International Symposium on Molecular Spectroscopy 71 st Meeting




- Slides: 15

International Symposium on Molecular Spectroscopy, 71 st Meeting Champaign-Urbana, June 20 -24, 2016 Large molecules structures by Broadband Fourier Transform Rotational Spectroscopy Luca Evangelistsi 1, Nathan Seifert 2, Lorenzo Spada 1, Brooks H. Pate Department of Chemistry University of Virginia 1 Dipartmento di Chimica “Giacomo Ciamician” Bologna 2 Department of Chemistry, University of Alberta http: //faculty. virginia. edu/bpate-lab/

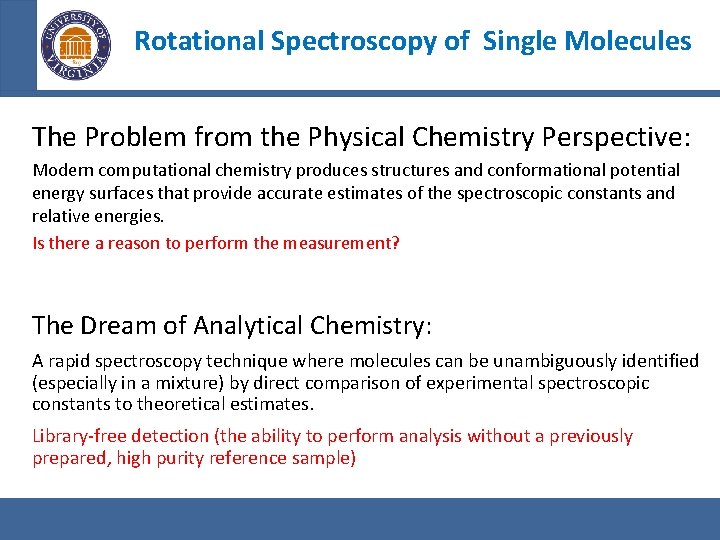
Rotational Spectroscopy of Single Molecules The Problem from the Physical Chemistry Perspective: Modern computational chemistry produces structures and conformational potential energy surfaces that provide accurate estimates of the spectroscopic constants and relative energies. Is there a reason to perform the measurement? The Dream of Analytical Chemistry: A rapid spectroscopy technique where molecules can be unambiguously identified (especially in a mixture) by direct comparison of experimental spectroscopic constants to theoretical estimates. Library-free detection (the ability to perform analysis without a previously prepared, high purity reference sample)

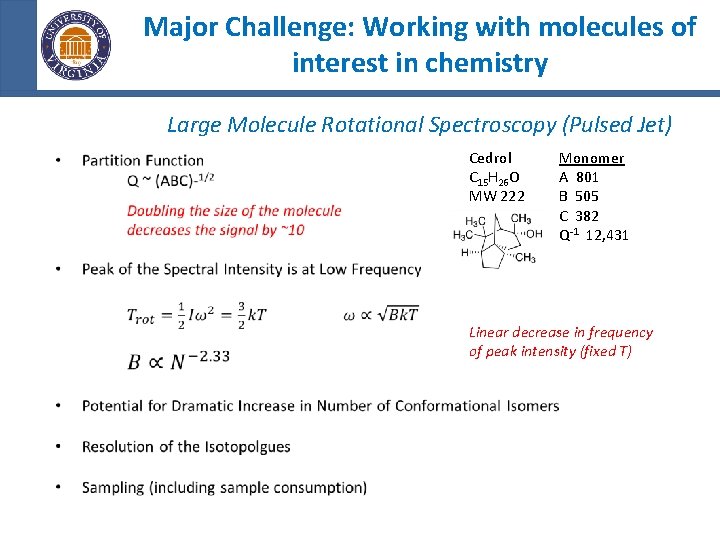
Major Challenge: Working with molecules of interest in chemistry Large Molecule Rotational Spectroscopy (Pulsed Jet) • Cedrol C 15 H 26 O MW 222 Monomer A 801 B 505 C 382 -1 Q 12, 431 Linear decrease in frequency of peak intensity (fixed T)

