International Pressure Ulcer Guidelines Outline Development process Whats





















































- Slides: 60

International Pressure Ulcer Guidelines

Outline � Development process � What’s new in the recommendations � Level A recommendations

Development process

Aim � Develop joint EPUAP/NPUAP pressure ulcer prevention and treatment guideline � Guideline is intended for ◦ Use of health care professionals ◦ Hospital, long term care, assisted living at home ◦ Guide patients and carers on range of prevention strategies

When in doubt – form a committee! � Guideline development GDG NPUAP Group (GDG)– from both EPUAP and NPUAP � Carol Dealey – Chair EPUAP GDG � Janet Cuddigan & Diane Langemo – Co-Chairs of NPUAP GDG � 6 members from each society GDG EPUAP

EPUAP GDG Dr. Carol Dealey United Kingdom Chair Dr. Katrien Vanderwee Belgium Coördinator Dr. Michael Clark United Kingdom Prof. Dr. Tom Defloor Belgium Dr. Lisette Schoonhoven The Netherlands Anne Witherow Northern Ireland European Launch International Pressure Ulcer Guidelines

NPUAP GDG Dr. Janet Cuddigan Co-Chair Nebraska, USA Dr. Diane Langemo Co-Chair North Dakota, USA Dr. Joyce Black Nebraska, USA Dr. Mona Baharestani Tennessee, USA Mary Ellen Posthauer Indiana, USA Evan Call Utah, USA

Who did what? � EPUAP were leading PU prevention guideline � NPUAP were leading PU treatment guideline ◦ In collaboration with the American NPUAP ◦ In collaboration with EPUAP

Methodology Overview 1. Identifying the Evidence ◦ Databases ◦ Search strategy 2. Evaluating the Evidence ◦ Evidence Tables ◦ Quality of Evidence ◦ Level of Evidence 3. Drafting Recommendations 4. Strength of Evidence Rating (A, B, C) 5. Summarizing supporting evidence 6. Peer Review – GDG – Stakeholders – EPUAP-NPUAP Boards

1. Identifying the Evidence � Identify scientific literature on pressure ulcer prevention and treatment in several electronic databases : �Pub. Med �Cinahl (Cumulative Index to Nursing & Allied Health Literature) �EMBASE (The Exerpta Medica Database) �AMED (Allied and Complementary Medicine Database) �Cochrane databases (Cochrane Database of Systematic Reviews, The Cochrane Central Register of Controlled Trials) �Health Technology Assessment database

1. Identifying the Evidence � Sensitive Pub. Med search strategy developed in ◦ Pressure ulcer AND ◦ Prevention OR Aetiology OR Prevention OR Skin assessment OR Risk assessment OR Nutrition OR Repositioning AND ◦ Design (including systematic review, meta-analysis, RCT, CCT, cohort, case-control, case series n>10) AND Period limitation: ◦ Human studies > 01/01/1998

1. Identifying the Evidence � All the references were screened by the GDG based on inclusion criteria: ◦ Published in a peer reviewed journal ◦ Primarily focuses on one of the following topics �Prevention or Treatment of PU �Causes of PU �PU risk assessment ◦ Abstract available

1. Identifying the Evidence ◦ Study �Has to use one of the following designs: �Randomised controlled trial �Controlled clinical trial �Cohort studies �Case-control studies �Case series �Should include at least 10 subjects ◦ Review / Meta-analysis �Has to use the Cochrane methodology

1. Identifying the Evidence � Exclusion criteria: ◦ Economic evaluations ◦ Animal studies (unless other not available) ◦ Studies of chronic wounds (unless sub- group of 10 or > subjects with pressure ulcers was analyzed separately) ◦ Note: If treatment was proven effective in other chronic wounds, the treatment was considered for use in pressure ulcers in the absence of studies of humans with pressure ulcers (SOE at C level).

1. Identifying the Evidence � Existing Evidence Summaries ◦ Evidence Tables from Previous Guidelines or other Sources: �AHCPR (AHRQ) �Paralyzed Veterans of America �Registered Nurses of Ontario �NICE ◦ Previous guidelines (AMDA, AWMA, EPUAP, NICE, PVA, RNAO, Singapore, WOCN, WHS)

2. Evaluating the Evidence � The full papers of relevant references were obtained � Small Working Groups (SWGs) reviewed the literature for specific topics � All papers were evaluated by 2 members of SWGs ◦ First reviewer �Evidence table �Methodology checklist ◦ Second reviewer �Checks evidence table �Checks methodology checklist

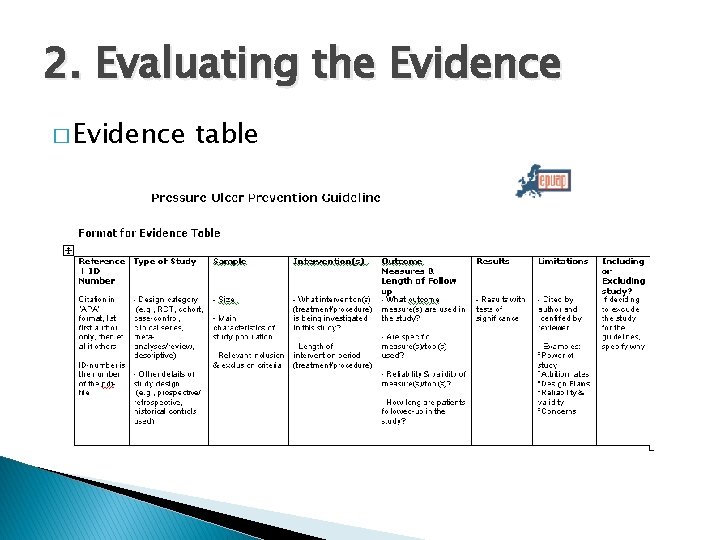
2. Evaluating the Evidence � Evidence table

