Interoperability in e Health Systems Asuman Dogac SRDC

Interoperability in e. Health Systems Asuman Dogac, SRDC Ltd. August 30, 2012 1
It was many and many a year ago… n There were standalone computers… q q q Then came the networks and the Internet… Then it became apparent that there would be huge benefits to be gained if the applications can talk to one another… Email is one such application although it is mostly for human consumption… August 30, 2012 VLDB 2012 2

What if… n What if Electronic Health Records are widely used and shared? Ø Ø What if Computerised Physician Order Entry are widely used? Ø Ø 100, 000 adverse drug events could be avoided yearly What if e. Prescriptions are widely used? Ø Ø 9 million bed-days could yearly be freed up corresponding to a value of € 3, 7 billion 5 million prescription errors could be avoided yearly What if Chronic Disease Management is used for diabetes patients? Ø 11, 000 diabetic deaths could be avoided every year Ref: a recent study covering 6 EU countries by realizing e. Health (ep. SOS) To achieve this, you need all the systems working together seamlessly: interoperability is needed Ø n August 30, 2012 VLDB 2012 3

Outline n Major Interoperability Standards and Profiles in e. Health q Interoperability Stack n n q Semantic Interoperability in e. Health n q n Communication and Transport Layer Document/Message Layer Business Process Layer Profiling Clinical Terminology Systems Medical Device Standards Real Life Uses q Sharing Electronic Health Records n q Monitoring Chronic Diseases n q Saglik-Net in Turkey and ep. SOS in Europe Patients with Cardiac Implants and Diabetes patients Secondary use of EHRs in Clinical Research August 30, 2012 VLDB 2012 4

Two points… n The talk is divided into two main parts: q q n Description of some of the interoperability standards (which may not be very thrilling ) Description of some real life uses seems more interesting It is not “all applied work”; it includes research August 30, 2012 VLDB 2012 5
Interoperability (Def’n) Interoperability with regard to a specific task is said to exist between two applications when one application can accept data (including data in the form of a service request) from the other and perform the task in an appropriate and satisfactory manner (as judged by the user of the receiving system) without the need for extra operator intervention n q n Ref: Brown, N. and Reynolds, M. 2000. Strategy for production and maintenance of standards for interoperability within and between service departments and other healthcare domains. Short Strategic Study CEN/TC 251/N 00 -047, CEN/TC 251 Health Informatics, Brussels, Belgium. This definition implies the following: q q The ability to communicate data (connectivity) The data received by the receiving system is sufficient to perform the task and The meaning attached to each data item is the same as that understood by the creators and users of the sending and receiving systems The task is performed to the satisfaction of the user of the receiving system August 30, 2012 VLDB 2012 6
Interoperability and Standards n Interoperability is possible by conforming to standards n Otherwise an application wishing to communicate with n different applications must develop that many different interfaces August 30, 2012 VLDB 2012 7

The nice thing about standards is that there are so many to choose from ! August 30, 2012 VLDB 2012 8

Some of the Main Standard Bodies in e. Health n HL 7 (Health Level Seven) q q q n Integrating the Healthcare Enterprise (IHE) q q q n Founded in 1987 A non-profit, ANSI accredited Standards Developing Organization Provides standards for the exchange, management, and integration of data that supports clinical patient care and the management, delivery, and evaluation of healthcare services Founded in 1998 in the USA by the Radiological Society of North America (RSNA) and the Healthcare Information and Management Systems Society (HIMSS) A not-for-profit initiative The goal is to stimulate integration of healthcare information resources CEN/TC 251 q q The technical committee on Health Informatics of the European Committee for Standardization Its mission is to achieve compatibility and interoperability between independent health systems August 30, 2012 VLDB 2012 9

Messages vs. Documents in Healthcare ITMessages n q q n They support an ongoing process in a real-time fashion They drive activities and may trigger further events They use the latest version of the data to support an ongoing process They are consumed to realize that event Documents q q q They have “static” content and have to be persisted They tend to be used “post occurrence”, i. e. once the actual process is done They are "passive“ They capture information and allow that information to be shared, but do not themselves drive any activity They are self contained such as an Electronic Health Record (EHR) n August 30, 2012 EHRs are documents also for medico-legal reasons: a medical professional takes the responsibility for the information contained in it VLDB 2012 10
HL 7 Message Standards n n The HL 7 standard is developed with the assumption that an event in the healthcare world, called the trigger event, causes the exchange of messages between a pair of applications When an event occurs in an HL 7 -compliant system: q q n An HL 7 message is prepared by collecting the necessary data from the underlying application systems and It is passed to the requestor, usually as an EDI (Electronic Data Interchange) message For example, a trigger event, called ADT^A 01 (admission/ discharge/ transfer—admission of an inpatient into a facility), occurs when a patient is admitted to a facility This event may cause the data about the patient to be collected and sent to a number of other application systems Currently, there are two message protocols supported by HL 7, Version 2 and Version 3 August 30, 2012 VLDB 2012 11
HL 7 Message Standards n n HL 7 Version 2 Messaging Standard is the most widely implemented standard for healthcare information in the world today However, Version 2 messages have no precisely defined underlying information model; q q n n n The definitions for many data fields are rather vague, and There a multitude of optional data fields Being HL 7 Version 2 -compliant does not imply direct interoperability between healthcare systems This optionality provides great flexibility but necessitates detailed bilateral agreements among the healthcare systems to achieve interoperability To remedy this problem, HL 7 developed Version 3 which is based on an object-oriented data model called Reference Information Model (RIM) Up to the current Version 2. 5, the scope of the HL 7 standard was limited to the exchange of messages between medical information systems Starting with Version 3. 0, a document markup standard, called the Clinical Document Architecture (CDA), is proposed August 30, 2012 VLDB 2012 12
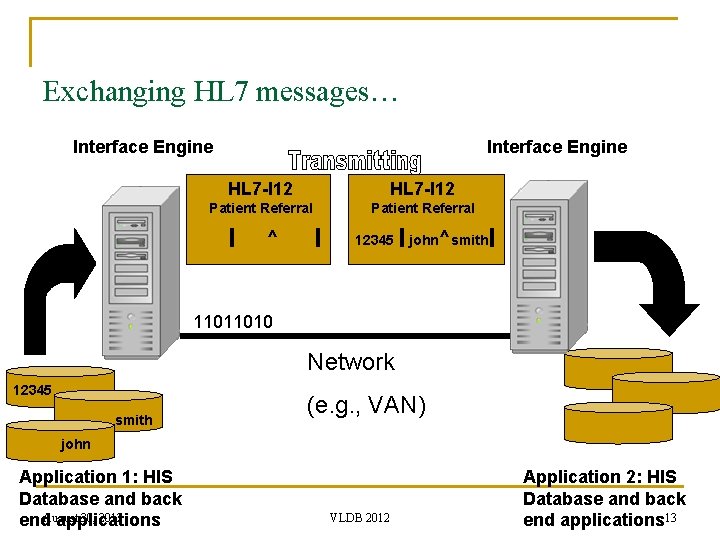
Exchanging HL 7 messages… Interface Engine HL 7 -I 12 Patient Referral ^ 12345 john ^ smith 11011010 Network 12345 smith (e. g. , VAN) john Application 1: HIS Database and back 30, 2012 end. August applications VLDB 2012 Application 2: HIS Database and back end applications 13

Interoperability Stack August 30, 2012 14
Interoperability Stack… n n n Interoperability involves not a single standard but a collection of standards addressing different layers in the interoperability stack There are several alternative standards to be chosen from for each layer Some standards specify a range of standards for a layer q n E. g. , HL 7 v 3 provides a number of normative specifications for the transport layer such as eb. MS or Web services Profiling avoids this problem by fixing the combination of the standards and even further restricting them to provide interoperability Integrated Healtcare Enterprises (IHE) n HITSP in USA for NHIN n Ministry of Health, Turkey for NHIS n 15 August 30, 2012 VLDB 2012 15
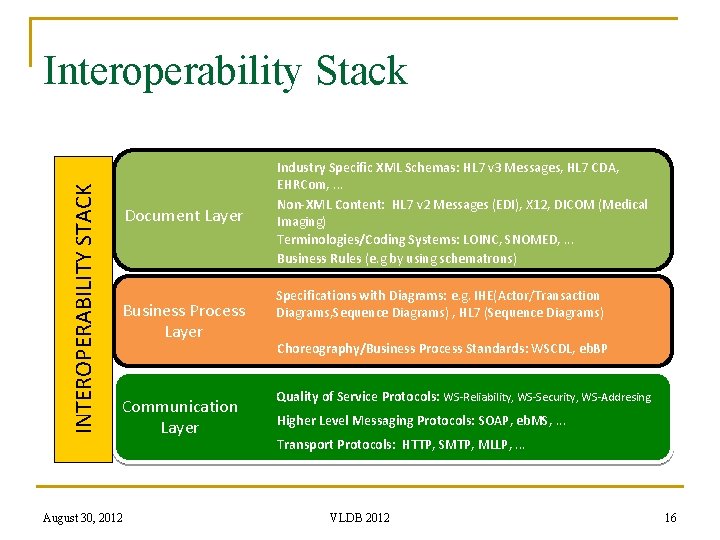
INTEROPERABILITY STACK Interoperability Stack Document Layer Business Process Layer Communication Layer Industry Specific XML Schemas: HL 7 v 3 Messages, HL 7 CDA, EHRCom, . . . Non-XML Content: HL 7 v 2 Messages (EDI), X 12, DICOM (Medical Imaging) Terminologies/Coding Systems: LOINC, SNOMED, . . . Business Rules (e. g by using schematrons) Specifications with Diagrams: e. g. IHE(Actor/Transaction Diagrams, Sequence Diagrams) , HL 7 (Sequence Diagrams) Choreography/Business Process Standards: WSCDL, eb. BP Quality of Service Protocols: WS-Reliability, WS-Security, WS-Addresing Higher Level Messaging Protocols: SOAP, eb. MS, . . . Transport Protocols: HTTP, SMTP, MLLP, . . . 16 August 30, 2012 VLDB 2012 16
Health Level 7 (HL 7) Message Standards n n HL 7 v 2. x q At the document layer, it defines the contents of messages with a lot of optional fields which provides flexibility but reduces interoperability q It recommends several protocol alternatives for the communication layer such as “Minimal Lower Layer Protocol (MLLP)” based on TCP/IP HL 7 v 3 q q q Defines a generic structure to express the concepts in a domain, called “Reference Information Model (RIM)” This generic RIM is then refined to subdomains and later to specific domain concepts For example, the HL 7 RIM can be specialized into: n n n q It recommends several protocol alternatives for the communication layer n n n q “CDA” for expressing clinical documents, “Clinical genomics” for expressing clinical and personalized genomics data, and “Claims and reimbursement” for handling claims and reimbursements eb. XML Message Specification Profile Web Services Profile TCP/IP based Minimal Lower Layer Protocol Profile There are sequence diagrams to describe the choreography of the interactions August 30, 2012 VLDB 2012 17
Some Document Standards… n HL 7 v 3 Clinical Document Architecture (CDA) q n open. EHR q q n Has a reference model and uses open. EHR archetypes to constrain them ASTM Continuity of Care Record (CCR) q q n First, a generic reference model that is specific to the healthcare domain which contains a few classes (e. g. , role, act, entity, participation) Then healthcare and application-specific concepts such as blood pressure, lab results are modeled as archetypes, that is, constraint rules that specialize the generic data structures ISO/CEN 13606 -1 q n Defines the structure and semantics of medical documents for the purpose of exchange in Extensible Markup Language (XML) Defines a core data set for the most relevant administrative, demographic, and clinical information facts about a patient’s healthcare, covering one or more healthcare encounters Contains various sections such as patient demographics, insurance information, diagnosis and problem list, medications, allergies and care plan All these standards, except CCR, define quite generic structures such as folder, section, entry that can be used to represent any kind of clinical statement August 30, 2012 VLDB 2012 18
Content Templates. . n Document standards are not enough for interoperability because there can be many different ways of organizing the same clinical information even when the same EHR content standard is used: q n Therefore, the templates/archetypes are necessary to constrain the structure and format of generic EHR content standards q n n The same information can be expressed through different components and these components can be nested differently For example, IHE defines several content templates such as Discharge Summary, Medical Summary or PHR Extract As another example, Continuity of Care Document (CCD) is defined by constraining HL 7 Clinical Document Architecture (CDA) with requirements set forward in CCR However, overlapping coding systems as well as the structural differences in document templates create semantic interoperability problems : q q Different code systems used by different healthcare applications in the same compositional structure within the same document template; Different compositional structures within the same document template that express the same meaning differently August 30, 2012 VLDB 2012 19
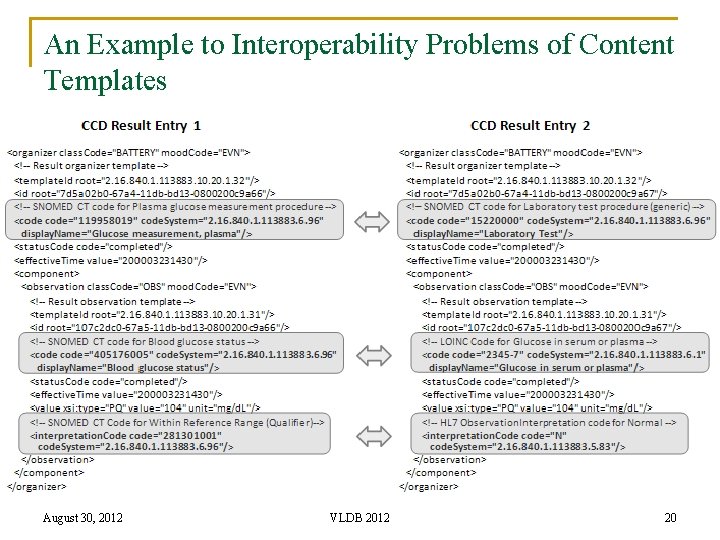
An Example to Interoperability Problems of Content Templates August 30, 2012 VLDB 2012 20
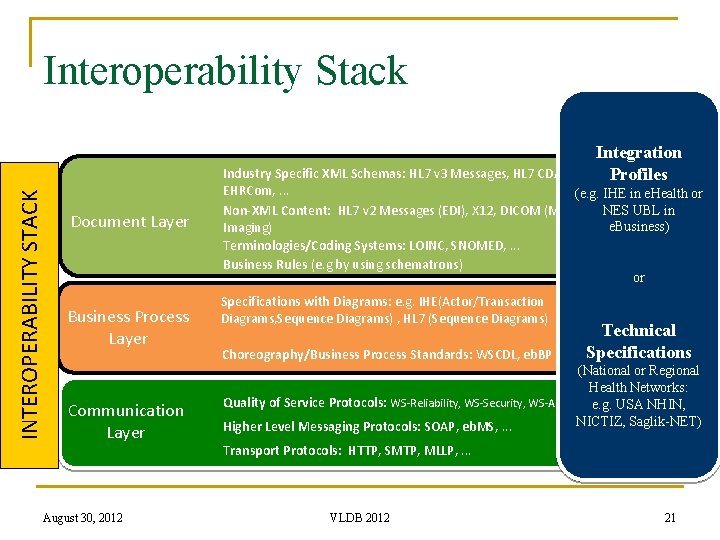
Interoperability Stack INTEROPERABILITY STACK Integration Profiles Document Layer Business Process Layer Communication Layer Industry Specific XML Schemas: HL 7 v 3 Messages, HL 7 CDA, EHRCom, . . . (e. g. IHE in e. Health or Non-XML Content: HL 7 v 2 Messages (EDI), X 12, DICOM (Medical NES UBL in e. Business) Imaging) Terminologies/Coding Systems: LOINC, SNOMED, . . . Business Rules (e. g by using schematrons) or Specifications with Diagrams: e. g. IHE(Actor/Transaction Diagrams, Sequence Diagrams) , HL 7 (Sequence Diagrams) Choreography/Business Process Standards: WSCDL, eb. BP Technical Specifications (National or Regional Health Networks: Quality of Service Protocols: WS-Reliability, WS-Security, WS-Addresinge. g. USA NHIN, NICTIZ, Saglik-NET) Higher Level Messaging Protocols: SOAP, eb. MS, . . . Transport Protocols: HTTP, SMTP, MLLP, . . . 21 August 30, 2012 VLDB 2012 21

