International System Of Units The SI system It

- Slides: 12

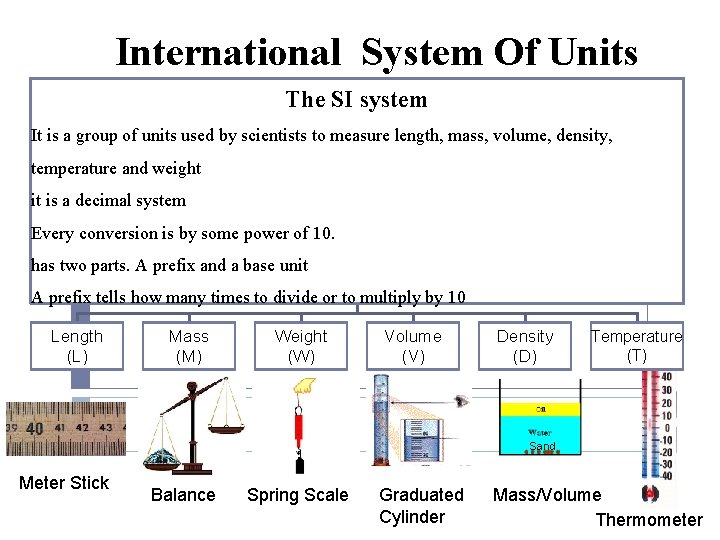
International System Of Units The SI system It is a group of units used by scientists to measure length, mass, volume, density, temperature and weight it is a decimal system Every conversion is by some power of 10. has two parts. A prefix and a base unit A prefix tells how many times to divide or to multiply by 10 Length (L) Mass (M) Weight (W) Volume (V) Density (D) Temperature (T) 1 cm Meter Stick Balance Spring Scale 1 cm Graduated Cylinder Sand Mass/Volume Thermometer

UNITS OF MEASUREMENT A. DEFINED UNITS B. DERIVED UNITS

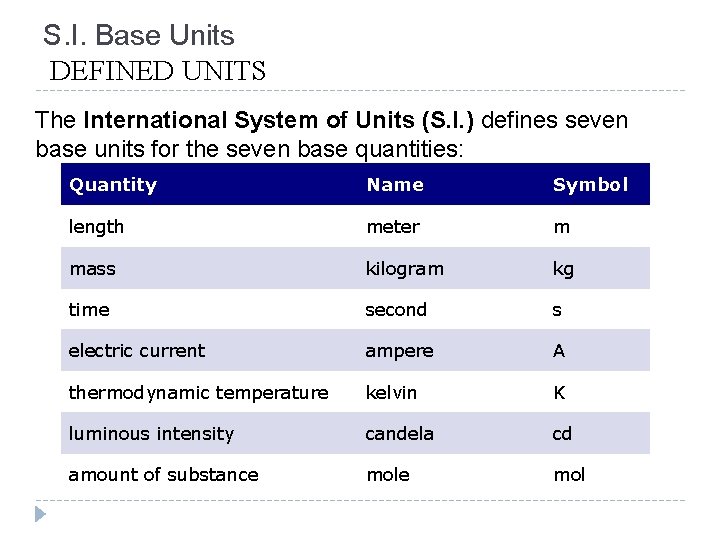
S. I. Base Units DEFINED UNITS The International System of Units (S. I. ) defines seven base units for the seven base quantities: Quantity Name Symbol length meter m mass kilogram kg time second s electric current ampere A thermodynamic temperature kelvin K luminous intensity candela cd amount of substance mol

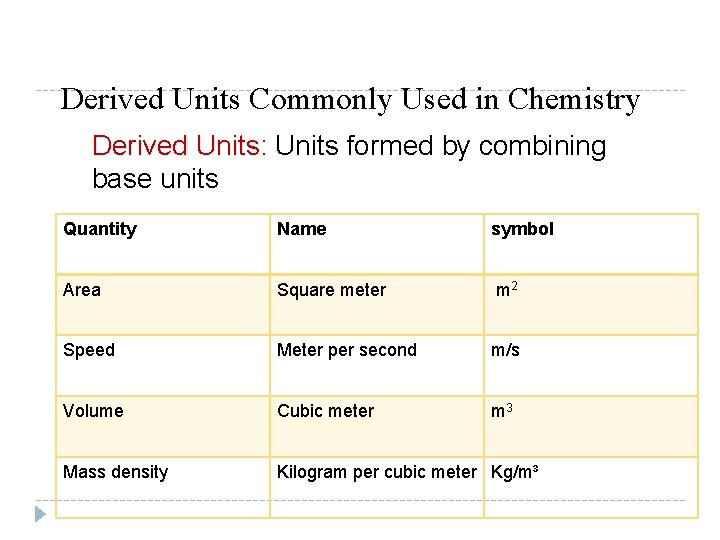
Derived Units Commonly Used in Chemistry Derived Units: Units formed by combining base units Quantity Name symbol Area Square meter m 2 Speed Meter per second m/s Volume Cubic meter m 3 Mass density Kilogram per cubic meter Kg/m³