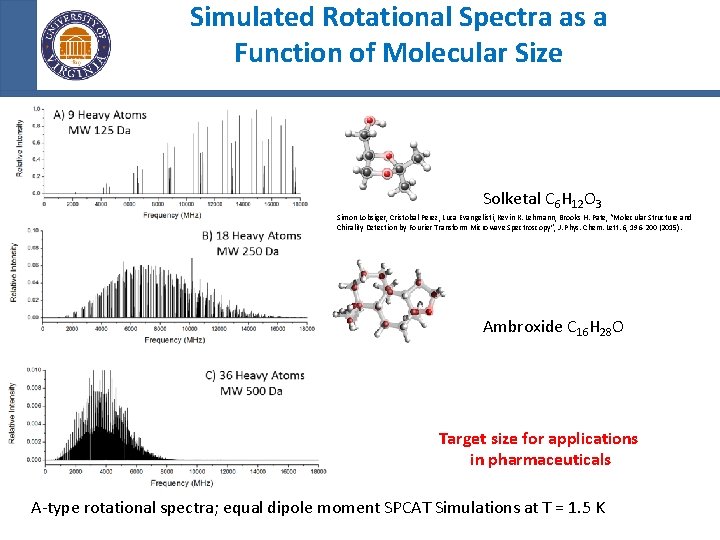
Simulated Rotational Spectra as a Function of Molecular Size Solketal C 6 H 12 O 3 Simon Lobsiger, Cristobal Perez, Luca Evangelisti, Kevin K. Lehmann, Brooks H. Pate, “Molecular Structure and Chirality Detection by Fourier Transform Microwave Spectroscopy”, J. Phys. Chem. Lett. 6, 196 -200 (2015). Ambroxide C 16 H 28 O Target size for applications in pharmaceuticals A-type rotational spectra; equal dipole moment SPCAT Simulations at T = 1. 5 K

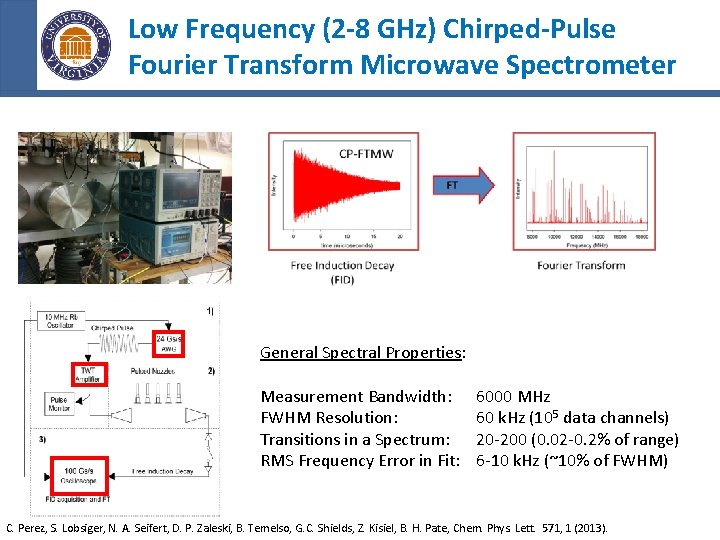
Low Frequency (2 -8 GHz) Chirped-Pulse Fourier Transform Microwave Spectrometer General Spectral Properties: Measurement Bandwidth: FWHM Resolution: Transitions in a Spectrum: RMS Frequency Error in Fit: 6000 MHz 60 k. Hz (105 data channels) 20 -200 (0. 02 -0. 2% of range) 6 -10 k. Hz (~10% of FWHM) C. Perez, S. Lobsiger, N. A. Seifert, D. P. Zaleski, B. Temelso, G. C. Shields, Z. Kisiel, B. H. Pate, Chem. Phys. Lett. 571, 1 (2013).

Sample in gas phase… Laser ablation Heatable nozzle - Quite expensive - Cheap - Need expertise - Easy to handle - Safety - Safe