Semantic Interoperability in e. Health August 30, 2012 22
Semantic Interoperability in e. Health n Ability for information shared by systems to be understood through formally defined domain concepts q q n In medicine, the clinical information is coded with “controlled vocabularies” or “terminologies” to express semantics, i. e. , the meaning of the terms used For example, the observation for a patient can be expressed as a “heart attack” or a “myocardial infarction”, and these mean the same thing to medical professionals But unless the term is associated with a unique code from a code system, automated processing of the exchanged term is very difficult because an application, programmed to use “heart attack”, would not understand “myocardial infarction” When a term from medical terminology system is used such as SNOMED CT and the code 22298006 for “Myocardial infarction”, the automated meaning exchange is possible Different coding systems used by data exchanging applications causes semantic interoperability problems August 30, 2012 VLDB 2012 23
Classification of Terminology Systems n The terminology systems can be classified based on how they express the semantics of the relationships between the coded terms: q q q Some are plain code lists, for example Rx. Norm Some are organized into a hierarchy, for example ICD-10 and hence give the parent-child information among the terms; Some express the relationships between the coded terms through ontological constructs, for example SNOMED CT or semantic networks like Unified Medical Language System (UMLS) August 30, 2012 VLDB 2012 24
SNOMED CT (Systematized Nomenclature of Medicine -- Clinical Terms) n SNOMED CT (http: //www. ihtsdo. org/snomed-ct/) is a computer processable collection of medical terminology covering most areas of clinical information such as q q q n n Diseases, Findings, Procedures, Microorganisms, and Pharmaceuticals It is the most comprehensive clinical vocabulary It is developed in native description logics (DL) formalism It contains approximately 350, 000 active concepts, more than one million terms (incl. synonyms) and about 1. 5 million relations between the concepts SNOMED CT crossmaps to other terminologies such as ICD-10 and LOINC as well August 30, 2012 VLDB 2012 25
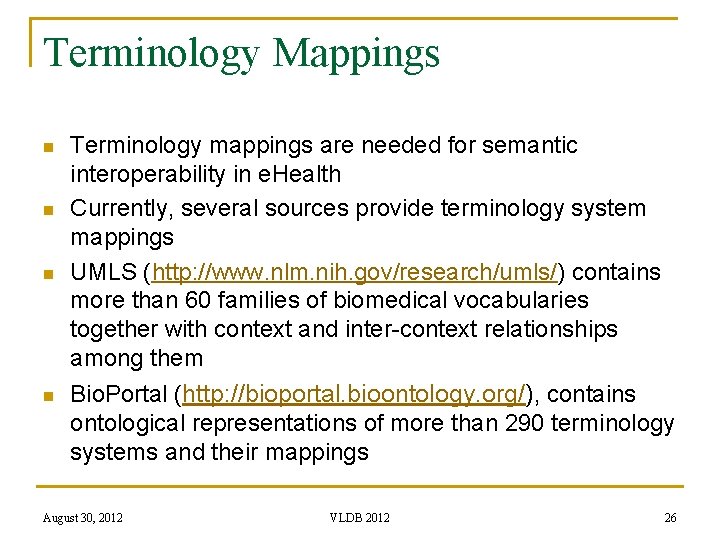
Terminology Mappings n n Terminology mappings are needed for semantic interoperability in e. Health Currently, several sources provide terminology system mappings UMLS (http: //www. nlm. nih. gov/research/umls/) contains more than 60 families of biomedical vocabularies together with context and inter-context relationships among them Bio. Portal (http: //bioportal. bioontology. org/), contains ontological representations of more than 290 terminology systems and their mappings August 30, 2012 VLDB 2012 26
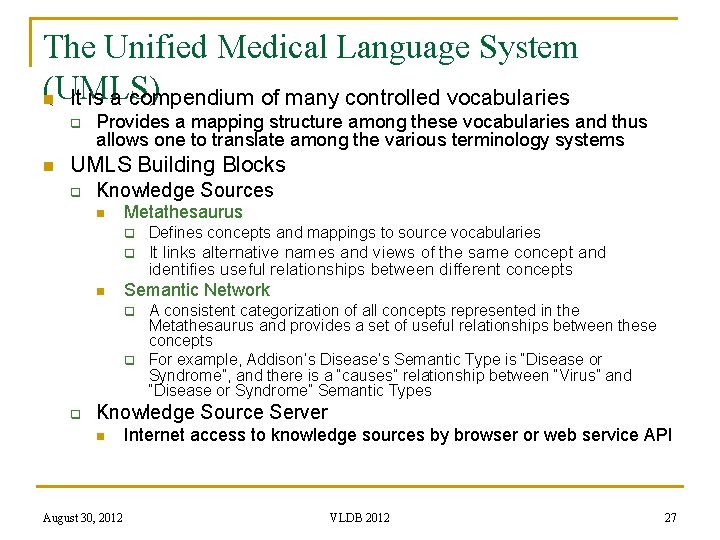
The Unified Medical Language System (UMLS) n It is a compendium of many controlled vocabularies q n Provides a mapping structure among these vocabularies and thus allows one to translate among the various terminology systems UMLS Building Blocks q Knowledge Sources n n Metathesaurus q Defines concepts and mappings to source vocabularies q It links alternative names and views of the same concept and identifies useful relationships between different concepts Semantic Network q q q A consistent categorization of all concepts represented in the Metathesaurus and provides a set of useful relationships between these concepts For example, Addison’s Disease’s Semantic Type is “Disease or Syndrome”, and there is a “causes” relationship between “Virus” and “Disease or Syndrome” Semantic Types Knowledge Source Server n August 30, 2012 Internet access to knowledge sources by browser or web service API VLDB 2012 27
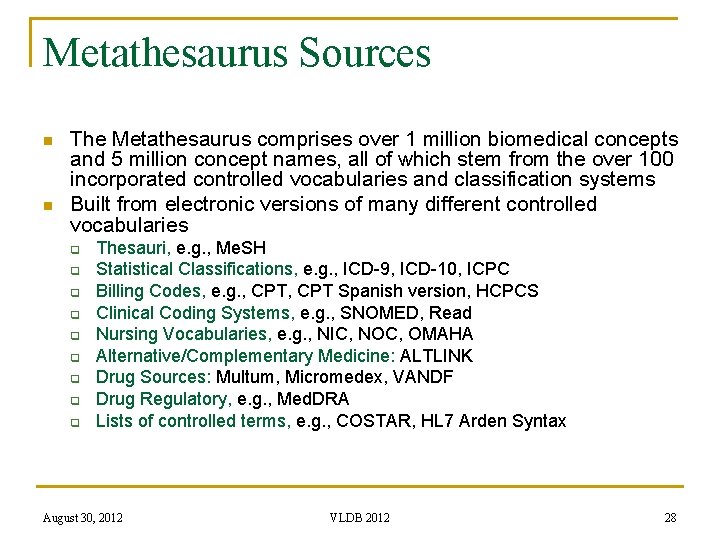
Metathesaurus Sources n n The Metathesaurus comprises over 1 million biomedical concepts and 5 million concept names, all of which stem from the over 100 incorporated controlled vocabularies and classification systems Built from electronic versions of many different controlled vocabularies q q q q q Thesauri, e. g. , Me. SH Statistical Classifications, e. g. , ICD-9, ICD-10, ICPC Billing Codes, e. g. , CPT Spanish version, HCPCS Clinical Coding Systems, e. g. , SNOMED, Read Nursing Vocabularies, e. g. , NIC, NOC, OMAHA Alternative/Complementary Medicine: ALTLINK Drug Sources: Multum, Micromedex, VANDF Drug Regulatory, e. g. , Med. DRA Lists of controlled terms, e. g. , COSTAR, HL 7 Arden Syntax August 30, 2012 VLDB 2012 28
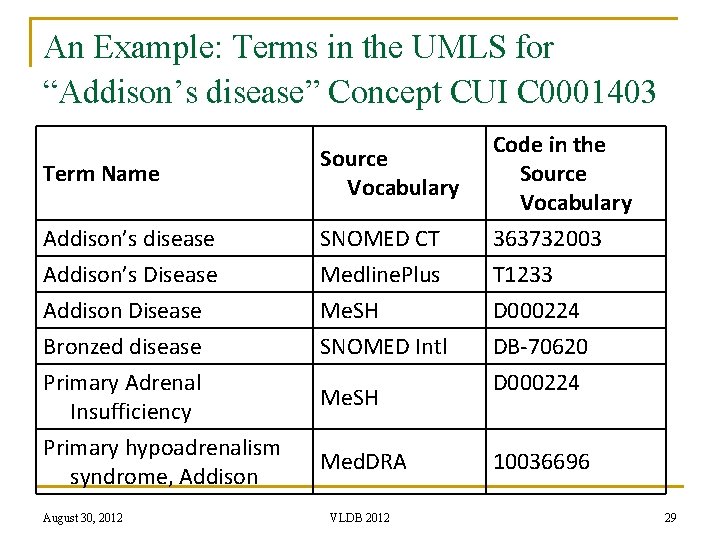
An Example: Terms in the UMLS for “Addison’s disease” Concept CUI C 0001403 Term Name Source Vocabulary Addison’s disease SNOMED CT Code in the Source Vocabulary 363732003 Addison’s Disease Medline. Plus T 1233 Addison Disease Me. SH D 000224 Bronzed disease SNOMED Intl DB-70620 Primary Adrenal Insufficiency Primary hypoadrenalism syndrome, Addison August 30, 2012 Me. SH Med. DRA VLDB 2012 D 000224 10036696 29
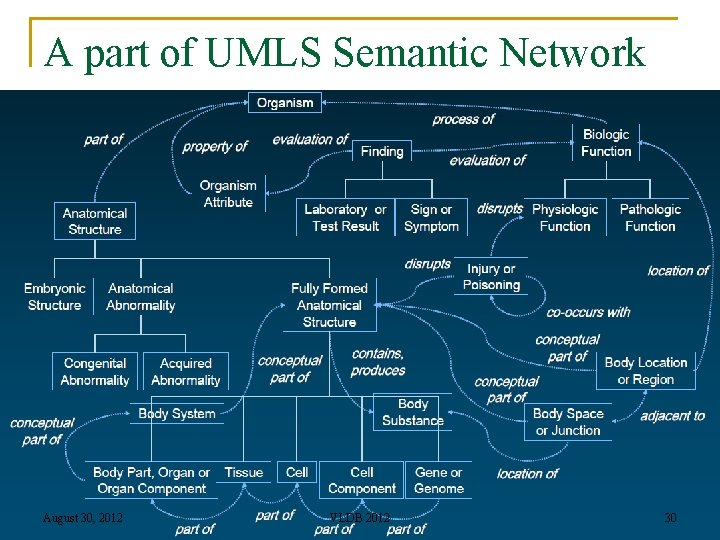
A part of UMLS Semantic Network August 30, 2012 VLDB 2012 30
Interoperability of Terminology Systems n To achieve interoperability among terminology systems q q n n n Not only the mapping information between them But also the semantic relationships among the terms (or, concepts) in a terminology system are necessary Because this helps discovering the implicit equivalences between two terms from different terminology systems by using reasoning However, the automated mappings cannot be fully reliable Therefore, continuous involvement of terminology experts and health care professionals in this process is necessary August 30, 2012 VLDB 2012 31
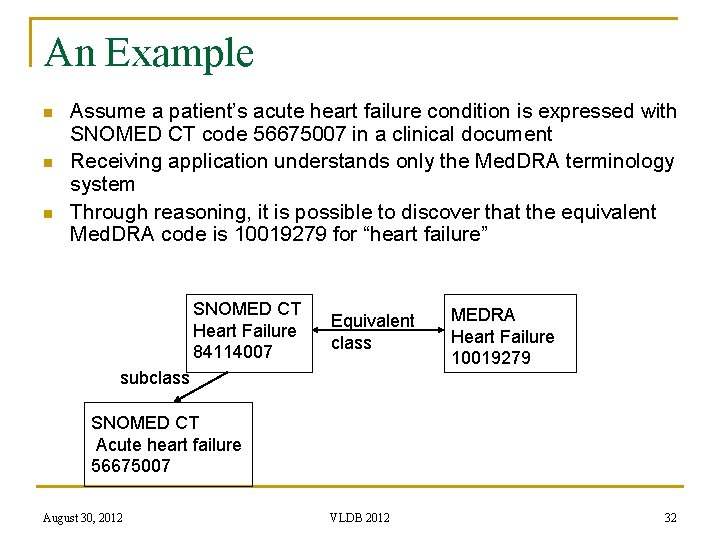
An Example n n n Assume a patient’s acute heart failure condition is expressed with SNOMED CT code 56675007 in a clinical document Receiving application understands only the Med. DRA terminology system Through reasoning, it is possible to discover that the equivalent Med. DRA code is 10019279 for “heart failure” SNOMED CT Heart Failure 84114007 Equivalent class subclass MEDRA Heart Failure 10019279 SNOMED CT Acute heart failure 56675007 August 30, 2012 VLDB 2012 32