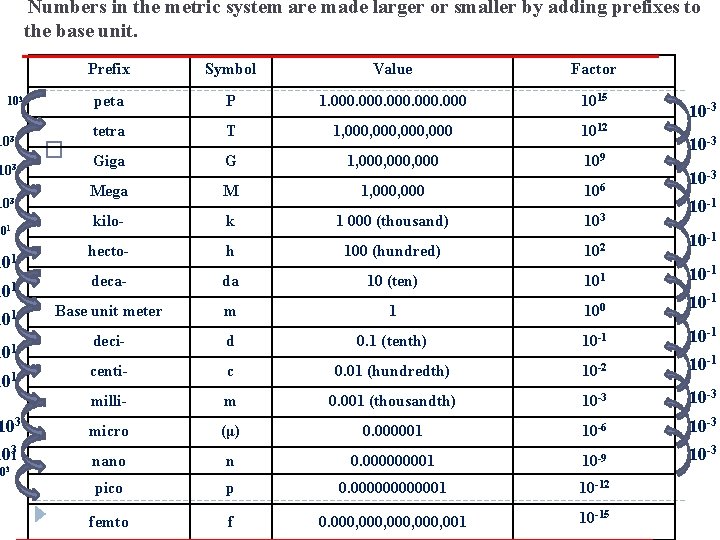
Numbers in the metric system are made larger or smaller by adding prefixes to the base unit. 103 103 101 101 Prefix Symbol Value Factor peta P 1. 000 1015 tetra T 1, 000, 000 1012 �The Golden Giga Ticket – B 1, 000, 000 G 109 10 -3 Mega M 1, 000 106 kilo- k 1 000 (thousand) 103 hecto- h 100 (hundred) 102 deca- da 10 (ten) 101 10 -1 Base unit meter m 1 100 deci- d 0. 1 (tenth) 10 -1 centi- c 0. 01 (hundredth) 10 -2 10 -1 milli- m 0. 001 (thousandth) 10 -3 103 micro (μ) 0. 000001 10 -6 10 -3 1031 nano n 0. 00001 10 -9 10 -3 pico p 0. 0000001 10 -12 femto f 0. 000, 001 10 -15 101 101 03

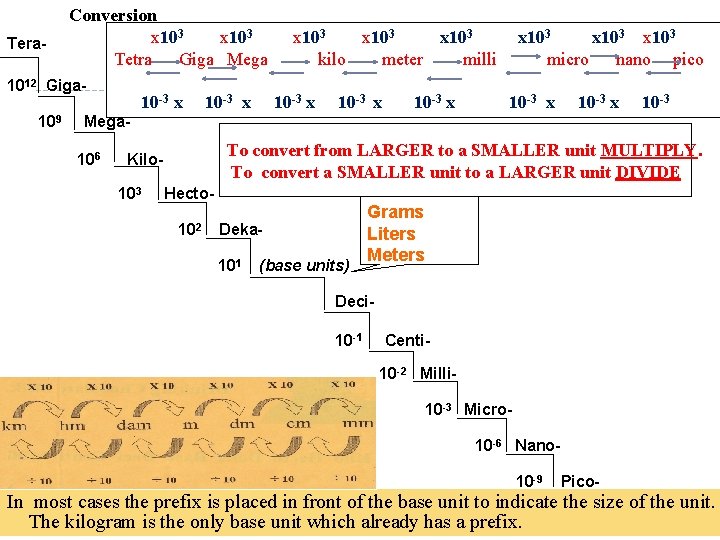
Tera- Conversion x 103 Tetra Giga Mega 1012 Giga 109 10 -3 x x 103 kilo meter milli 10 -3 x x 103 micro nano pico 10 -3 x 10 -3 Mega 106 To convert from LARGER to a SMALLER unit MULTIPLY. To convert a SMALLER unit to a LARGER unit DIVIDE Kilo 103 Hecto 102 Deka 101 (base units) Grams Liters Meters Deci 10 -1 Centi 10 -2 Milli 10 -3 Micro 10 -6 Nano 10 -9 Pico- In most cases the prefix is placed in front of the base unit to indicate the size of the unit. The kilogram is the only base unit which already has a prefix.