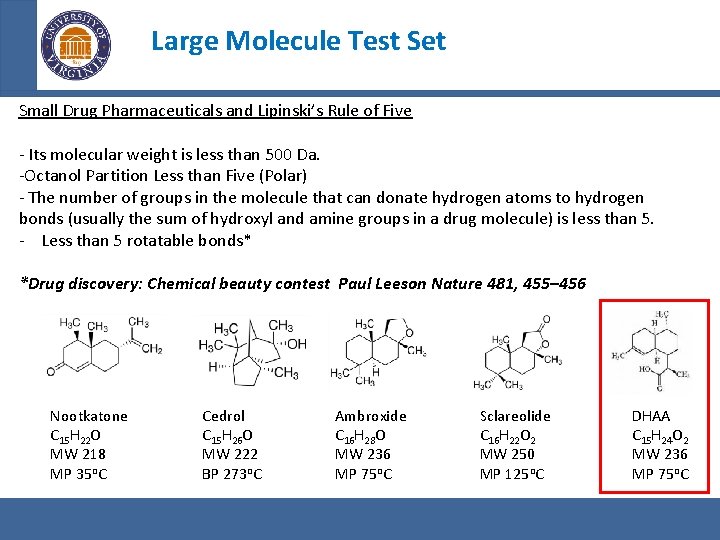
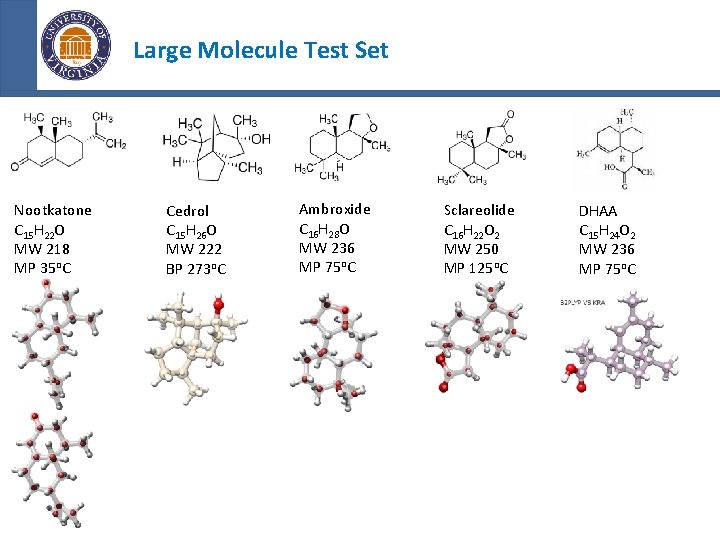
Large Molecule Test Set Small Drug Pharmaceuticals and Lipinski’s Rule of Five - Its molecular weight is less than 500 Da. -Octanol Partition Less than Five (Polar) - The number of groups in the molecule that can donate hydrogen atoms to hydrogen bonds (usually the sum of hydroxyl and amine groups in a drug molecule) is less than 5. - Less than 5 rotatable bonds* *Drug discovery: Chemical beauty contest Paul Leeson Nature 481, 455– 456 Nootkatone C 15 H 22 O MW 218 MP 35 o. C Cedrol C 15 H 26 O MW 222 BP 273 o. C Ambroxide C 16 H 28 O MW 236 MP 75 o. C Sclareolide C 16 H 22 O 2 MW 250 MP 125 o. C DHAA C 15 H 24 O 2 MW 236 MP 75 o. C

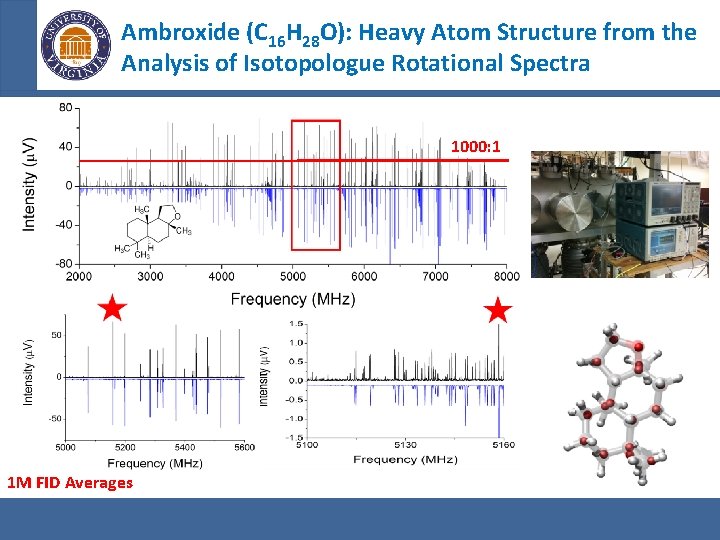
Ambroxide (C 16 H 28 O): Heavy Atom Structure from the Analysis of Isotopologue Rotational Spectra 1000: 1 1 M FID Averages

Molecular Structure from Isotopic Substitution Structure Information through Principal Moments of Inertia: Measure: A, B, C “normal species” A, B, C singly substituted isotopomer From : ΔIa, ΔIb, ΔIc Obtain: (|Ra|, |Rb|, |Rc|) J. Kraitchman, Am. J. Phys. 21, 17 (1953). Kraitchman Analysis 1) Build up the molecular structure “atom-by-atom” 2) No model assumptions required (but guidance on sign is helpful) 3) A single answer for a single data set 4) Other methods for refinement Structures of Phenol Dimer and Trimer from Isotopes in Natural Abundance Phys. Chem. Phys. , 2013, 15, 11468 Alberto Lesarri, Valladolid Reproducibility and Repeatability

Large Molecule Test Set Nootkatone C 15 H 22 O MW 218 MP 35 o. C Cedrol C 15 H 26 O MW 222 BP 273 o. C Ambroxide C 16 H 28 O MW 236 MP 75 o. C Sclareolide C 16 H 22 O 2 MW 250 MP 125 o. C DHAA C 15 H 24 O 2 MW 236 MP 75 o. C