Semantic Mediation ! ED M O SN CT August 30, 2012 VLDB 2012 33

An Example to How Some of the Mentioned e. Health Standards Used in Practice Sağlık-Net: The National Health Information System (NHIS), Turkey August 30, 2012 34
National Health Information System of Turkey (NHIS-T) n n n National Health Information System of Turkey (NHIS-T) is a nation-wide infrastructure for sharing patients’ electronic health records (EHRs) The NHIS-T became operational on January 15, 2009 By Feb. 2012, 98% of the public hospitals and 60% of the private and university hospitals were connected to NHIS-T with daily feeds of their patients’ EHRs Out of 74 million citizens of Turkey, electronic healthcare records of 60 million citizens have already been created in NHIS-T As of Feb. 2012, there are 661 million EHRs in the system q ~ 427 million Observation records q ~ 110 million Mouth and Teeth examination records q ~ 21 million In-patient records August 30, 2012 VLDB 2012 35
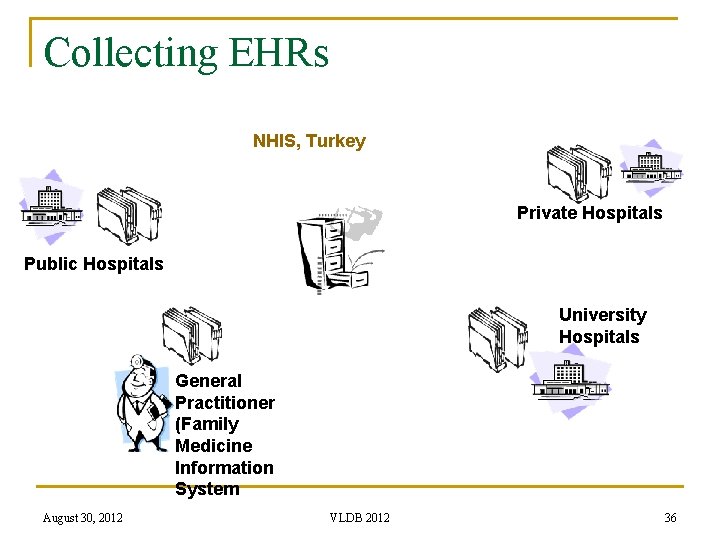
Collecting EHRs NHIS, Turkey Private Hospitals Public Hospitals University Hospitals General Practitioner (Family Medicine Information System August 30, 2012 VLDB 2012 36
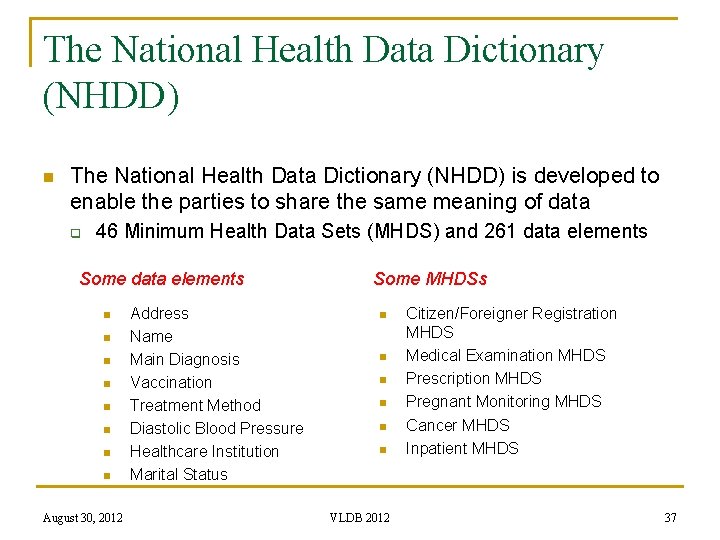
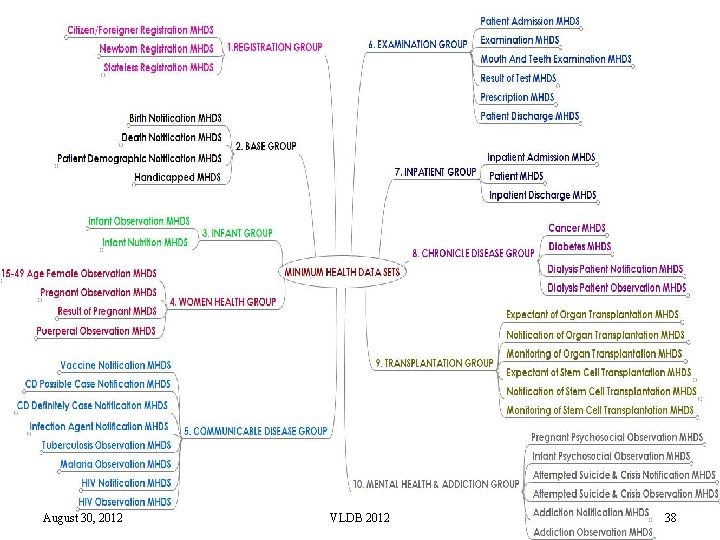
The National Health Data Dictionary (NHDD) n The National Health Data Dictionary (NHDD) is developed to enable the parties to share the same meaning of data q 46 Minimum Health Data Sets (MHDS) and 261 data elements Some data elements n n n n August 30, 2012 Address Name Main Diagnosis Vaccination Treatment Method Diastolic Blood Pressure Healthcare Institution Marital Status Some MHDSs n n n VLDB 2012 Citizen/Foreigner Registration MHDS Medical Examination MHDS Prescription MHDS Pregnant Monitoring MHDS Cancer MHDS Inpatient MHDS 37
August 30, 2012 VLDB 2012 38
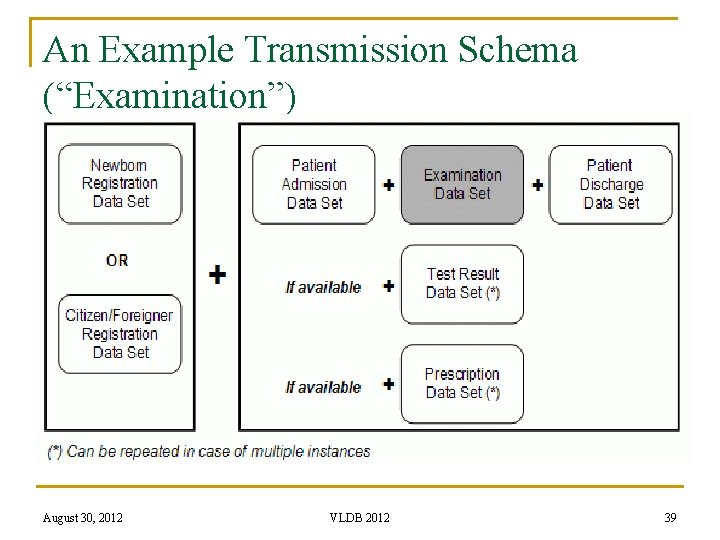
An Example Transmission Schema (“Examination”) August 30, 2012 VLDB 2012 39
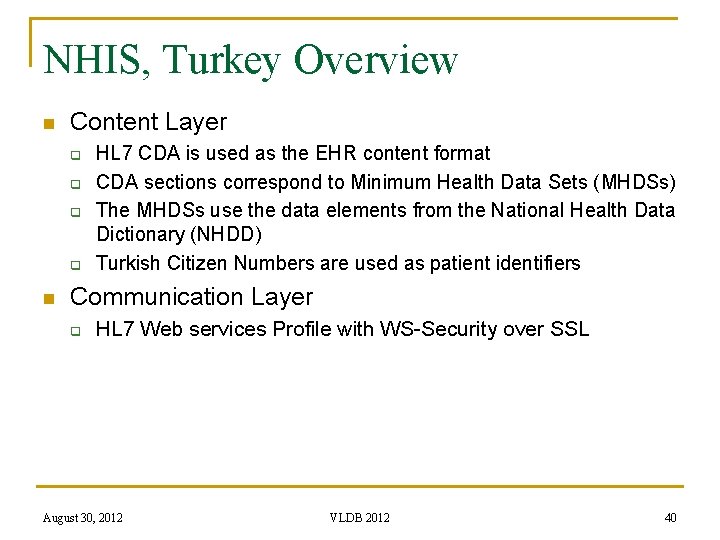
NHIS, Turkey Overview n Content Layer q q n HL 7 CDA is used as the EHR content format CDA sections correspond to Minimum Health Data Sets (MHDSs) The MHDSs use the data elements from the National Health Data Dictionary (NHDD) Turkish Citizen Numbers are used as patient identifiers Communication Layer q HL 7 Web services Profile with WS-Security over SSL August 30, 2012 VLDB 2012 40
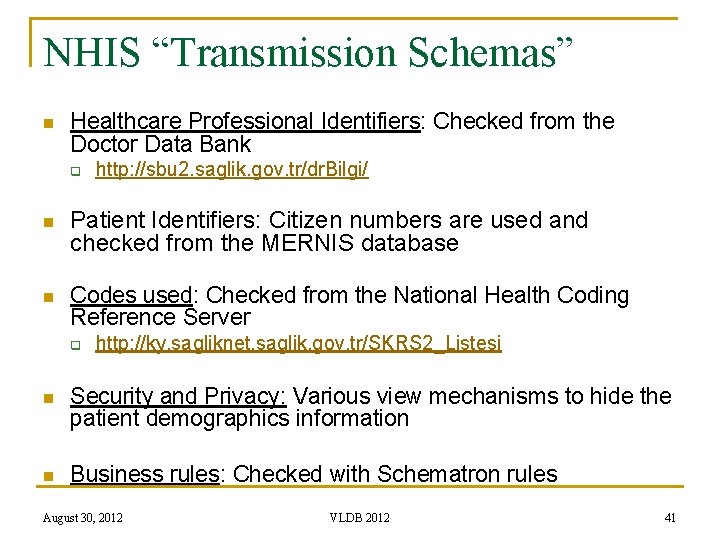
NHIS “Transmission Schemas” n Healthcare Professional Identifiers: Checked from the Doctor Data Bank q http: //sbu 2. saglik. gov. tr/dr. Bilgi/ n Patient Identifiers: Citizen numbers are used and checked from the MERNIS database n Codes used: Checked from the National Health Coding Reference Server q http: //ky. sagliknet. saglik. gov. tr/SKRS 2_Listesi n Security and Privacy: Various view mechanisms to hide the patient demographics information n Business rules: Checked with Schematron rules August 30, 2012 VLDB 2012 41

Healthcare Professional Registry n Ministry of Health is authorized to provide the work licenses to the physicians in Turkey n The diploma/specialty information of the medical professionals is recorded together with their Turkish citizenship numbers in the Doctor Data Bank (DDB) n As of October 2007, there are 162, 446 registered doctors in the data bank n The Doctor Data Bank is for checking the validity of the healthcare professional identity in the “Transmission Schema” n Later it will be used authorizing access to the EHRs of the patients August 30, 2012 VLDB 2012 42
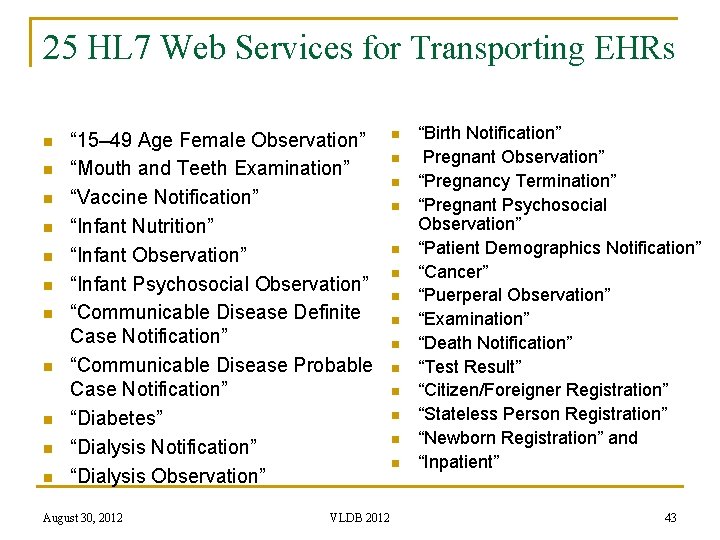
25 HL 7 Web Services for Transporting EHRs n n n “ 15– 49 Age Female Observation” “Mouth and Teeth Examination” “Vaccine Notification” “Infant Nutrition” “Infant Observation” “Infant Psychosocial Observation” “Communicable Disease Definite Case Notification” “Communicable Disease Probable Case Notification” “Diabetes” “Dialysis Notification” “Dialysis Observation” August 30, 2012 VLDB 2012 n n n n “Birth Notification” Pregnant Observation” “Pregnancy Termination” “Pregnant Psychosocial Observation” “Patient Demographics Notification” “Cancer” “Puerperal Observation” “Examination” “Death Notification” “Test Result” “Citizen/Foreigner Registration” “Stateless Person Registration” “Newborn Registration” and “Inpatient” 43
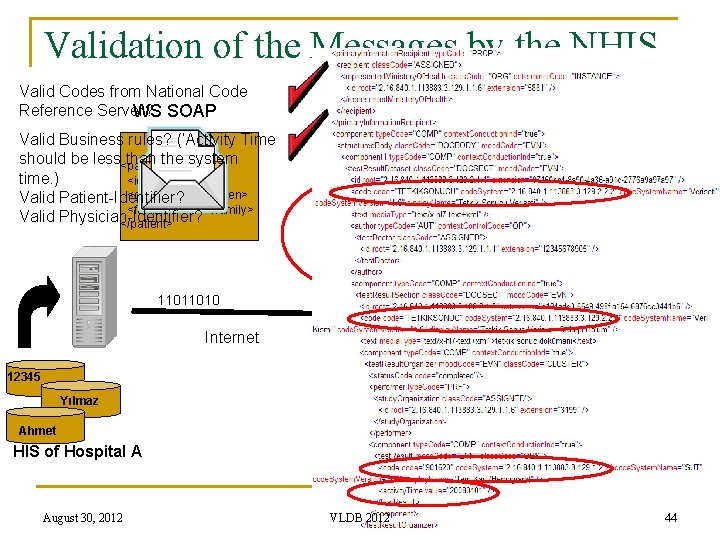
Validation of the Messages by the NHIS Valid Codes from National Code Reference Server? WS SOAP Valid SOAP message? Valid use of WS-Security User Name Token Profile? Valid Business rules? (‘Activity Time HL 7 -V 3 should be less than the system <patient> time. ) <id> </id> <given> </given> Valid Patient-Identifier? <family> </family> Valid Physician-Identifier? </patient> Valid HL 7 CDA Schema? 11011010 Internet Observation Service 12345 NHIS Yılmaz Ahmet HIS of Hospital A August 30, 2012 VLDB 2012 44
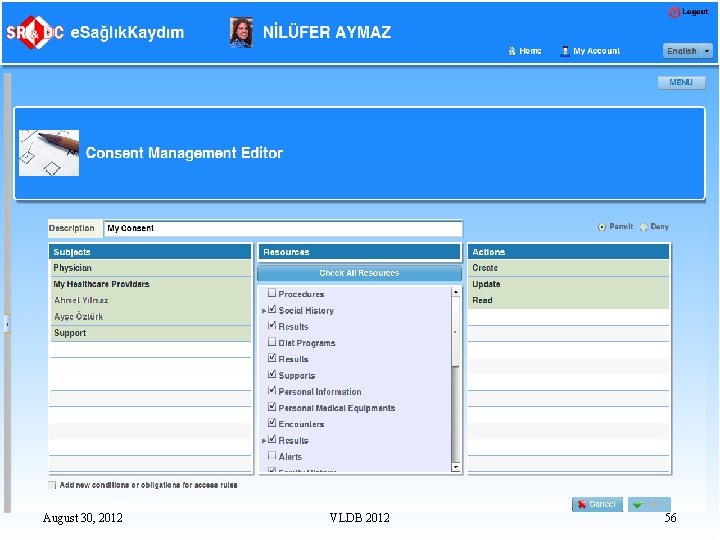
Handling Security and Privacy n n n There are two types of administrators in the system: q Security Administrator is in charge of granting rights to the Database Administrators but they themselves have no right to access the database Various “View” mechanisms are developed to hide the patient demographics data from the unauthorized users The Mo. H has selected Oracle Identity Management System Access to NHIS data is audited by logging all the user events Currently the work is going on for determining the legal ground about the access rights of various types of users August 30, 2012 VLDB 2012 45
Decision Support System Child mortality rate under the age of 12 months in Bilecik? Number of diarrhea incidents in Istanbul during the last three days? Number of cancer incidents during last year in all of the provinces of Turkey? Decision Support System NHIS August 30, 2012 VLDB 2012 46

