Interplay between spin charge lattice and orbital degrees

Interplay between spin, charge, lattice and orbital degrees of freedom Lecture notes Les Houches June 2006 George Sawatzky

Rough content of 3 lectures • Basics of the electronic structure of correlated systems • Some theoretical and experimental methods • Towards real materials involving charge, orbital, spin and lattice degrees of freedom • Some new experimental methods and new ideas for magnetic materials
Content Lecture 1 • Electronic structure of correlated electron systems – Why are TM compounds and rare earths special – Quasi atomic vs band structure approaches – Hund’s rule, spin orbit interactions – DFT, LDA+U, DMFT, Model H exact diagonalization – Spectral weight transfer

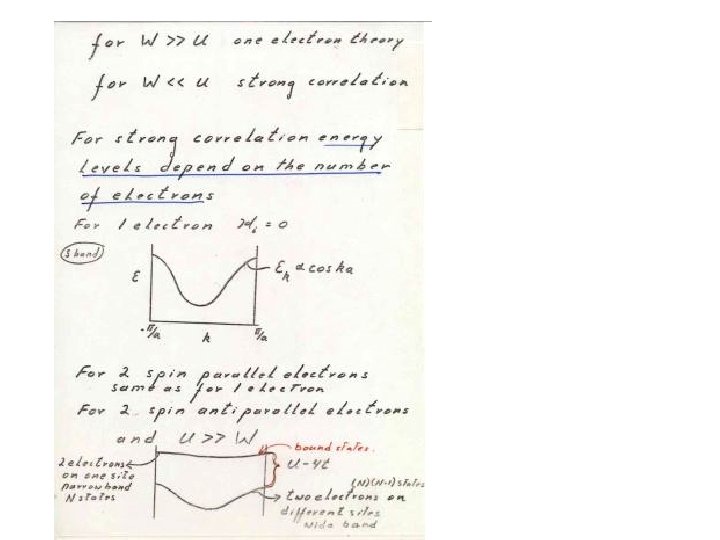
Some Historical notes • 1929 -1931 Bloch Wilson theory of solids • 1937 De Boer and Verwey ( Ni. O-Co. O breakdown of band theory • 1937 Peierls 3 d electrons avoid each other ( basically the Hubbard model) • 1950 Jonker van Zanten - Zener Pervoskites double exchange • 1959 Anderson superexchange (U>>W) • 1964 Hubbard model- Hohenberg Kohn DFTGoodenough Transition metal compounds