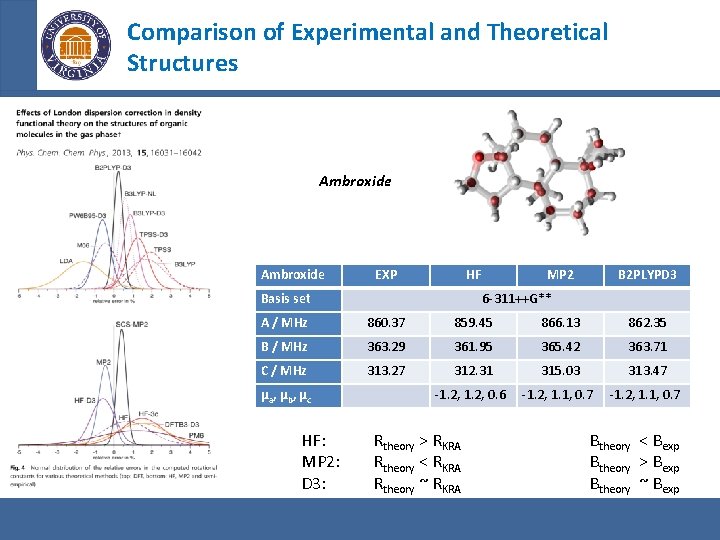
Comparison of Experimental and Theoretical Structures Ambroxide EXP HF Basis set MP 2 B 2 PLYPD 3 6 -311++G** A / MHz 860. 37 859. 45 866. 13 862. 35 B / MHz 363. 29 361. 95 365. 42 363. 71 C / MHz 313. 27 312. 31 315. 03 313. 47 -1. 2, 0. 6 -1. 2, 1. 1, 0. 7 µa, µb, µc HF: MP 2: D 3: Rtheory > RKRA Rtheory < RKRA Rtheory ~ RKRA Btheory < Bexp Btheory > Bexp Btheory ~ Bexp

Rotational spectroscopy VS X-ray structures rs structure for Sclareolide Absolute configuration: -Optical rotatory dispersion -Vibrational circular dichroism -Proton NMR (chiral shift reagent) -X-ray crystallography X-ray structure for Sclareolide

Direct Analysis of Diastereomer Ratios by Molecular Rotational Spectroscopy • Identification of diastereomers without the need for a known, pure sample • Sensitivity of the structures to epimerization for large molecules • Conformational flexibility • Accuracy of theoretical structures and rotational constants (and dipole moment)

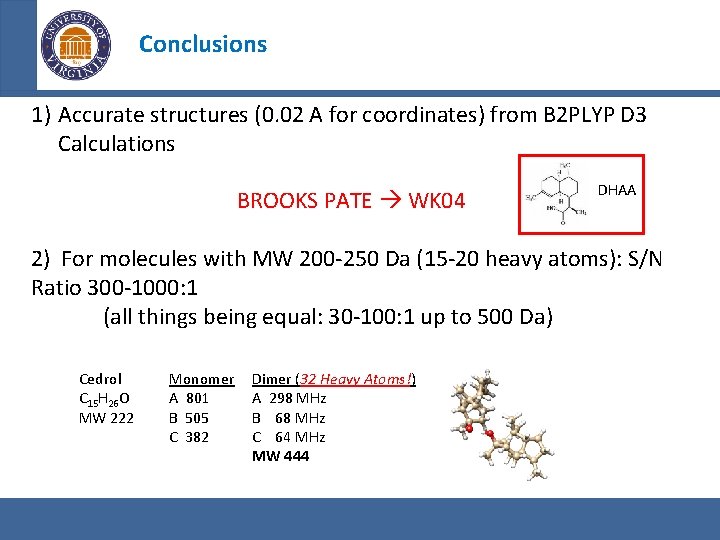
Conclusions 1) Accurate structures (0. 02 A for coordinates) from B 2 PLYP D 3 Calculations BROOKS PATE WK 04 DHAA 2) For molecules with MW 200 -250 Da (15 -20 heavy atoms): S/N Ratio 300 -1000: 1 (all things being equal: 30 -100: 1 up to 500 Da) Cedrol C 15 H 26 O MW 222 Monomer Dimer (32 Heavy Atoms!) A 801 A 298 MHz B 505 B 68 MHz C 382 C 64 MHz MW 444

Acknowledgements Ø Pate Group Ø Bright. Spec Ø NSF Ø MC-IOF 328405 Ø RSC
 International symposium on molecular spectroscopy
International symposium on molecular spectroscopy Introduction to molecular spectroscopy
Introduction to molecular spectroscopy Difference between atomic and molecular spectroscopy
Difference between atomic and molecular spectroscopy Catalysis lecture notes
Catalysis lecture notes Upcdms
Upcdms International police executive symposium
International police executive symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium Ips perforating
Ips perforating Ips perforating
Ips perforating International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium Covalent bond
Covalent bond Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Today meeting or today's meeting
Today meeting or today's meeting