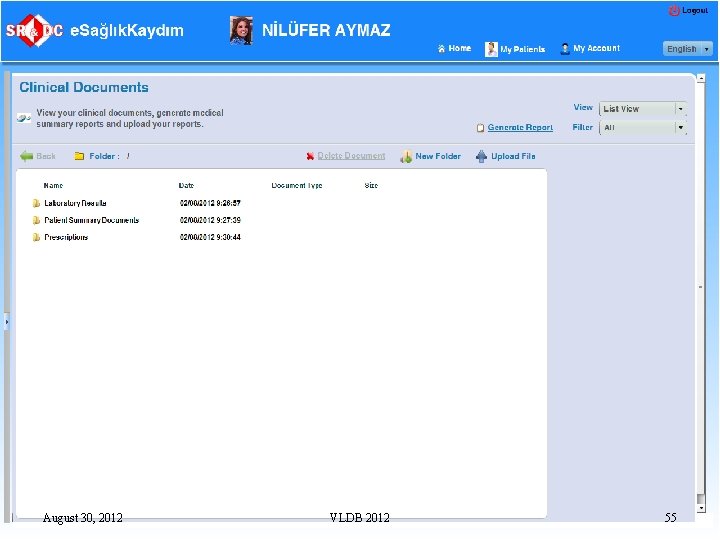
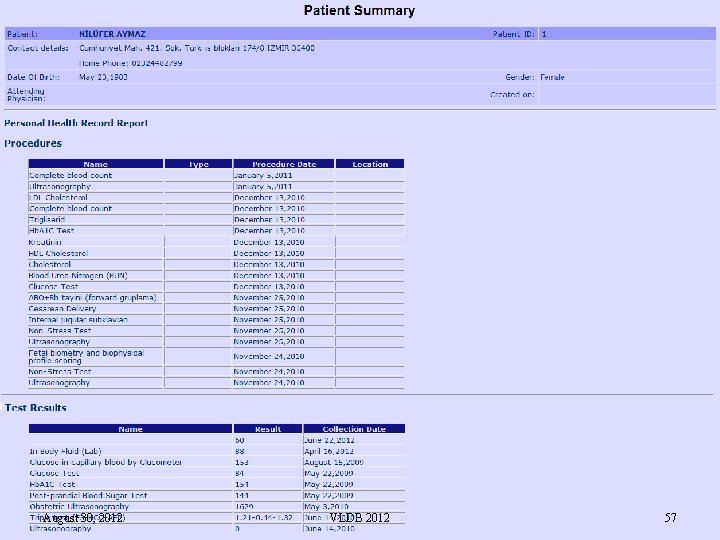
Note. . n n n In Turkey, although the EHRs are collected, they are shared only with General Practitioners but not with the physicians in the secondary and tertiary care This is because there is a need for a consent mechanism for the patients The next step is to allow sharing of EHRs not only with authorized medical practitioners but also with the patients themselves q Through a Personal Health Record system, called, e. Saglik. Kaydim August 30, 2012 VLDB 2012 47

Personal Health Record Systems n n The Personal Health Record (PHR) systems have evolved from Web pages where patients entered their own data manually Currently, they are classified as: q q q Provider-tethered PHR system: Data from a medical provider’s healthcare information system is entered into the PHRs automatically Payer-tethered PHR systems provide patients access to claims data Third party PHR systems such as Microsoft Health. Vault that provides a secure storage for PHR data together with data exchange interfaces so that third parties can develop applications to upload patient data, for example, home health devices Interoperable PHR systems, on the other hand, represent a future type of PHR in which all entities have access to data A recent report by Google, HIMSS, Kaiser Permanente and Microsoft concludes that from the perspective of the healthcare system, interoperable PHRs provide the greatest value August 30, 2012 VLDB 2012 48
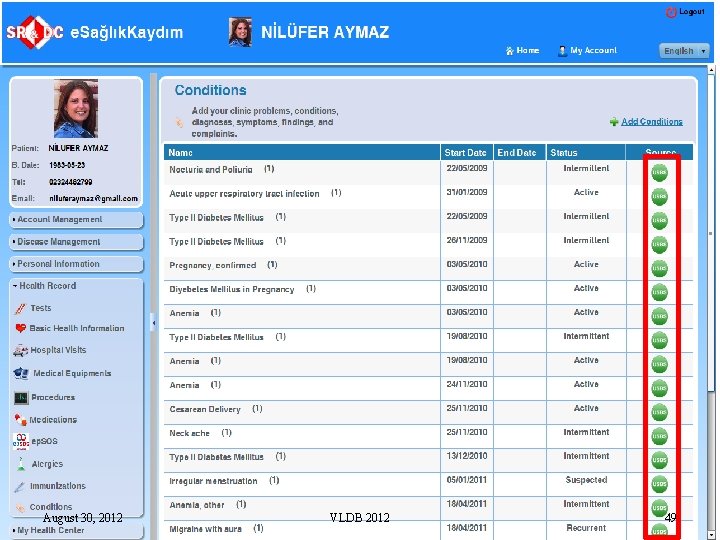
August 30, 2012 VLDB 2012 49
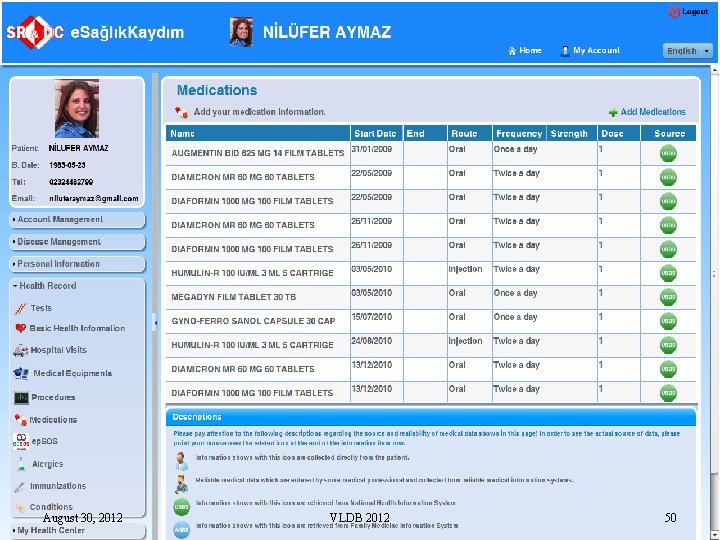
August 30, 2012 VLDB 2012 50
August 30, 2012 VLDB 2012 51
August 30, 2012 VLDB 2012 52
August 30, 2012 VLDB 2012 53
August 30, 2012 VLDB 2012 54
August 30, 2012 VLDB 2012 55
August 30, 2012 VLDB 2012 56
August 30, 2012 VLDB 2012 57

Factors Affecting the Success of NHIS, Turkey n n The Ministry of Health, being the national authority to decide on the e. Health standards in Turkey, was able to enforce the standards that have led to their fast adoption Building a national common data dictionary consisting of e. Health data elements and minimum health data sets has helped to q q q n n Identify clearly the meaning of data elements; Giving their explanation in the local language and, more importantly, Making it possible to share and re-use these components Finally, the comprehensive testing, especially the conformance and interoperability testing of vendor-based hospital information systems contributed to the fast integration of the majority of the 65 HIS vendors in Turkey Note however that while addressing the national level EHR interoperability may be relatively easy, it is much more difficult to do this in an international environment August 30, 2012 VLDB 2012 58

Related Publications n Although this is applied work, it also involved research: q q q Asuman Dogac, Mustafa Yuksel, et. al. , ”Electronic Health Record Interoperability as Realized in Turkey’s National Health Information System”, Methods of Information in Medicine, Vol. 50, No. 2, March 2011, pp. 140– 149. Namli, T. , Dogac, A. , “Testing Conformance and Interoperability of e. Health Applications”, Methods of Information in Medicine, Vol. 49, No. 3, May 2010, pp. 281– 289. Namli T. , Aluc G. , Dogac A. , “An Interoperability Test Framework for HL 7 based Systems”, IEEE Transactions on Information Technology in Biomedicine Vol. 13, No. 3, May 2009, pp. 389 -399. Namli T. , et. al. , “Testing the Conformance and Interoperability of NHIS to Turkey’s HL 7 Profile”, 9 th International HL 7 Interoperability Conference (IHIC) 2008, Crete, Greece, October, 2008, pp. 63 -68. Kabak Y. , Dogac A. , et. al. , “The Use of HL 7 CDA in the National Health Information System (NHIS) of Turkey, 9 th International HL 7 Interoperability Conference (IHIC) 2008, Crete, Greece, October, 2008 pp. 49 -55. Eichelberg M. , Aden T. , Riesmeier J. , Dogac A. , Laleci G. , “A Survey and Analysis of Electronic Healthcare Record Standards”, ACM Computing Surveys, Vol. 37, No: 4, December 2005. August 30, 2012 VLDB 2012 59

Some Other Related Publications n n n n ARTEMIS: An Infrastructure for the Interoperability of Medical Information Systems, Healthcare IT Management Journal, Volume 1, Issue 2, Summer 2006, pp. 26 -27. “Artemis: Deploying Semantically Enriched Web Services in the Healthcare Domain”, Information Systems Journal (Elsevier), Volume 31, Issues 4 -5, June-July 2006, pp. 321339 Collaborative Business Process Support in IHE XDS through eb. XML Business Processes, In proc. of International Conference on Data Engineering (ICDE 2006) Exploiting eb. XML Registry Semantic Constructs for Handling Archetype Metadata in Healthcare Informatics, International Journal of Metadata, Semantics and Ontologies, Volume 1, No. 1, 2006. The Need for Semantic Web Service in the e. Health, W 3 C workshop on Frameworks for Semantics in Web Services Archetype-based Semantic Interoperability of Web Service Messages in the Healthcare Domain, Int'l Journal on Semantic Web & Information Systems, Vol. 1, No. 4, October 2005, pp. 1 -22. Artemis Message Exchange Framework: Semantic Interoperability of Exchanged Messages in the Healthcare Domain, ACM Sigmod Record, Vol. 34, No. 3, September 2005 August 30, 2012 VLDB 2012 60