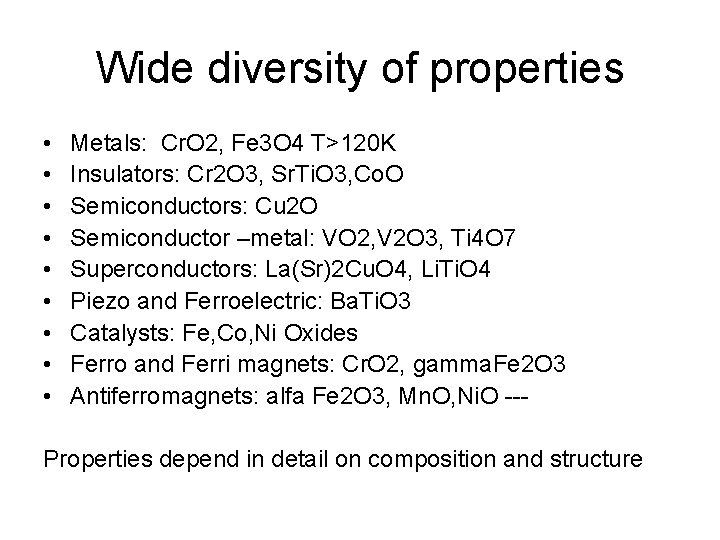
Wide diversity of properties • • • Metals: Cr. O 2, Fe 3 O 4 T>120 K Insulators: Cr 2 O 3, Sr. Ti. O 3, Co. O Semiconductors: Cu 2 O Semiconductor –metal: VO 2, V 2 O 3, Ti 4 O 7 Superconductors: La(Sr)2 Cu. O 4, Li. Ti. O 4 Piezo and Ferroelectric: Ba. Ti. O 3 Catalysts: Fe, Co, Ni Oxides Ferro and Ferri magnets: Cr. O 2, gamma. Fe 2 O 3 Antiferromagnets: alfa Fe 2 O 3, Mn. O, Ni. O --- Properties depend in detail on composition and structure
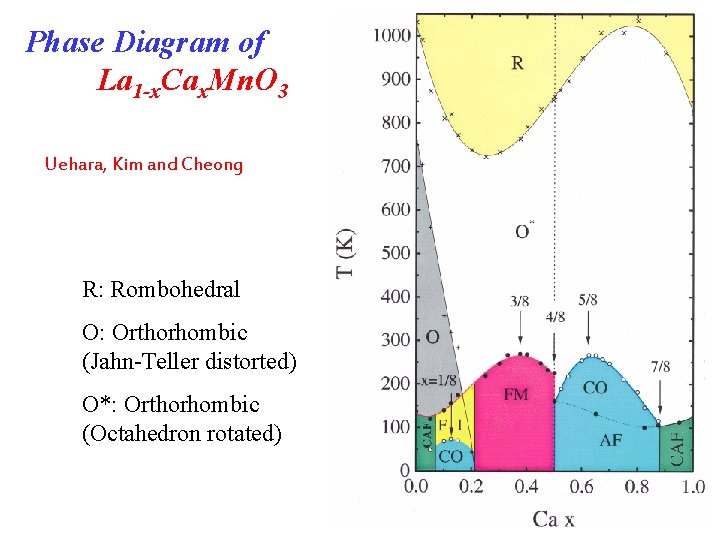
Phase Diagram of La 1 -x. Cax. Mn. O 3 Uehara, Kim and Cheong R: Rombohedral O: Orthorhombic (Jahn-Teller distorted) O*: Orthorhombic (Octahedron rotated)
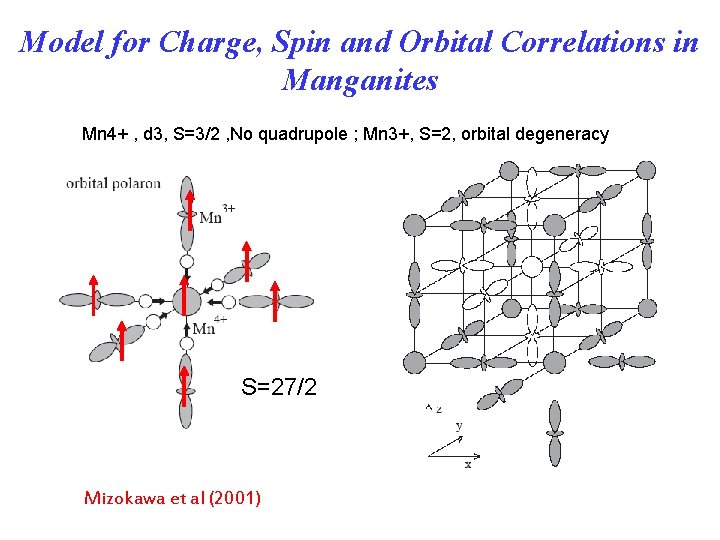
Model for Charge, Spin and Orbital Correlations in Manganites Mn 4+ , d 3, S=3/2 , No quadrupole ; Mn 3+, S=2, orbital degeneracy S=27/2 Mizokawa et al (2001)

