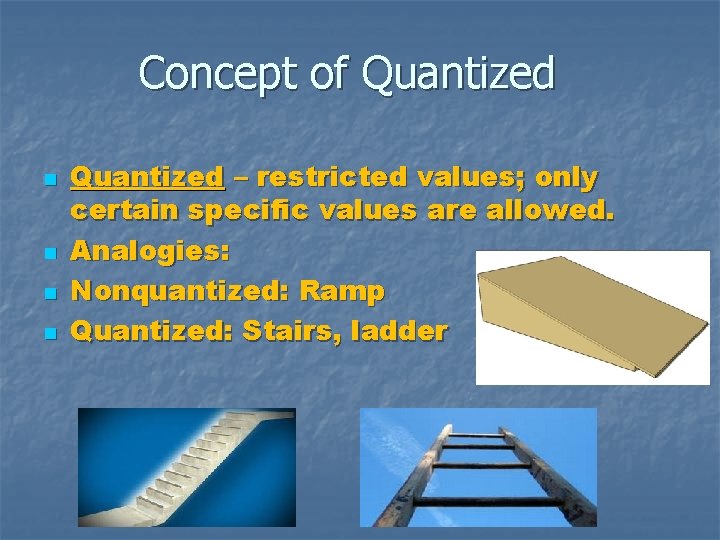
Concept of Quantized n n Quantized – restricted values; only certain specific values are allowed. Analogies: Nonquantized: Ramp Quantized: Stairs, ladder

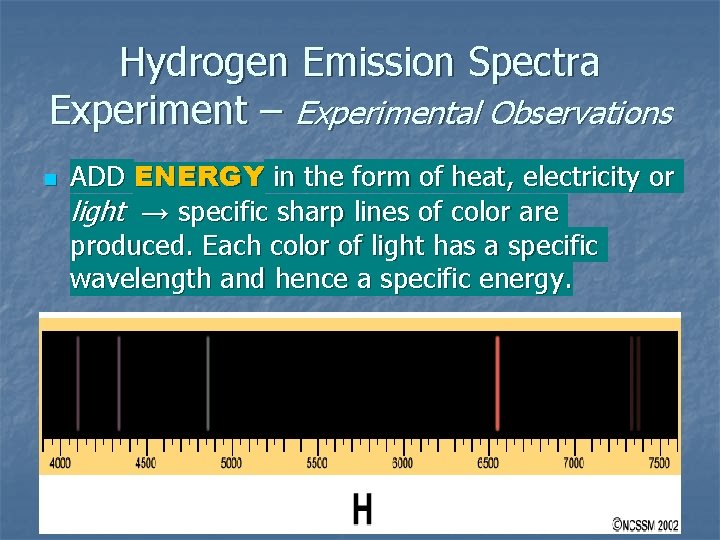
Hydrogen Emission Spectra Experiment – Experimental Observations n ADD ENERGY in the form of heat, electricity or light → specific sharp lines of color are produced. Each color of light has a specific wavelength and hence a specific energy.

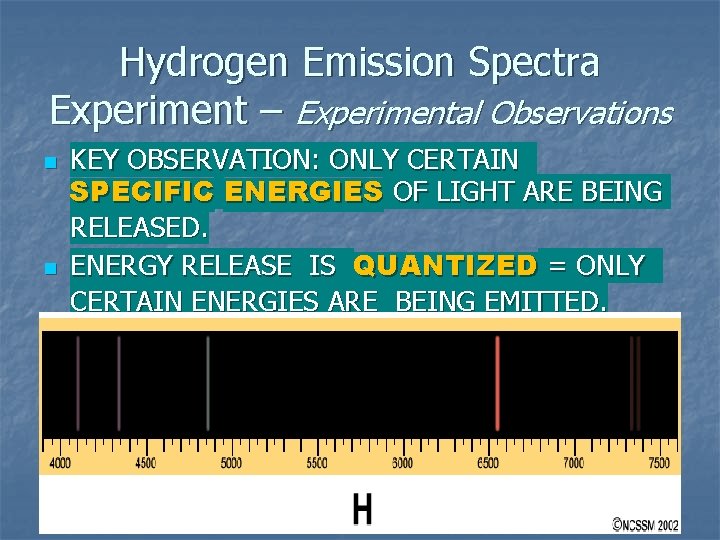
Hydrogen Emission Spectra Experiment – Experimental Observations n n KEY OBSERVATION: ONLY CERTAIN SPECIFIC ENERGIES OF LIGHT ARE BEING RELEASED. ENERGY RELEASE IS QUANTIZED = ONLY CERTAIN ENERGIES ARE BEING EMITTED.