Some example Integrating Healthcare Enterprise (IHE) Profiles August 30, 2012 61
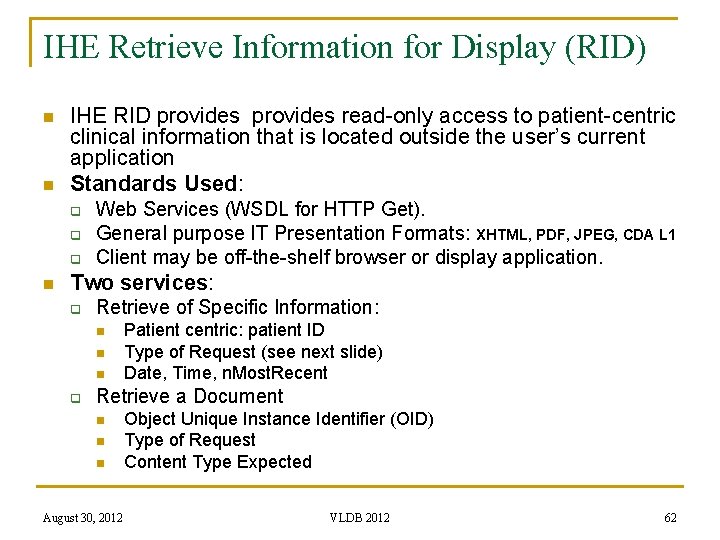
IHE Retrieve Information for Display (RID) n n IHE RID provides read-only access to patient-centric clinical information that is located outside the user’s current application Standards Used: q q q n Web Services (WSDL for HTTP Get). General purpose IT Presentation Formats: XHTML, PDF, JPEG, CDA L 1 Client may be off-the-shelf browser or display application. Two services: q Retrieve of Specific Information: n n n q Patient centric: patient ID Type of Request (see next slide) Date, Time, n. Most. Recent Retrieve a Document n n n August 30, 2012 Object Unique Instance Identifier (OID) Type of Request Content Type Expected VLDB 2012 62
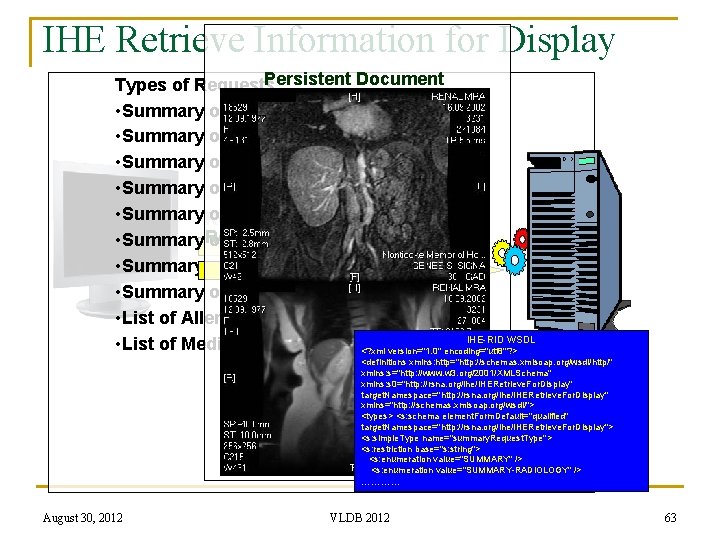
IHE Retrieve Information for Display Persistent Document Types of Requests • Summary of All Reports • Summary of Laboratory Reports • Summary of Radiology Reports • Summary of Cardiology Reports • Summary Retrieve of Surgery Reports Specific Info for Display Document for Display • Summary Retrieve of Intensive Care Reports • Summary of Emergency Reports • Summary of Discharge Reports • List of Allergies IHE-RID WSDL • List of Medications <? xml version="1. 0" encoding="utf 8"? > <definitions xmlns: http="http: //schemas. xmlsoap. org/wsdl/http/" xmlns: s="http: //www. w 3. org/2001/XMLSchema" xmlns: s 0="http: //rsna. org/ihe/IHERetrieve. For. Display" target. Namespace="http: //rsna. org/ihe/IHERetrieve. For. Display" xmlns="http: //schemas. xmlsoap. org/wsdl/"> <types> <s: schema element. Form. Default="qualified" target. Namespace="http: //rsna. org/ihe/IHERetrieve. For. Display"> <s: simple. Type name="summary. Request. Type"> <s: restriction base="s: string"> <s: enumeration value="SUMMARY" /> <s: enumeration value="SUMMARY-RADIOLOGY" /> ………… August 30, 2012 VLDB 2012 63
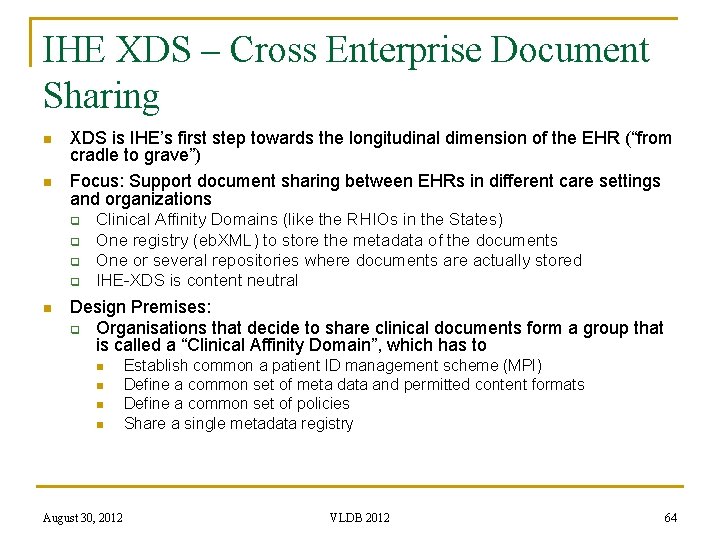
IHE XDS – Cross Enterprise Document Sharing n n XDS is IHE’s first step towards the longitudinal dimension of the EHR (“from cradle to grave”) Focus: Support document sharing between EHRs in different care settings and organizations q q n Clinical Affinity Domains (like the RHIOs in the States) One registry (eb. XML) to store the metadata of the documents One or several repositories where documents are actually stored IHE-XDS is content neutral Design Premises: q Organisations that decide to share clinical documents form a group that is called a “Clinical Affinity Domain”, which has to n n August 30, 2012 Establish common a patient ID management scheme (MPI) Define a common set of meta data and permitted content formats Define a common set of policies Share a single metadata registry VLDB 2012 64
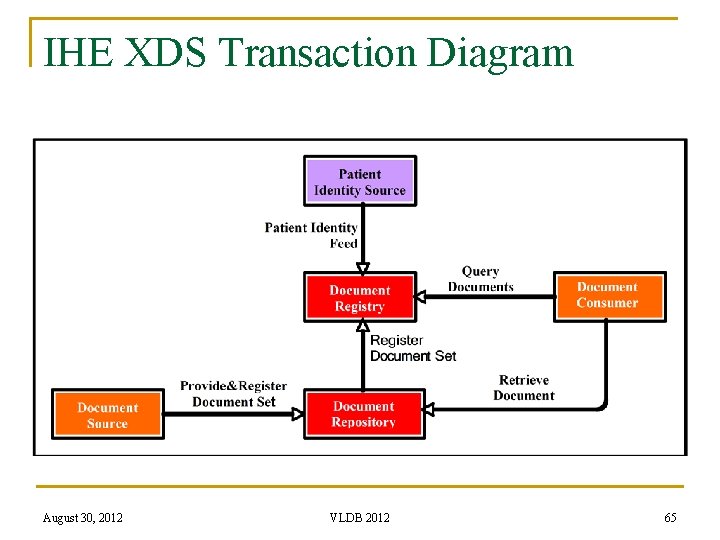
IHE XDS Transaction Diagram August 30, 2012 VLDB 2012 65
IHE XDS n Business Process Layer: There are five actors in this profile q q q n n Document Source Actor submits an EHR with the “Provide and Register Document Set” transaction to the Document Repository contains the EHRs and registers the metadata of the documents to the Document Registry with “Register Document Set” transaction Document Registry contains the metadata of the documents Patient Identity Source aligns the different Patient IDs Document Consumer queries the registry with “Query Documents” transaction with metadata to obtain the pointer to the EHR in the Document Repository Document Consumer uses the “Retrieve Document” transaction to obtain the document from the Document Repository using the pointer Document Layer: To be decided by each Clinical Affinity Domain (CDA, pdf, …) Communication Layer: eb. XML Registry specification 3. 0 (MTOM based Web services) August 30, 2012 VLDB 2012 66

An Example to How IHE Profiles are Used in Practice ep. SOS - European Patients Smart open Services August 30, 2012 70
Exchanging Patient Summaries and e. Prescriptions in Europe: The ep. SOS initiative • Different languages • Different e. Health processes • Different grade of development • Different Legislation • Different concepts • No European Nomenclature for Medicines August 30, 2012 VLDB 2012 71
The ep. SOS participating nations August 30, 2012 VLDB 2012 72