Ordering in strongly correlated systems Stripes in Nd-LSCO rivers of Charge— Antiferro/ Antiphase DQ < 0. 5 e Quadrupole moment ordering D QC ~ 1 e D QO ~ 0 Charge inhomogeneity in Bi 2212 Pan, Nature, 413, 282 (2001); Hoffman, Science, 295, 466 (2002) DQ ~ 0. 1 e
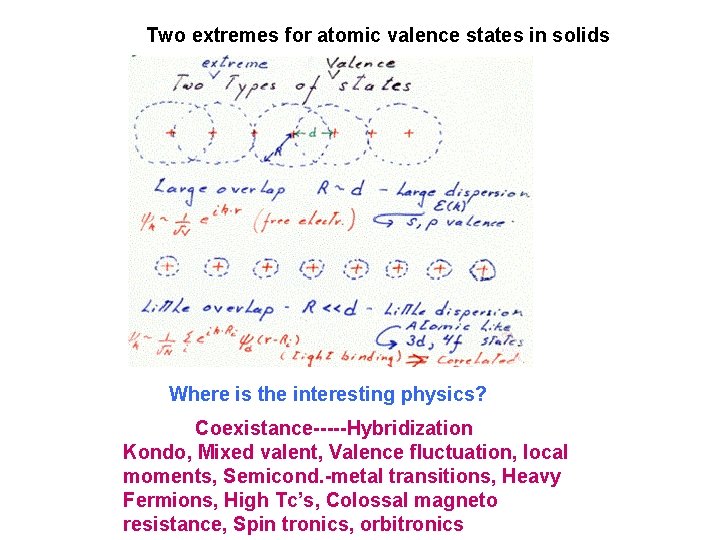
Two extremes for atomic valence states in solids Where is the interesting physics? Coexistance-----Hybridization Kondo, Mixed valent, Valence fluctuation, local moments, Semicond. -metal transitions, Heavy Fermions, High Tc’s, Colossal magneto resistance, Spin tronics, orbitronics
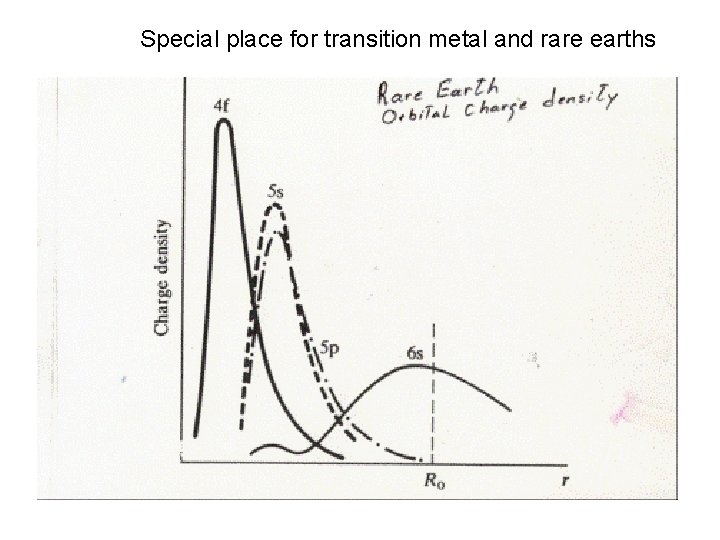
Special place for transition metal and rare earths

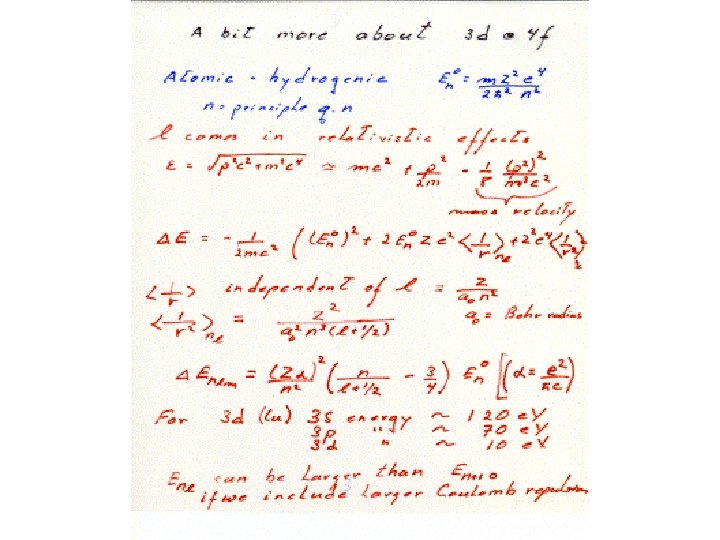
Why are 3 d and 4 f orbitals special • Lowest principle q. n. for that l value • Large centrifugal barrier l=2, 3 • Small radial extent, no radial nodes orthogonal to all other core orbitals via angular nodes • High kinetic energy ( angular nodes) • Relativistic effects • Look like core orb. But have high energy and form open shells like valence orb.