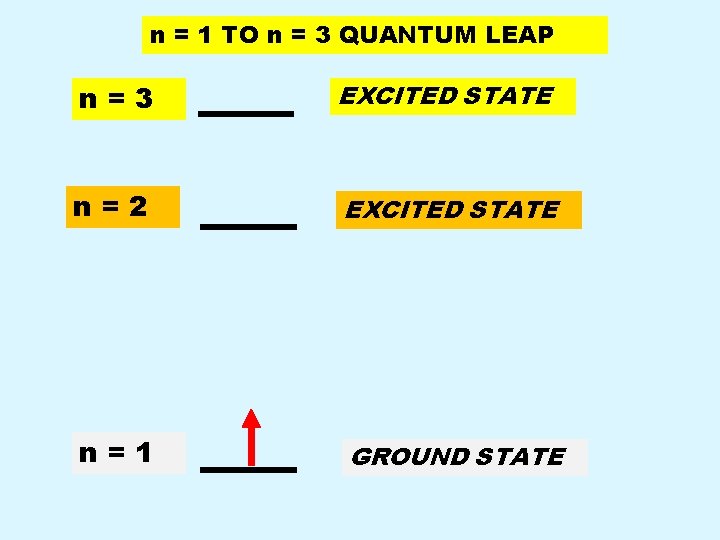
Interpretation of Experiment n n Energy added to atoms is absorbed by ELECTRONS. Electron absorbs specific quantity of energy, it makes a quantum leap from its lowest energy GROUND state to higher energy EXCITED state.

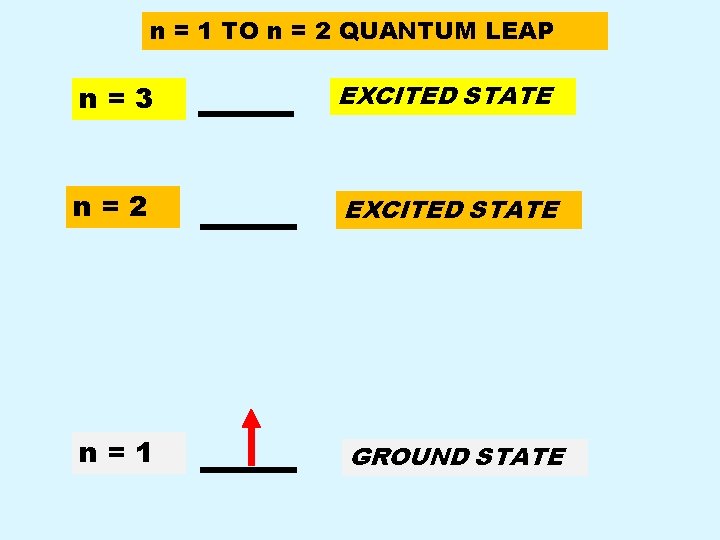
n = 1 TO n = 2 QUANTUM LEAP n=3 EXCITED STATE n=2 EXCITED STATE n=1 GROUND STATE

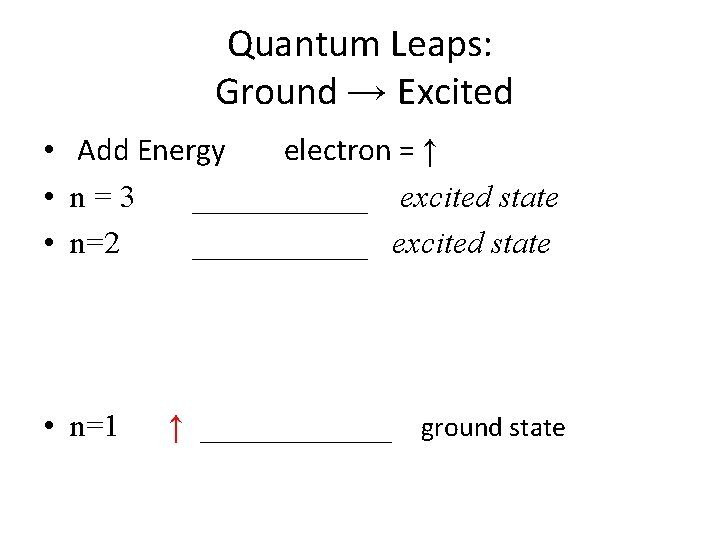
Quantum Leaps: Ground → Excited • Add Energy electron = ↑ • n=3 ______ excited state • n=2 ______ excited state • n=1 ↑ ______ ground state