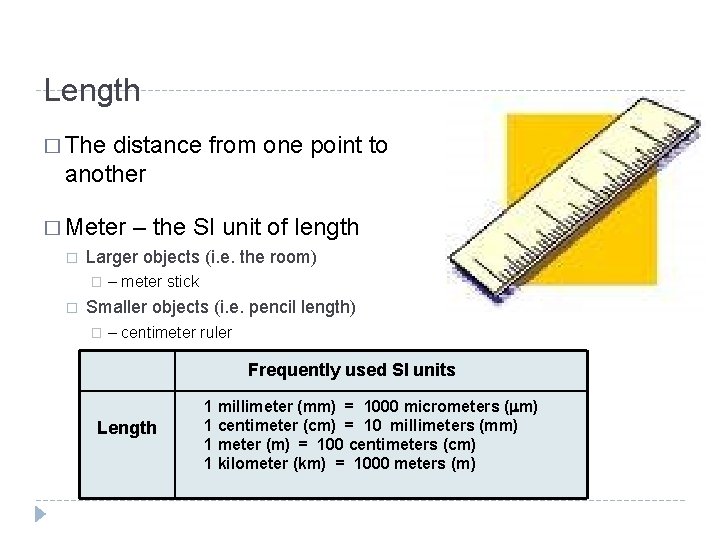
Length � The distance from one point to another � Meter – the SI unit of length � Larger objects (i. e. the room) � � – meter stick Smaller objects (i. e. pencil length) � – centimeter ruler Frequently used SI units Length 1 millimeter (mm) = 1000 micrometers (mm) 1 centimeter (cm) = 10 millimeters (mm) 1 meter (m) = 100 centimeters (cm) 1 kilometer (km) = 1000 meters (m)

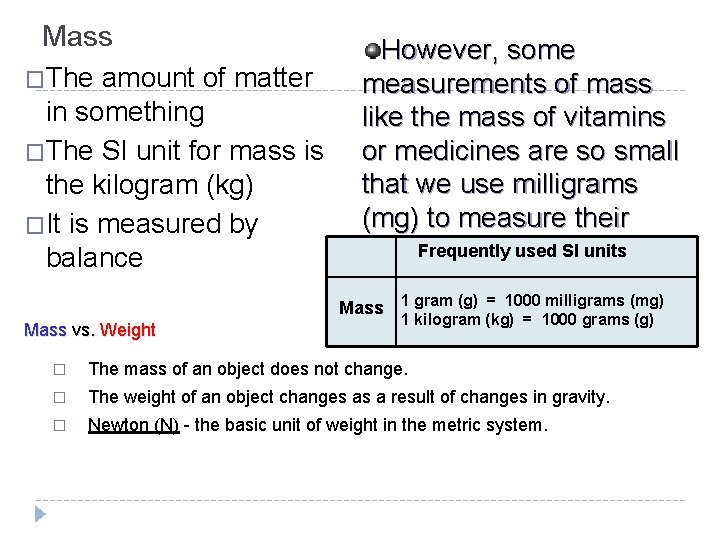
Mass �The amount of matter in something �The SI unit for mass is the kilogram (kg) �It is measured by balance However, some measurements of mass like the mass of vitamins or medicines are so small that we use milligrams (mg) to measure their mass. Frequently used SI units Mass 1 gram (g) = 1000 milligrams (mg) Mass vs. Weight 1 kilogram (kg) = 1000 grams (g) � The mass of an object does not change. � The weight of an object changes as a result of changes in gravity. � Newton (N) - the basic unit of weight in the metric system.

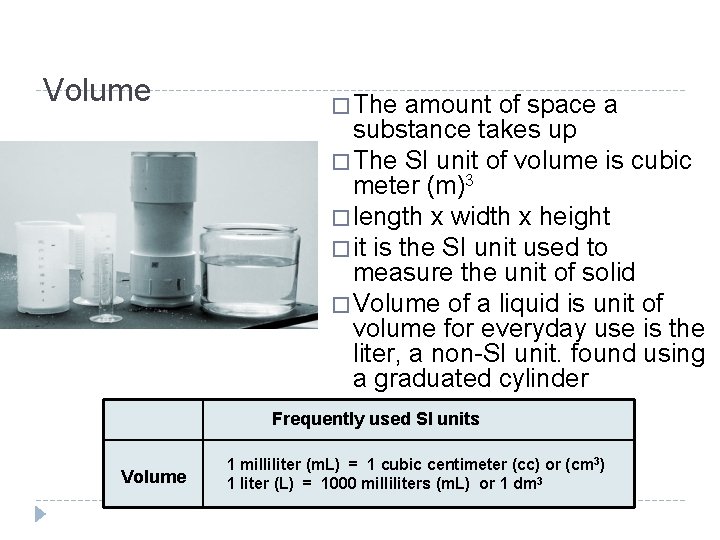
Volume � The amount of space a substance takes up � The SI unit of volume is cubic meter (m)3 � length x width x height � it is the SI unit used to measure the unit of solid � Volume of a liquid is unit of volume for everyday use is the liter, a non-SI unit. found using a graduated cylinder Frequently used SI units Volume 1 milliliter (m. L) = 1 cubic centimeter (cc) or (cm 3) 1 liter (L) = 1000 milliliters (m. L) or 1 dm 3