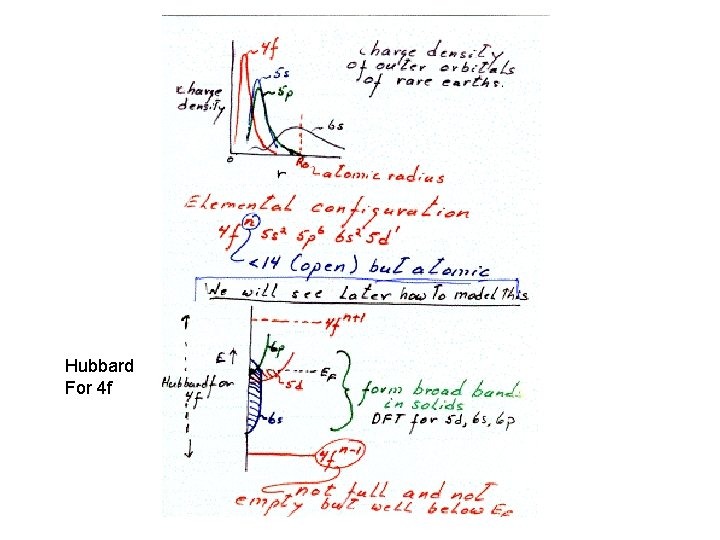
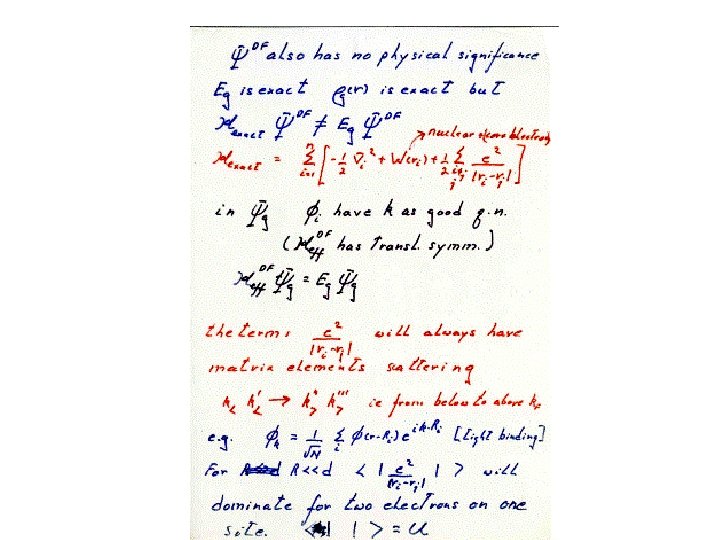
Hubbard For 4 f



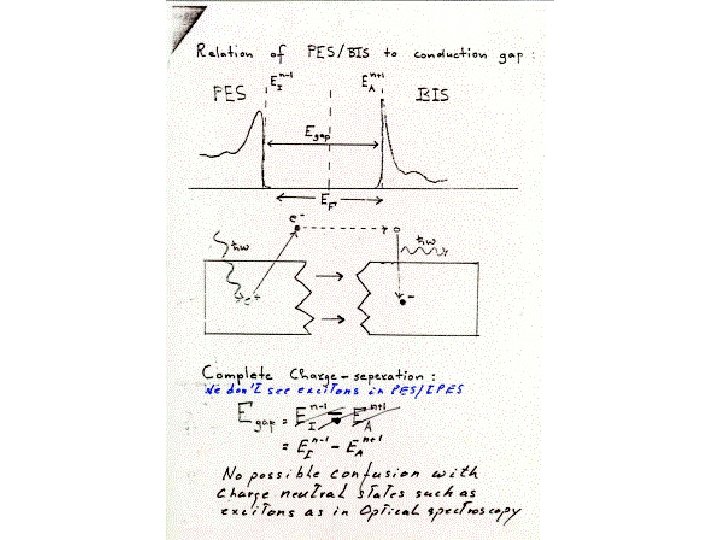
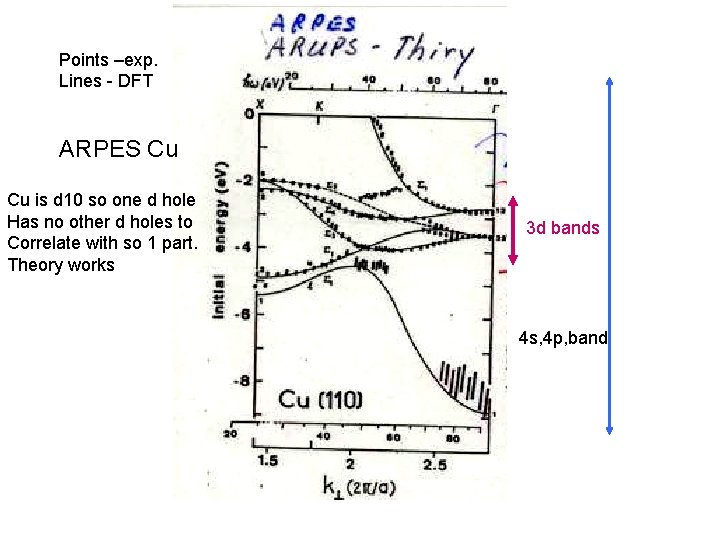
Points –exp. Lines - DFT ARPES Cu Cu is d 10 so one d hole Has no other d holes to Correlate with so 1 part. Theory works 3 d bands 4 s, 4 p, band
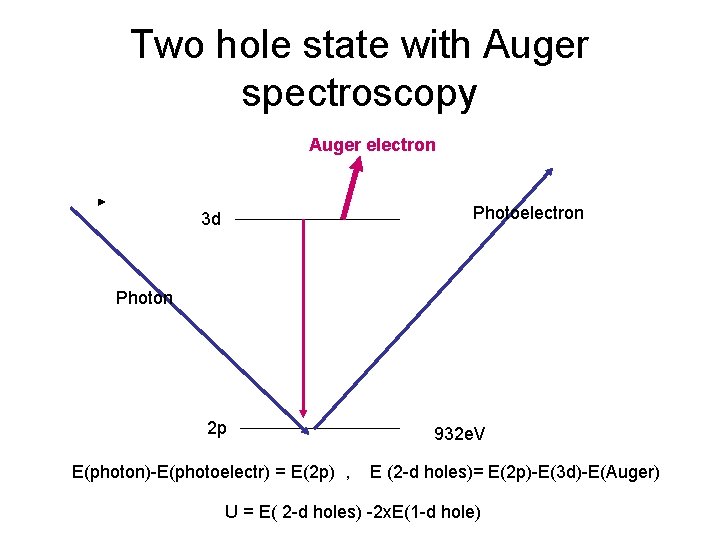
Two hole state with Auger spectroscopy Auger electron Photoelectron 3 d Photon 2 p