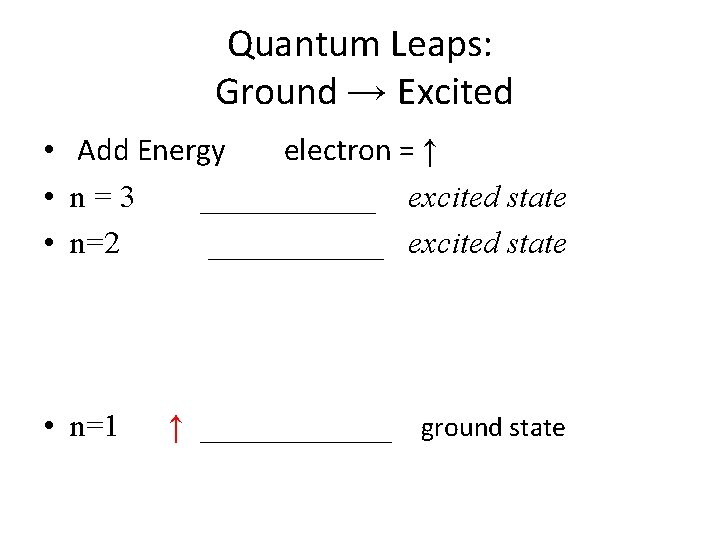
Quantum Leaps: Ground → Excited • Add Energy electron = ↑ • n=3 ______ excited state • n=2 ______ excited state • n=1 ↑ ______ ground state

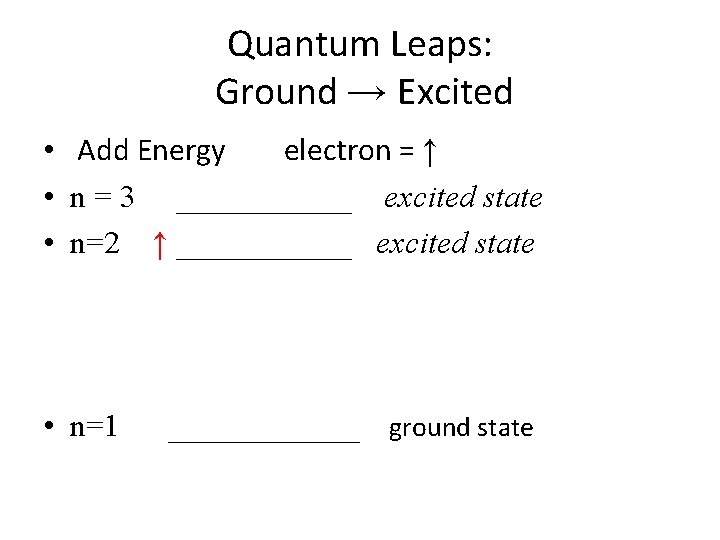
Quantum Leaps: Ground → Excited • Add Energy electron = ↑ • n = 3 ______ excited state • n=2 ↑ ______ excited state • n=1 ______ ground state