The ep. SOS Solution n n Patient Summary for European Citizens e. Prescription service for European Citizens q q n The content templates for the three document types q q q n n e. Prescribing is the electronic prescribing of medicine using software to transmit the prescription data to the pharmacy where it is being retrieved e. Dispensing is the electronic retrieval of an e. Prescription, the dispensing of the medicine to the patient and the submission of an electronic report for the medicine dispensed Patient summary, e. Prescription and e. Dispensation These pivot documents to be exchanged are defind by using and further restricting IHE PCC templates Each country defines its own mapping from its national data structures onto these pivot document schemas August 30, 2012 VLDB 2012 73
Important Standards Used in ep. SOS n Content models for Patient Summary, e. Prescription and e. Dispensation documents q q q n Clinical Terminology Systems q n ICD-10, SNOMED CT, LOINC, ATC, … Interoperability Profiles q q q n HL 7 Clinical Document Architecture – CDA v 2 HL 7/ASTM Continuity of Care Document (CCD) Content templates Integrating the Healthcare Enterprise (IHE) Patient Care Coordination (PCC) Content templates IHE Cross-Community Patient Discovery (XCPD) profile IHE Cross-Community Access (XCA) profile IHE Cross-Enterprise Document Reliable Interchange (XDR) profile Security q q q IHE Cross-Enterprise User Assertion (XUA) profile IHE Audit Trail & Node Authentication (ATNA) profile … August 30, 2012 VLDB 2012 74
The ep. SOS Solution n For the coded representation of clinical content within these schemas, subsets from existing terminology systems are used q q n n n ep. SOS Master Value Sets Catalogue (MVC) is created The latest version 1. 7 of MVC contains 46 value sets For example, q q n SNOMED CT, ICD-10, LOINC, ATC, … The procedures value set contains 102 terms from SNOMED CT and Illnesses and disorders value set contains 1685 terms from ICD-10 Each ep. SOS participating nation provides q q Mapping of any locally used terminology system to MVC content and also Translation of the MVC content into its own language, resulting in the ep. SOS Master Translation/Transcoding Catalogue (MTC) August 30, 2012 VLDB 2012 75
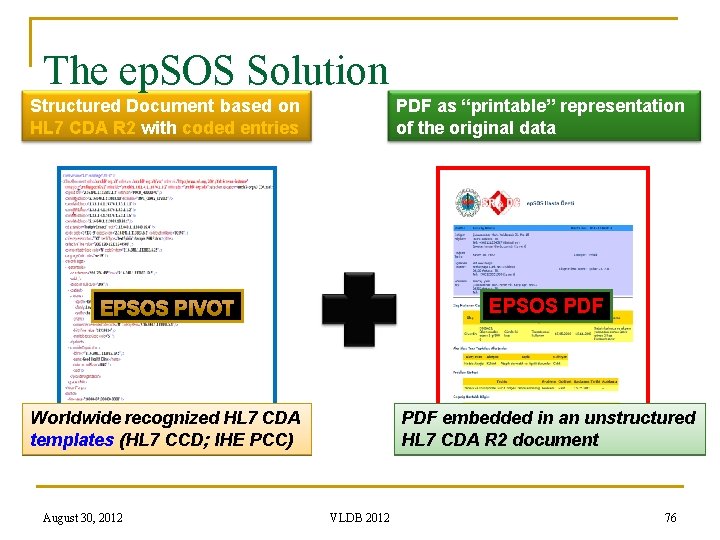
The ep. SOS Solution Structured Document based on HL 7 CDA R 2 with coded entries PDF as “printable” representation of the original data EPSOS PDF Worldwide recognized HL 7 CDA templates (HL 7 CCD; IHE PCC) August 30, 2012 PDF embedded in an unstructured HL 7 CDA R 2 document VLDB 2012 76
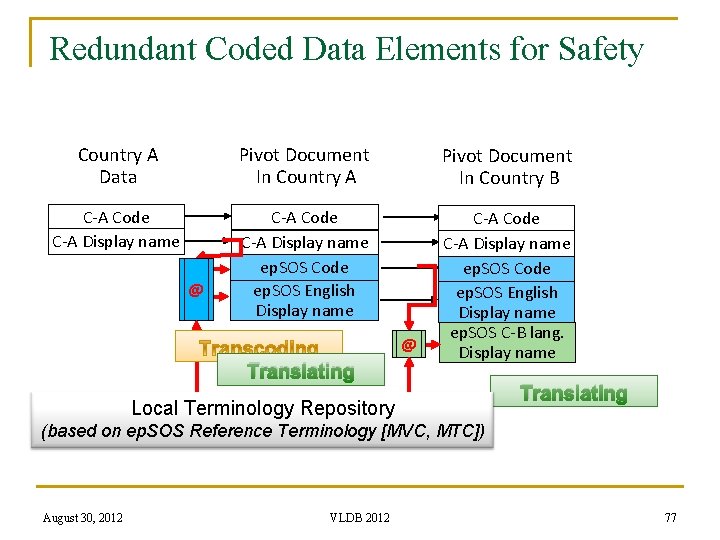
Redundant Coded Data Elements for Safety Country A Data Pivot Document In Country A Pivot Document In Country B C-A Code C-A Display name ep. SOS Code ep. SOS English Display name ep. SOS C-B lang. Display name @ @ Translating Local Terminology Repository Translating (based on ep. SOS Reference Terminology [MVC, MTC]) August 30, 2012 VLDB 2012 77
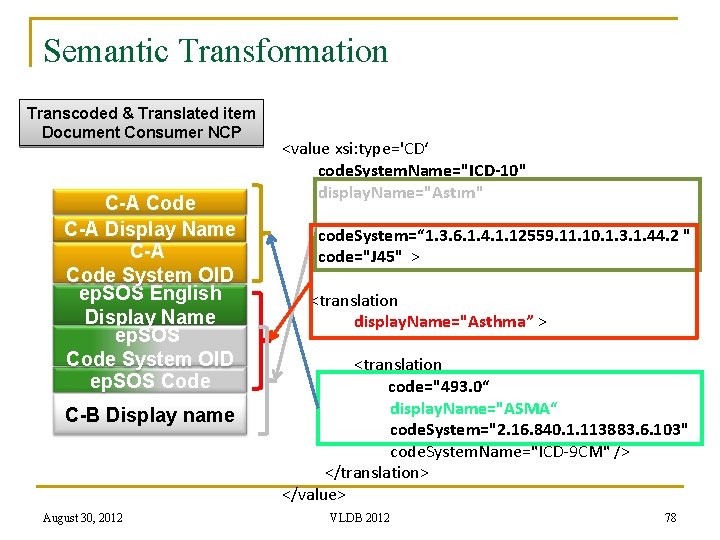
Semantic Transformation Transcoded & Translated item Document Consumer NCP C-A Code C-A Display Name C-A Code System OID ep. SOS English Display Name ep. SOS Code System OID ep. SOS Code C-B Display name August 30, 2012 <value xsi: type='CD‘ code. System. Name="ICD-10" display. Name="Astım" code. System=“ 1. 3. 6. 1. 4. 1. 12559. 11. 10. 1. 3. 1. 44. 2 " code="J 45" > <translation display. Name="Asthma” > <translation code="493. 0“ display. Name="ASMA“ code. System="2. 16. 840. 1. 113883. 6. 103" code. System. Name="ICD-9 CM" /> </translation> </value> VLDB 2012 78
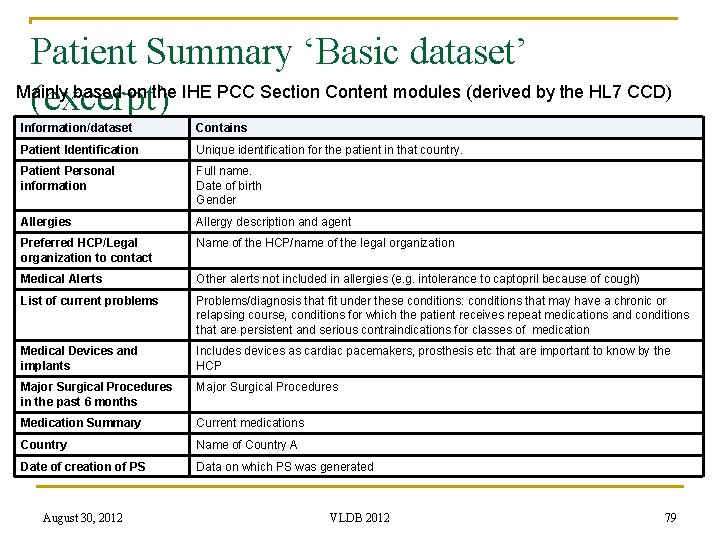
Patient Summary ‘Basic dataset’ Mainly based on the IHE PCC Section Content modules (derived by the HL 7 CCD) (excerpt) Information/dataset Contains Patient Identification Unique identification for the patient in that country. Patient Personal information Full name. Date of birth Gender Allergies Allergy description and agent Preferred HCP/Legal organization to contact Name of the HCP/name of the legal organization Medical Alerts Other alerts not included in allergies (e. g. intolerance to captopril because of cough) List of current problems Problems/diagnosis that fit under these conditions: conditions that may have a chronic or relapsing course, conditions for which the patient receives repeat medications and conditions that are persistent and serious contraindications for classes of medication Medical Devices and implants Includes devices as cardiac pacemakers, prosthesis etc that are important to know by the HCP Major Surgical Procedures in the past 6 months Major Surgical Procedures Medication Summary Current medications Country Name of Country A Date of creation of PS Data on which PS was generated August 30, 2012 VLDB 2012 79
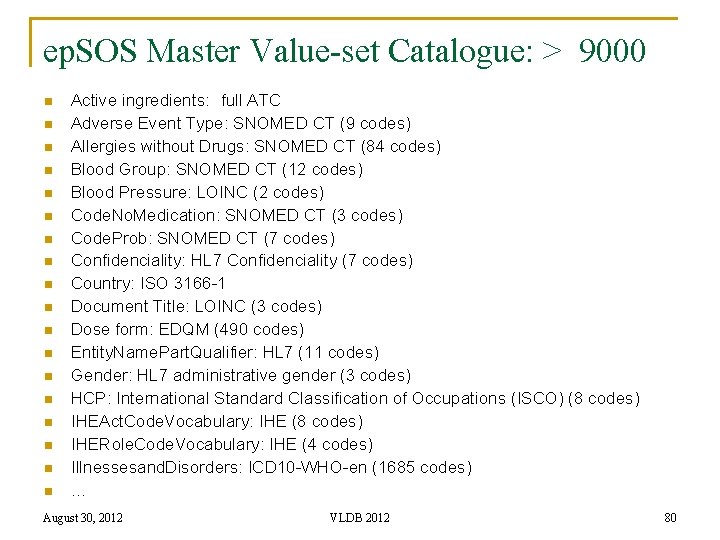
ep. SOS Master Value-set Catalogue: > 9000 n n n n n Active ingredients: full ATC Adverse Event Type: SNOMED CT (9 codes) Allergies without Drugs: SNOMED CT (84 codes) Blood Group: SNOMED CT (12 codes) Blood Pressure: LOINC (2 codes) Code. No. Medication: SNOMED CT (3 codes) Code. Prob: SNOMED CT (7 codes) Confidenciality: HL 7 Confidenciality (7 codes) Country: ISO 3166 -1 Document Title: LOINC (3 codes) Dose form: EDQM (490 codes) Entity. Name. Part. Qualifier: HL 7 (11 codes) Gender: HL 7 administrative gender (3 codes) HCP: International Standard Classification of Occupations (ISCO) (8 codes) IHEAct. Code. Vocabulary: IHE (8 codes) IHERole. Code. Vocabulary: IHE (4 codes) Illnessesand. Disorders: ICD 10 -WHO-en (1685 codes) … August 30, 2012 VLDB 2012 80
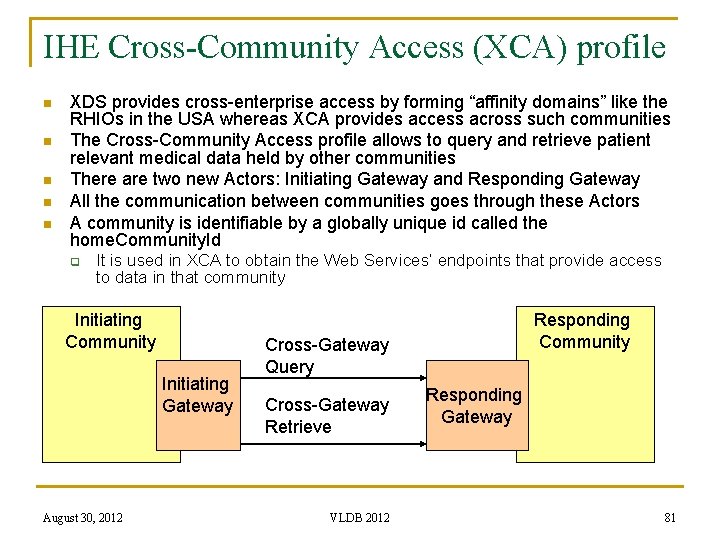
IHE Cross-Community Access (XCA) profile n n n XDS provides cross-enterprise access by forming “affinity domains” like the RHIOs in the USA whereas XCA provides access across such communities The Cross-Community Access profile allows to query and retrieve patient relevant medical data held by other communities There are two new Actors: Initiating Gateway and Responding Gateway All the communication between communities goes through these Actors A community is identifiable by a globally unique id called the home. Community. Id q It is used in XCA to obtain the Web Services’ endpoints that provide access to data in that community Initiating Community Initiating Gateway August 30, 2012 Responding Community Cross-Gateway Query Cross-Gateway Retrieve VLDB 2012 Responding Gateway 81
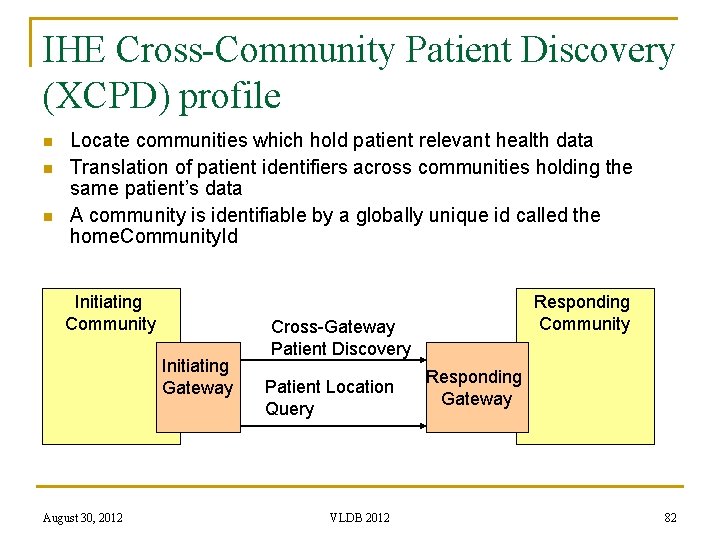
IHE Cross-Community Patient Discovery (XCPD) profile n n n Locate communities which hold patient relevant health data Translation of patient identifiers across communities holding the same patient’s data A community is identifiable by a globally unique id called the home. Community. Id Initiating Community Initiating Gateway August 30, 2012 Responding Community Cross-Gateway Patient Discovery Patient Location Query VLDB 2012 Responding Gateway 82
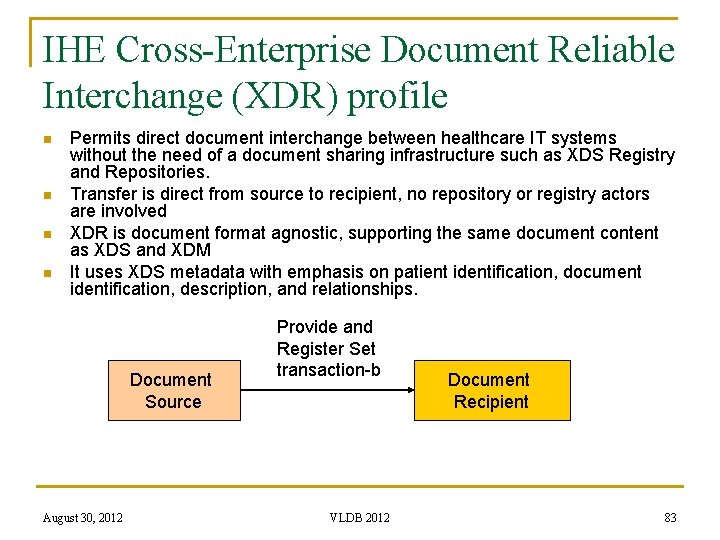
IHE Cross-Enterprise Document Reliable Interchange (XDR) profile n n Permits direct document interchange between healthcare IT systems without the need of a document sharing infrastructure such as XDS Registry and Repositories. Transfer is direct from source to recipient, no repository or registry actors are involved XDR is document format agnostic, supporting the same document content as XDS and XDM It uses XDS metadata with emphasis on patient identification, document identification, description, and relationships. Document Source August 30, 2012 Provide and Register Set transaction-b VLDB 2012 Document Recipient 83
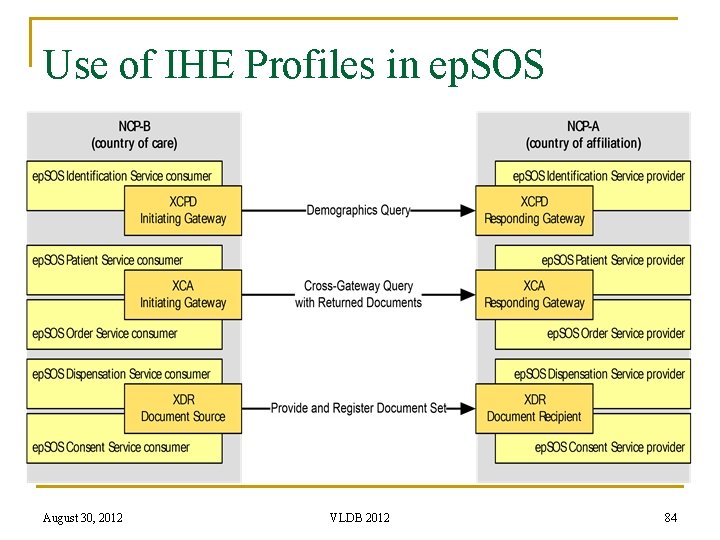
Use of IHE Profiles in ep. SOS August 30, 2012 VLDB 2012 84

Medical Device Interoperability August 30, 2012 85
Medical Device- Healthcare Application Interoperability Standards n n Personal medical devices are essential to the practice of modern health care services The prominent standards for the integration of medical device data into electronic health records (EHR/PHR) include: q q n IHE Patient Care Device (PCD) integration profiles and Electronic/Personal Health Record Network Interface (x. HRN-IF) by the Continua Health Alliance Both of these standards address how to map q q The device data obtained through the ISO/IEEE 11073 standard To the healthcare application interfaces August 30, 2012 VLDB 2012 86
IHE Device Enterprise Communication (DEC) Profile n n Used for transmitting information from medical devices to the enterprise applications For this purpose: q q n n ISO/IEEE 11073 Domain Information Model is mapped to HL 7 v 2. 5 Observation Report and The ISO/IEEE 11073 Data Types are mapped to HL 7 v 2. 5 Data Types For semantic interoperability, IHE has developed the Rosetta Terminology Mapping (RTM) Profile to provide the mapping between the proprietary device parameters to ISO/IEEE 11073 Nomenclature A special case of this profile for cardiac implantable devices q IHE Implantable Device – Cardiac – Observation (IDCO) Profile q Defines a standard based translation and transfer of device interrogation information from the interrogation system to the healthcare application August 30, 2012 VLDB 2012 87
An Example to How Medical Device Standards are Used in Practice i. Cardea: An Intelligent Platform for Personalized Remote Monitoring of the Cardiac Patients with Electronic Implant Devices August 30, 2012 88
e. Health Situation with Cardiac Patients n There has been an exponential growth in the number of cardiac implantable devices: 800. 000 CIED patients in EU with 5. 8 million follow-up visits n CIED electronic and software complexity have widen their function and application n However, due to their limited processing capabilities restricted by their size, CIEDs need to be supported with external software running on “data centers” n Currently, the data center processing is standalone with their own custom software and proprietary interfaces q q q Patient and device data is stored in data centres operated by the vendors Presented via secure Web-sites to the access of responsible healthcare professionals Access to follow-up information often requires clinicians to use multiple vendor specific systems and interfaces, reducing efficiency August 30, 2012 VLDB 2012 89
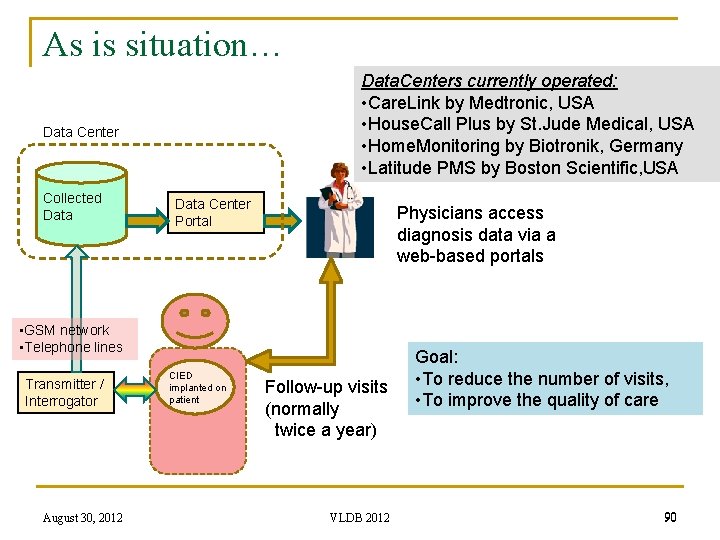
As is situation… Data. Centers currently operated: • Care. Link by Medtronic, USA • House. Call Plus by St. Jude Medical, USA • Home. Monitoring by Biotronik, Germany • Latitude PMS by Boston Scientific, USA Data Center Collected Data Center Portal Physicians access diagnosis data via a web-based portals • GSM network • Telephone lines Transmitter / Interrogator August 30, 2012 CIED implanted on patient Follow-up visits (normally twice a year) VLDB 2012 Goal: • To reduce the number of visits, • To improve the quality of care 90
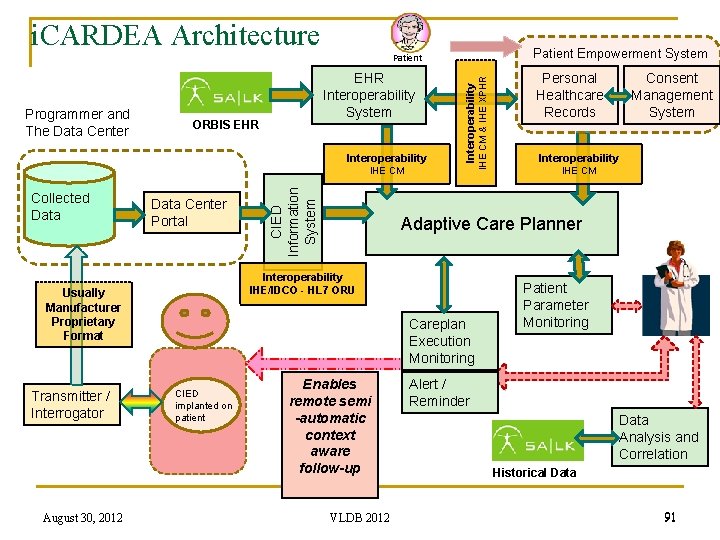
i. CARDEA Architecture Patient Empowerment System ORBIS EHR Interoperability Programmer and The Data Center EHR Interoperability System Collected Data Center Portal August 30, 2012 Careplan Execution Monitoring CIED implanted on patient Personal Healthcare Records Consent Management System Interoperability IHE CM Adaptive Care Planner Interoperability IHE/IDCO - HL 7 ORU Usually Manufacturer Proprietary Format Transmitter / Interrogator CIED Information System IHE CM & IHE XPHR Patient Enables remote semi -automatic context aware follow-up VLDB 2012 Patient Parameter Monitoring Alert / Reminder Data Analysis and Correlation Historical Data 91