E(photon)-E(photoelectr) = E(2 p) , 932 e. V E (2 -d holes)= E(2 p)-E(3 d)-E(Auger) U = E( 2 -d holes) -2 x. E(1 -d hole)
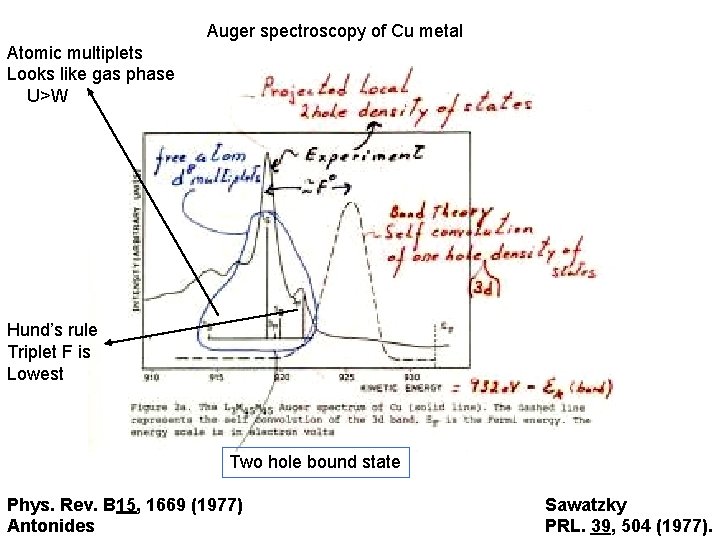
Auger spectroscopy of Cu metal Atomic multiplets Looks like gas phase U>W Hund’s rule Triplet F is Lowest Two hole bound state Phys. Rev. B 15, 1669 (1977) Antonides Sawatzky PRL. 39, 504 (1977).




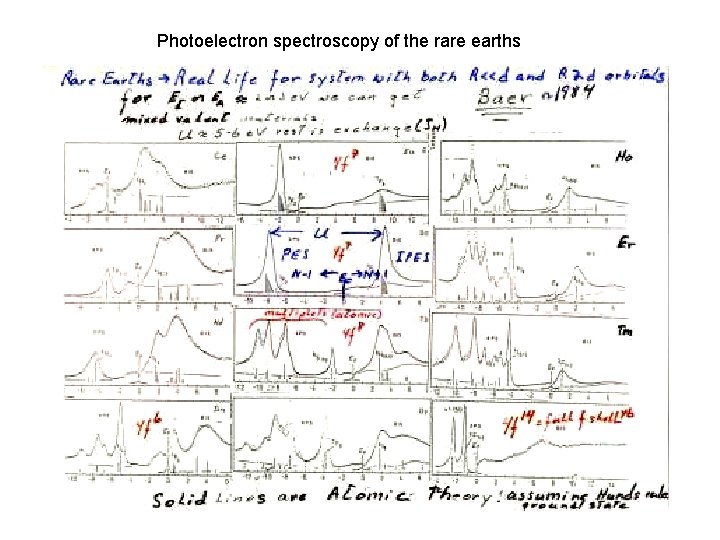
Photoelectron spectroscopy of the rare earths
- Slides: 28