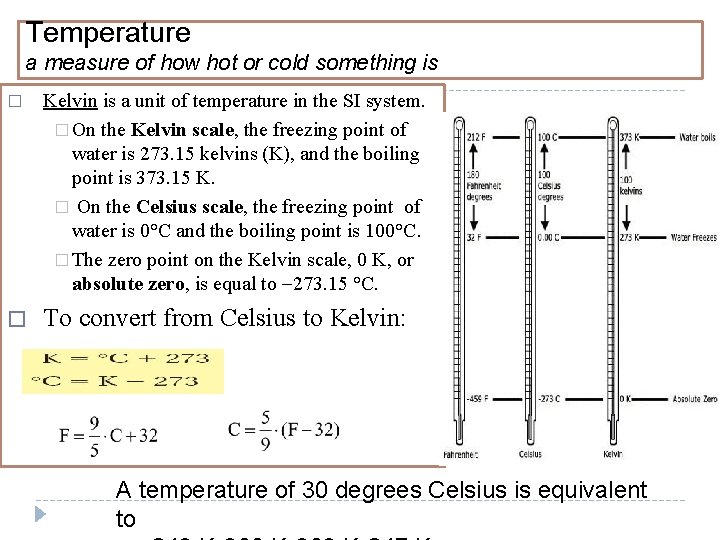
Temperature a measure of how hot or cold something is � Kelvin is a unit of temperature in the SI system. � On the Kelvin scale, the freezing point of water is 273. 15 kelvins (K), and the boiling point is 373. 15 K. � On the Celsius scale, the freezing point of water is 0°C and the boiling point is 100°C. � The zero point on the Kelvin scale, 0 K, or absolute zero, is equal to 273. 15 °C. � To convert from Celsius to Kelvin: A temperature of 30 degrees Celsius is equivalent to

Density a measure of the amount of matter that occupies a certain volume Density = mass/volume (units are usually g/m. L) � Mass= density x volume � Volume= mass/density The density of water = 1. 00 g/m. L The density of oil = 0. 91 g/m. L If, an object has a mass of 15 grams and occupies a volume of 5. 0 cm 3 � density is SPECIFIC GRAVITY This is the ratio of the density of the mineral to the density of water. Specific gravity= density of substance/ density of water Sp. gr= d sub/ d water Area = length x width M²= m x m Most areas are measured in square (base) meters Its

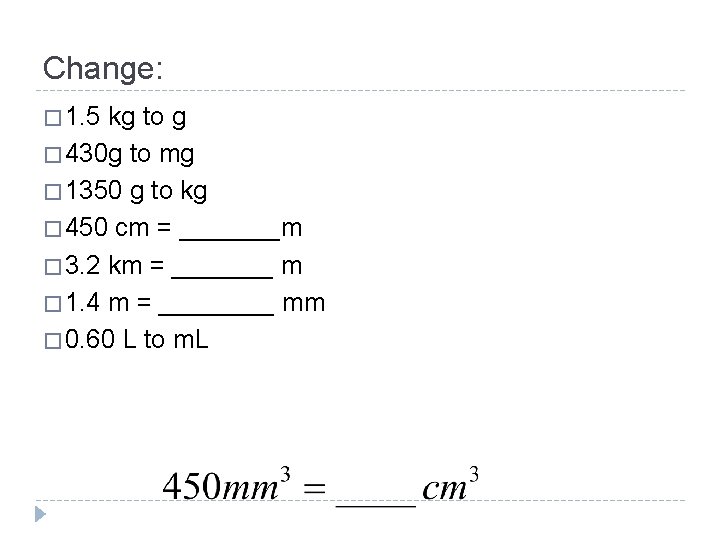
Change: � 1. 5 kg to g � 430 g to mg � 1350 g to kg � 450 cm = _______m � 3. 2 km = _______ m � 1. 4 m = ____ mm � 0. 60 L to m. L