Clinical Guidelines n n n n Clinical guidelines are developed to assist general practitioners in making clinical decisions They usually include plans for treatment and are used in developing the “Clinical Decision Support” systems Clinical guidelines aim to reduce inter-practice variations and improve the quality of care and standardize clinical procedures A variety of government and professional organizations are producing and disseminating clinical guidelines A comprehensive database of evidence-based clinical practice guidelines and related documents exist, (NGC, http: //www. guideline. gov) Several computer interpretable models of Clinical Guidelines have been proposed such as GLIF, ASBRU, ARDEN and EON Additionally, there are several guideline execution engines processing these models, such as GLEE, GLARE and De. Gel August 30, 2012 VLDB 2012 92
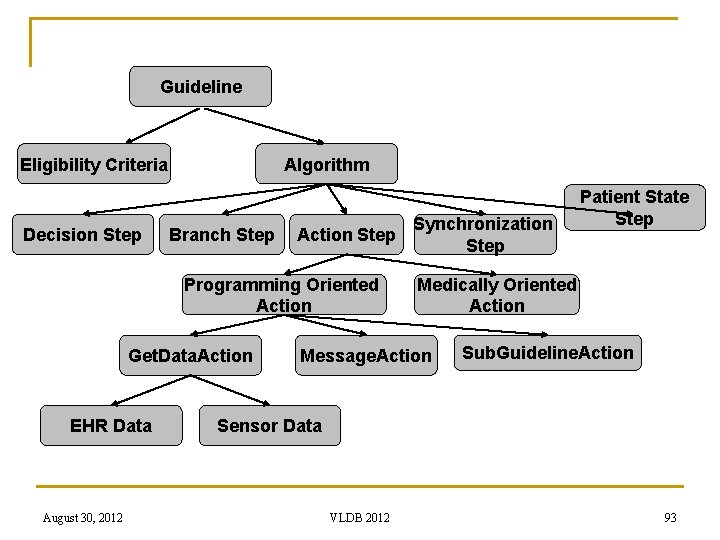
Guideline Eligibility Criteria Decision Step Algorithm Branch Step Action Step Programming Oriented Action Get. Data. Action EHR Data August 30, 2012 Synchronization Step Patient State Step Medically Oriented Action Message. Action Sub. Guideline. Action Sensor Data VLDB 2012 93
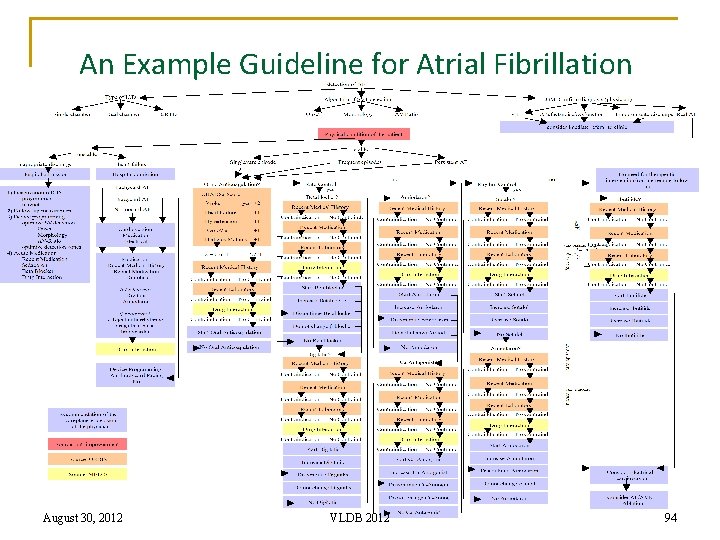
An Example Guideline for Atrial Fibrillation 94 August 30, 2012 VLDB 2012 94

Personalized Adaptive Care Plan n Personalized follow-up of CIED patients is coordinated through a “care plan” q q q n An executable definition of a care plan that consists of computer interpretable clinical guideline models Control flow of the care plan is dynamically adapted based on the patient’s context – PHR, EHR, Diagnosis data, etc. Provides reminder and personalized guidance services to the medical professionals Careplan Engine q q Executes machine processable care plan definition Subscribes to EHR and PHR interoperability Systems for retrieving most recent relevant clinical parameters related with the care plan n q q Care Plan Engine is kept up-to date about these clinical parameters Most recent CIED data is continuously retrieved as a result of pre-programmed remote follow-ups and alerts Care Plan Engine executes the care plan for: n n Regular pre-programmed follow-ups Each alert situation to assess the patient’s condition in full context and react as soon as possible in guidance of clinical guidelines q August 30, 2012 A monitoring interface is presented to the Medical professional VLDB 2012 95
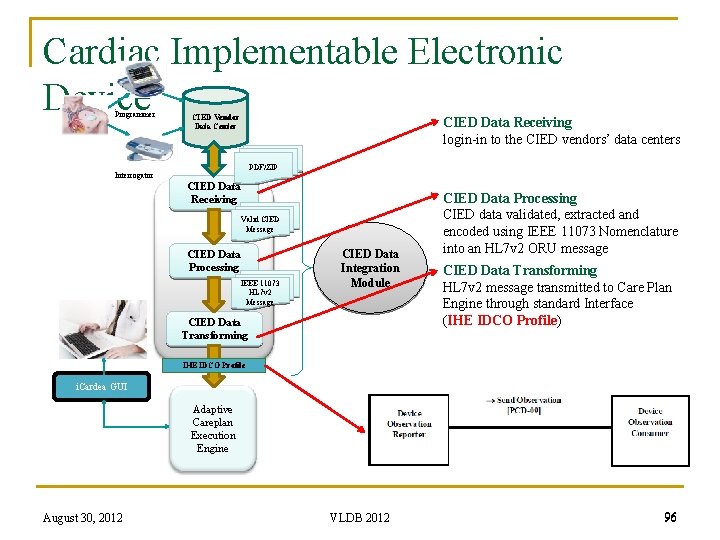
Cardiac Implementable Electronic Device Programmer CIED Vendor Data Center CIED Data Receiving login-in to the CIED vendors’ data centers PDF/ZIP Interrogator CIED Data Receiving Valid CIED Message CIED Data Processing IEEE 11073 HL 7 v 2 Message CIED Data Integration Module CIED Data Transforming CIED Data Processing CIED data validated, extracted and encoded using IEEE 11073 Nomenclature into an HL 7 v 2 ORU message CIED Data Transforming HL 7 v 2 message transmitted to Care Plan Engine through standard Interface (IHE IDCO Profile) IHE IDCO Profile i. Cardea GUI Adaptive Careplan Execution Engine August 30, 2012 VLDB 2012 96
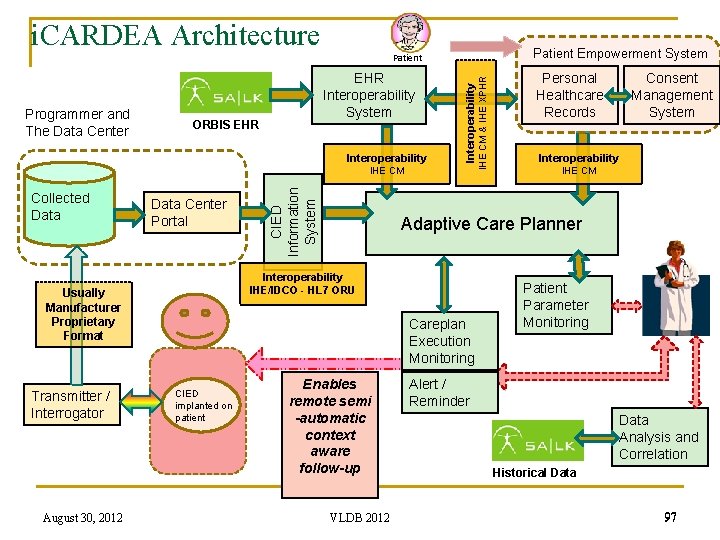
i. CARDEA Architecture Patient Empowerment System ORBIS EHR Interoperability Programmer and The Data Center EHR Interoperability System Collected Data Center Portal August 30, 2012 Careplan Execution Monitoring CIED implanted on patient Personal Healthcare Records Consent Management System Interoperability IHE CM Adaptive Care Planner Interoperability IHE/IDCO - HL 7 ORU Usually Manufacturer Proprietary Format Transmitter / Interrogator CIED Information System IHE CM & IHE XPHR Patient Enables remote semi -automatic context aware follow-up VLDB 2012 Patient Parameter Monitoring Alert / Reminder Data Analysis and Correlation Historical Data 97

Interoperability Challenges Addressed n Interoperability with Cardiovascular Implantable Electronic Devices q q To collect Pacing parameters, EGM, Shocks/Therapies provided, Alerts IHE Implantable Device Cardiac Observations (IDCO) profile implementation n n ISO/IEEE 11073 (Point of Care Medical Device Communication Standards) Nomencalature and HL 7 v 2 ORU Messages Interoperability with Legacy EHR Systems q q To collect previous or ongoing healthcare problems, medications, lab results IHE XDS, HL 7 CDA documents and IHE Care Management Profile Implementations August 30, 2012 VLDB 2012 98
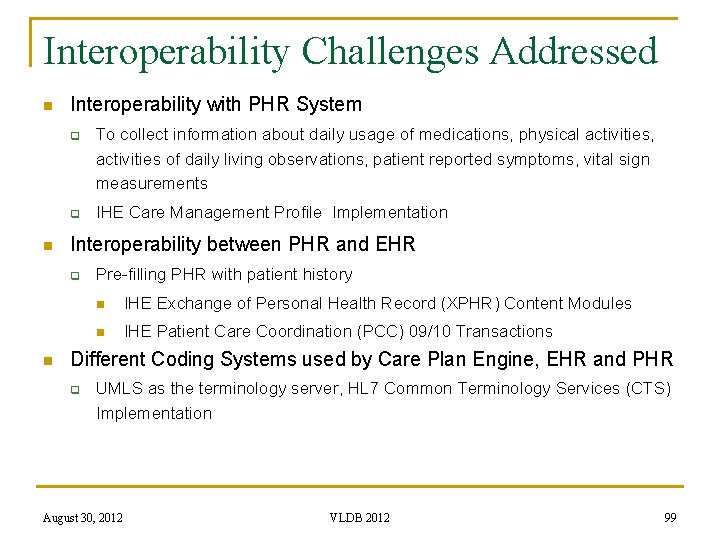
Interoperability Challenges Addressed n Interoperability with PHR System q q n IHE Care Management Profile Implementation Interoperability between PHR and EHR q n To collect information about daily usage of medications, physical activities, activities of daily living observations, patient reported symptoms, vital sign measurements Pre-filling PHR with patient history n IHE Exchange of Personal Health Record (XPHR) Content Modules n IHE Patient Care Coordination (PCC) 09/10 Transactions Different Coding Systems used by Care Plan Engine, EHR and PHR q UMLS as the terminology server, HL 7 Common Terminology Services (CTS) Implementation August 30, 2012 VLDB 2012 99
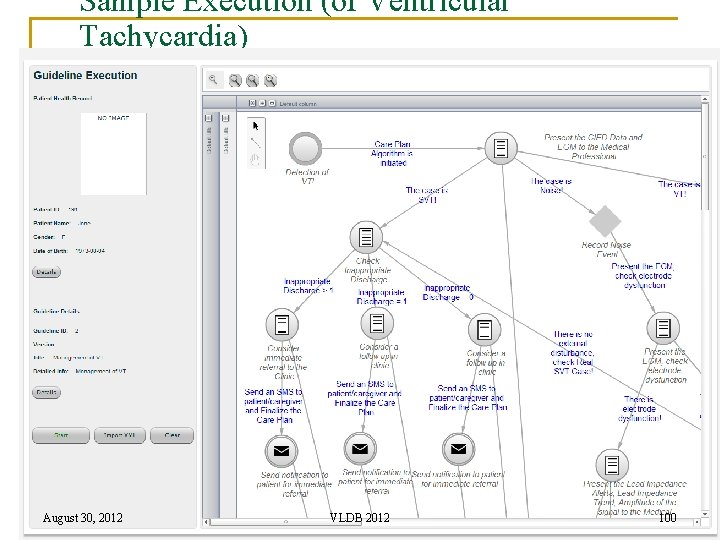
Sample Execution (of Ventricular Tachycardia) August 30, 2012 VLDB 2012 100
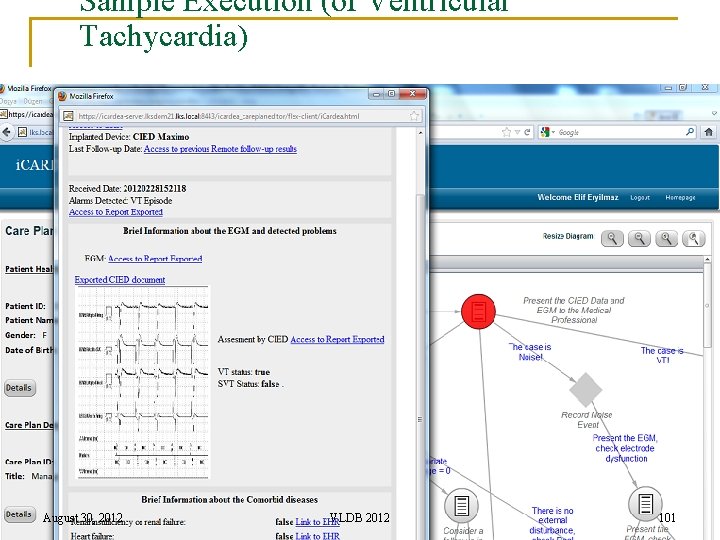
Sample Execution (of Ventricular Tachycardia) August 30, 2012 VLDB 2012 101