n = 1 TO n = 3 QUANTUM LEAP n=3 EXCITED STATE n=2 EXCITED STATE n=1 GROUND STATE

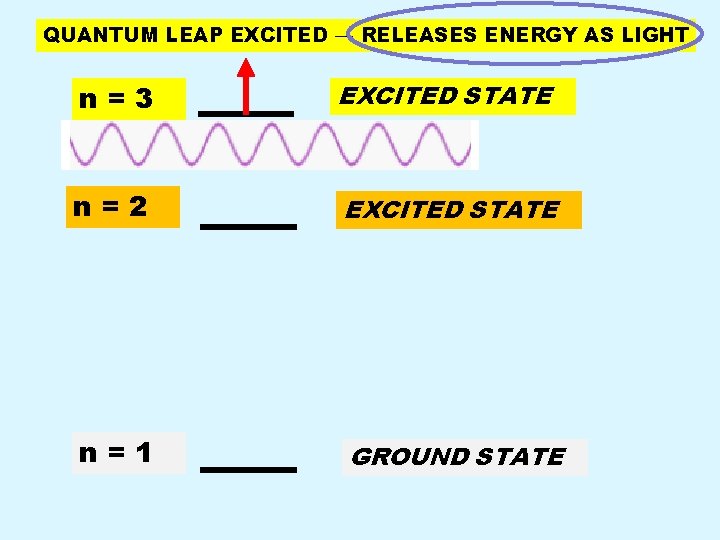
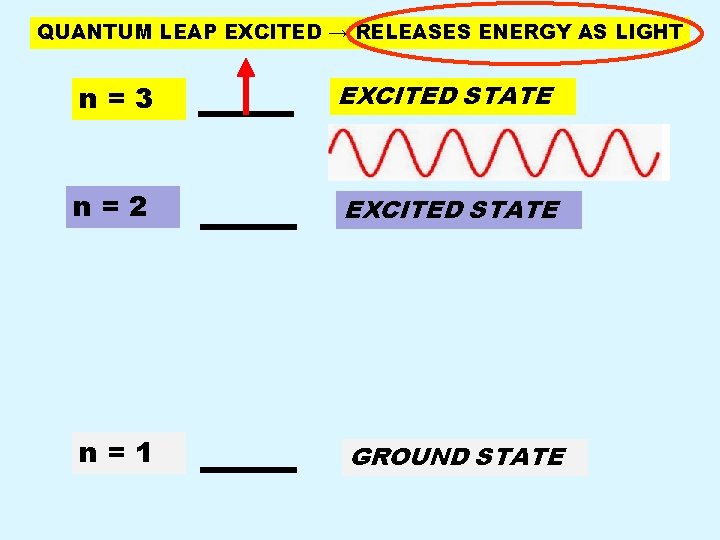
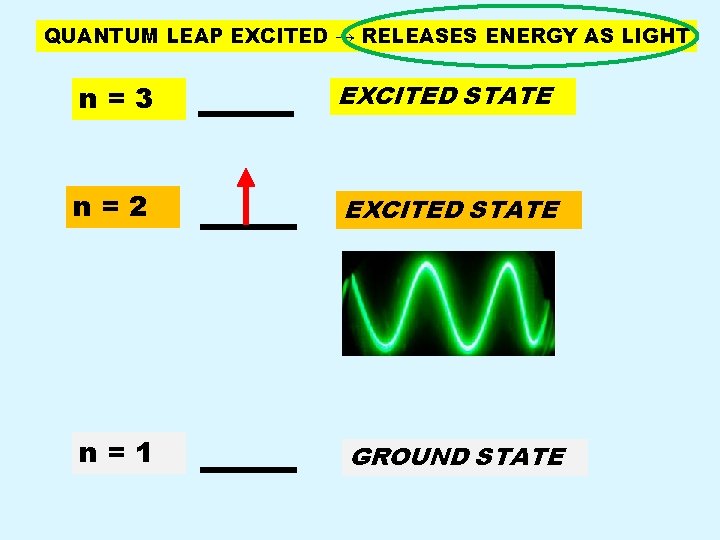
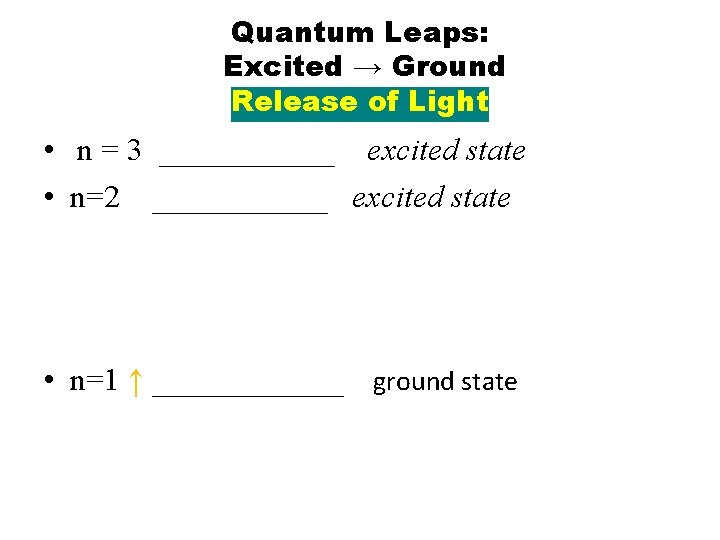
Back Page n n n Electrons CANNOT remain in an excited state for any significant time period. Electron in excited state returns to the ground state by emitting a specific amount of energy in the form of LIGHT. Because only certain specific jumps from excited to ground state are possible, only certain specific energies and hence colors of light can be emitted.

QUANTUM LEAP EXCITED → RELEASES ENERGY AS LIGHT n=3 EXCITED STATE n=2 EXCITED STATE n=1 GROUND STATE

QUANTUM LEAP EXCITED → RELEASES ENERGY AS LIGHT n=3 EXCITED STATE n=2 EXCITED STATE n=1 GROUND STATE

QUANTUM LEAP EXCITED → RELEASES ENERGY AS LIGHT n=3 EXCITED STATE n=2 EXCITED STATE n=1 GROUND STATE

Quantum Leaps: Excited → Ground Release of Light • n = 3 ______ excited state • n=2 ______ excited state • n=1 ↑ ______ ground state