Related Publications n This is applied work involving research: q q q q Laleci G. B. , Dogac A. , “A Semantically Enriched Clinical Guideline Model Enabling Deployment in Heterogeneous Healthcare Environments”, IEEE Transactions on Information Technology in Biomedicine Vol. 13, No. 2, pp. 263 -273, March, 2009. Yuksel M. , Dogac A. , “Interoperability of Medical Device Information and the Clinical Applications: An HL 7 RMIM based on the ISO/IEEE 11073 DIM”, IEEE Transactions on Information Technology in Biomedicine, Vol. 15, No. 4, July 2011, pp. 557 - 566. Chronaki, C. , Sfakianakis, S. G. , Petrakis, Y. , Yang, M. , Radulescu, M. , Eichelberg, M. , Erturkmen, G. L. , Hinterbuchner, L. , Arbelo, E. , & Dogac, A, “Integrating Out-Patient and Remote Follow-Up of Cardiovascular Implantable Electronic Device Patients”, ICPES 2011 – World Society of Arrhythmias, PACE (ICPES 2011). 34 (11), (1357 -8). December 11 -14, 2011, Athens, Greece. Arbelo, E. , Trucco, E. , Dogac, A. , Luepkes, C. , Chronaki, C. , Hinterbuchner, L. , Ploessnig, M. , Yang, M. , Guillén, A. , & Brugada, J. , “i. CARDEA: Personalized Remote Monitoring of Atrial Fibrillation in Patients with Electronic Implant Devices”, ICPES 2011 – World Society of Arrhythmias, PACE (ICPES 2011, P 193). 34 (11), (1445 -6). December 11 -14, 2011, Athens, Greece. Trucco E; Arbelo E; Laleci GB; Yang M; Kabak Y; Chronaki C; Hinterbuchner L; Guillen A; Dogac A; Brugada J, “Personalized Remote Monitoring of Atrial Fibrillation in Patients with Electronic Implant Devices”, ICPES 2011 – World Society of Arrhythmias, PACE (ICPES 2011, P 189). 34 (11), (1443 -4). December 11 -14, 2011, Athens, Greece. Yang M. , Chronaki C. E. , Lüpkes C. , Thiel A. , Plößnig M. , Hinterbuchner L. , Arbelo E. , Laleci G. B. , Kabak Y. , Duarte F. , Guillén A. , Pfeifer B. , Pecho W. , Dogac A. , Eichelberg M. , Hein A. , “Guideline-Driven Telemonitoring and Follow-up of Cardiovascu-lar Implantable Electronic Devices using ISO/IEEE 11073, HL 7 & IHE Profiles”, 33 rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC ’ 11), Boston, USA, August 30 th - September 3 rd, 2011. Further Information on i. Cardea is Available from http: //www. srdc. com. tr/icardea/ August 30, 2012 VLDB 2012 102

Empowering European Diabetes Patients Support of Patient Empowerment by an intelligent self-management pathway for patients – The EMPOWER Project August 30, 2012 103
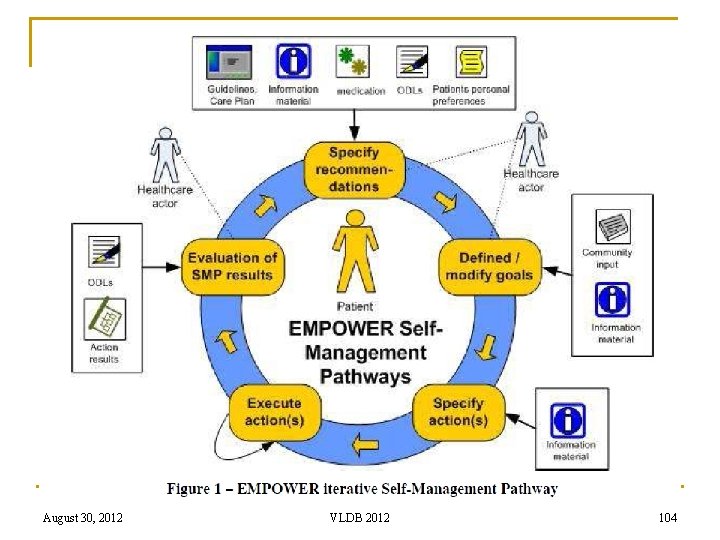
104 August 30, 2012 VLDB 2012 104
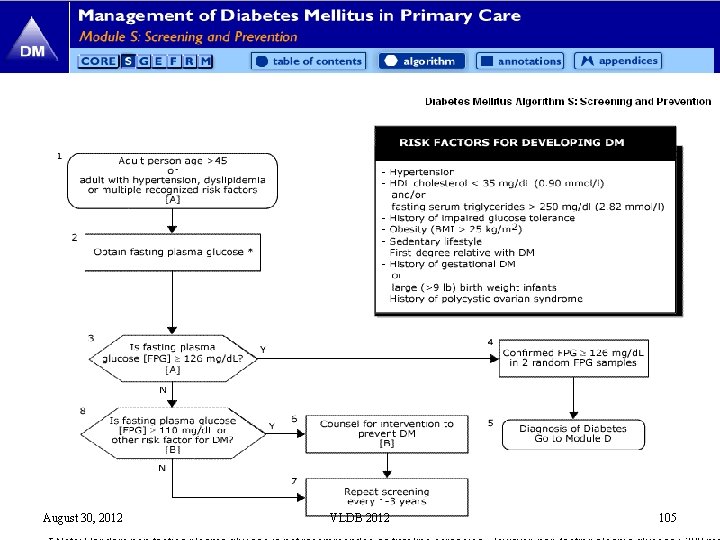
August 30, 2012 VLDB 2012 105

Interoperability in the Clinical Research Domain August 30, 2012 106

Clinical Research n Clinical research is a field of biomedical research addressing the assessment of new q q n Pharmaceutical and biological drugs Diagnostic methods Medical devices and Vaccines in humans In this way, their safety is checked by the regulatory authorities such as: n n Food and Drug Administration (FDA) in the USA and The European Medicines Agency (EMA) in the European Union It is driven by clinical trials or post market studies Clinical trials investigate the role of some factor or agent in the prevention or treatment of a disease August 30, 2012 VLDB 2012 107
How the Clinical Trials are Conducted? n The sponsor, usually a biopharmaceutical company, creates the trial protocol (Study Design) specifying instructions on q q n n n The data to be captured Tests to be ordered, Inclusion and exclusion criteria for subjects, and The number and type of visits This data is sent both to the regulatory body for approval and to the clinical investigators Based on the inclusion and exclusion criteria specified in the trial protocol, eligible patients are selected A clinical investigator takes the responsibility of conducting the trial at a particular trial site, and generates, collects and records data The collected data to be sent to the sponsor includes the Case Report Forms (CRFs) Electronic Case Report Forms are collected through computerized Electronic Data Capture (EDC) systems August 30, 2012 VLDB 2012 108

How the Clinical Trials are Conducted? n n n Sponsors use copies of the data recorded at the clinical site, and perform various analyses to reach their conclusions Collected trial data and the results of the analysis are then sent to the regulators During the lifetime of the clinical trials, investigators immediately report any serious Adverse Event (AE) to the sponsor Regulators need to reconstruct the trial by comparing the data submitted to the agency by the sponsor with the source data maintained at the investigational site After the drug, medical device, diagnostic product or treatment regimen is authorized to be used in market, the healthcare institutes can voluntarily report AE incidents to the national competent authorities August 30, 2012 VLDB 2012 109

Interoperability Standards for Clinical Research n The parties involved in a regulated clinical research study are q q q n Clinical Data Interchange Standards Consortium (CDISC, http: //www. cdisc. org) develops standards covering almost all the steps within a regulated clinical research study including q q n n n The sponsor, The clinical investigator and The regulatory body, each with their own software applications, Study Design (CDISC SDM), Study Data Collection (CDISC ODM and CDASH), Study Data Analysis (CDISC ADAM) and Submission to the regulatory bodies (CDISC SDTM) There are Individual Case Safety Report (ICSR) Standards for the exchange of adverse event reports during and after clinical research studies These set of standards proved to be very useful and are being widely used in managing clinical research studies However, the vast majority of clinical research study protocols also need data from the clinical care domain August 30, 2012 VLDB 2012 110

Clinical Trials vs Post Market Safety Studies n Clinical trials are not adequate to ensure comprehensive drug safety q q n Limited size and scope Do not include patients with comorbid conditions and those being treated with concomitant medications Designed to pick-up common problems not rare adverse events Cannot detect long-term adverse events Post market safety studies address this problem, but q Based on voluntarily sent spontaneous case safety reports n Medical professionals do not always see reporting a priority & detecting adverse events may not always be straightforward It is estimated that medical practitioners report only about 5% of harmful drug side effects Approximately 5% of all hospital admissions in Europe are due to an adverse drug reaction (ADR), and ADRs are the fifth most common cause of hospital deaths n q August 30, 2012 VLDB 2012 111

The Interoperability Problems of Clinical Care and Clinical Research n To improve the effectiveness of clinical research processes, the information from the clinical care must be re-used: q q Most of the data needed by clinical research is already available in the clinical care records (EHRs) However clinical research and the clinical care domains use different standards and hence are not interoperable: n Clinical research uses CDISC standards n Clinical care is dominated by HL 7 standards n The terminology systems used are also different For example, currently the clinicians copy the results of therapeutic procedures and examinations from an EHR system in the Case Report Form (CRF) manually As another example, the investigators have to manually select the eligible patients from the underlying EHR systems, by examining the inclusion/exclusion criteria listed in trial design documents August 30, 2012 VLDB 2012 112

IHE Standards for the Interoperability of Clinical Care and Clinical Research Domains n n An Initiative for the interoperability of clinical care and clinical research domains - two profiles from IHE: q IHE Drug Safety Content Profile (DSC) q IHE Clinical Research Document Profile (CRD) These profiles reuse the available standards in clinical care and research domains to facilitate their interoperability However, the interoperability is achieved through hard-coded mappings between clinical research and care standards q For example, in CRD, a direct XSLT mapping between IHE PCC templates and the CDASH annotated ODM structure is provided Also the XSLT mappings are only valid for the given fixed formats defined in PCC/CCD and ODM models; once these formats are modified due to emerging requirements, new mappings will be needed August 30, 2012 VLDB 2012 113

The SALUS Approach n n An effort in this direction: The SALUS Project q Enable post-market analysis for different subpopulations selected from multiple, distributed EHRs as target cohorts q Automated ADE detection tools screening EHRs in a hospital to facilitate ADE detection q Enable ADE reporting by automatically extracting the available information from the EHRs into the individual case safety reports, to avoid double data entry q Enable real time screening of multiple, distributed, heterogeneous EHRs for early detection of adverse event signals SALUS (Scalable, Standard based Interoperability Framework for Sustainable Proactive Post Market Safety Studies) q q Supported by the European Commission under the FP 7 Program For further information: http: //www. srdc. com. tr/projects/salus/ August 30, 2012 VLDB 2012 114
The SALUS Approach n n The SALUS framework is based on the “semantic mediation” of clinical care and clinical research domains q A process of matching schemas and mapping attributes and values using semantics In achieving this, the mediator needs q A shared conceptual reference model to serve as the common ground n n To correlate the concepts from different sources to reconcile their differences and To establish some well-defined relationships among them Building a coherent conceptual reference model to be used in “semantic mediation”, on the other hand, requires a ‘‘semantic harmonization’’ process Semantic Harmonization involves q Investigating semantically connected domain analysis models q Deriving common semantics and q Expressing this semantics through explicit and formal knowledge representations techniques August 30, 2012 VLDB 2012 115
The SALUS Approach n Fortunately, this shared conceptual model exists for the clinical care and research domains through the efforts by the BRIDG Project q q n The BRIDG model unifies various aspects of all the concepts in the clinical care and research domains and Creates a shared generic representation for each concept In the SALUS framework, the RDF representation of the BRIDG model is used as the core ontology to have the common shared semantics in a formal, machine processable form August 30, 2012 VLDB 2012 116
Biomedical Research Integrated Domain Group (BRIDG) n n n BRIDG initiative aims to enable the re-use of information from the clinical care domain in clinical research It has developed a domain analysis model (DAM) which harmonizes q CDISC data standards with q The HL 7 Reference Information Model (RIM) This facilitates data exchange between the EHR systems and the sponsor clinical research systems Currently the BRIDG model semantics is available only to human experts because it is not in a machine processable form Mappings between q The standards harmonized by the BRIDG initiative and q The BRIDG DAM are provided through spreadsheets August 30, 2012 VLDB 2012 117
The SALUS Approach n n The BRIDG DAM semantics has already been mapped to more than one implementation model (such as CDISC SDTM and HL 7 Study Design RMIM) using spreadsheets To be able to use these mapping information automatically, we have converted them into machine processable format as follows: q The mappings of the terms in a dataset standard (such as CDASH) to BRIDG Model is rather straight forward and we used SPARQL queries for this purpose q For standards that have a compositional nature enabling multidimensional representation of clinical data, such as HL 7 RIM based models, a more complex mapping mechanism is needed n n August 30, 2012 For this, we utilized ontology mapping mechanisms For example, when the value of an attribute in a source class needs to be processed to obtain the value of the attribute in the target class VLDB 2012 118
The SALUS Approach n n Another challenge is the different terminology systems used by clinical care and clinical research institutes Bio. Portal (http: //bioportal. bioontology. org) initiative is an important effort in this direction: q n n More importantly, it also serves mapping information between the coded terms of these terminology systems as an ontology We have implemented an export functionality q q n It serves more than 290 biomedical ontologies including ontological representations of major terminology systems like SNOMED CT, LOINC, ICD 10, Med. DRA, WHO-ART and Rx. NORM To add these ontologies together with their mappings to our Semantic Framework, and To utilize them to address end-to-end semantic interoperability of clinical care and research systems Ref: Gokce B. Laleci, Mustafa Yuksel, Asuman Dogac, “Providing Semantic Interoperability between Clinical Care and Clinical Research Domains”, currently under revision, IEEE Transactions on Information Technology in Biomedicine August 30, 2012 VLDB 2012 119

Thank you. . . Questions? Slides are available from: www. srdc. com. tr/~asuman/e. Health. Interoperability. ppt August 30, 2012 VLDB 2012 120
- Slides: 117