2. Evaluating the Evidence � Methodology checklist

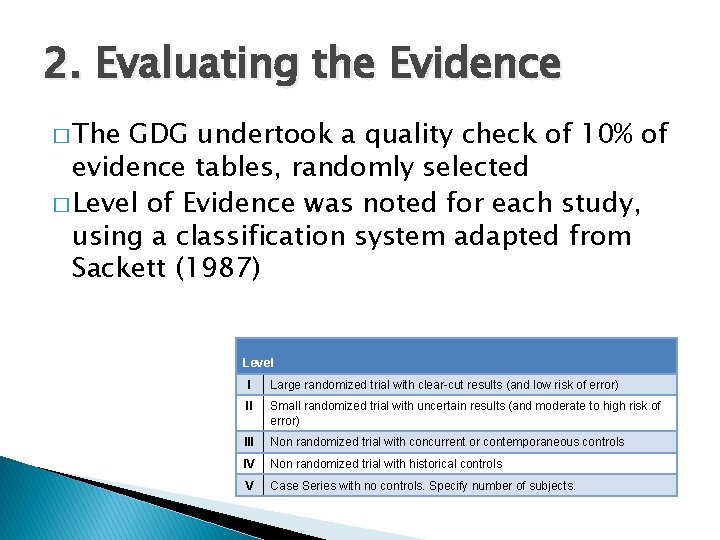
2. Evaluating the Evidence � The GDG undertook a quality check of 10% of evidence tables, randomly selected � Level of Evidence was noted for each study, using a classification system adapted from Sackett (1987) Level I Large randomized trial with clear-cut results (and low risk of error) II Small randomized trial with uncertain results (and moderate to high risk of error) III Non randomized trial with concurrent or contemporaneous controls IV Non randomized trial with historical controls V Case Series with no controls. Specify number of subjects.

3. Drafting Recommendations � Evidence tables allowed the SWGs to develop guideline statements � Uniformity and internal consistency � Guideline statements and supporting text were sent to GDG for review

4. Assigning Strength of Evidence Ratings � Identifies the strength of cumulative evidence supporting a recommendation Strength of Evidence Ratings for Each Recommendation Level Criteria A The recommendation is supported by direct scientific evidence from properly designed & implemented controlled trials on pressure ulcers in humans (or humans at risk for pressure ulcers) providing statistical results that consistently support the guideline statement. (Level I studies required) B Recommendation are supported by direct scientific evidence from properly designed & implemented clinical series on pressure ulcers in humans (or humans at risk for pressure ulcers) providing statistical results that consistently support the recommendation. (Level II, IV, V studies) C The recommendation is supported by indirect evidence (e. g. , studies in normal human subjects, humans with other types of chronic wounds, animal models) and/or expert opinion.

5. Summarizing Supporting Evidence � SWGs summarized the evidence supporting each recommendations � Recommendations with SOE rating A or B required an explicit summary of one or more studies of human subjects with a PU or at risk for PU development. � The level of evidence for each study is also identified

6. Stakeholders � Whole process could be followed by stakeholders on a website http: //www. pressureulcerguideline. org

6. Stakeholders � Register ◦ ◦ as stakeholder Interest in PU Contribute to guideline by reading draft guideline Ensuring all relevant evidence has been included Commenting on guideline � Stakeholders ◦ Individual ◦ Representative for a society /organisation

6. Stakeholders � Every stakeholder was informed ◦ About search filter, inclusion criteria, retrieved references and given the opportunity to comment ◦ When statements were available for review and given the opportunity to comment on the statements both by suggesting literature that might have been missed and commenting on the text � The full GDG met to discuss each comment and review the suggested literature � The guideline text was then revised in the light of the literature/comments as appropriate

146 Representatives from 32 Countries

And 903 individuals from 53 countries

Guideline documents

Versions of the Guidelines � The guidelines will be in several formats: ◦ Quick Reference Guide ◦ Clinical Practice Guideline ◦ Technical version ◦ Patient and carer version

Quick Reference Guide � This version has all the guideline statements and some text. It does not include references. � It will be available on the website for free download as separate prevention and treatment guidelines. � It will also be available in hard copy with both prevention and treatment included – for purchase

Clinical Practice Guideline � This version will have the methodology, the statements and the underpinning evidence and all the references. � It will include both prevention and treatment will be available for purchase in a number of formats: book, CD-Rom, on-line

Technical Version � Will contain a brief description of the methodology, all the papers reviewed, the evidence tables, list of papers reviewed but not used. � It will be on the websites will probably only be accessed by the seriously mad or those doing Ph. D studies!

Patient and Carer Version � This will not form part of the international guideline products – it is too difficult to produce a document that reflects all the varied healthcare symptoms. � We were advised that this was best undertaken at a national level. NPUAP will produce a version for the USA � It would be good to have details of any national versions on the EPUAP website so that people can be directed to them

Volume � Full guideline documentation runs to several hundred pages! � ‘Quick’ versions run to over 80 pages!

Accessing the Guidelines � When can you get your hands on them? � The QRG treatment and CPG are with the copy editor at present. We have just agreed on the artwork for the covers. � We hope to have them on the website by mid. September, but an email alert will be sent out

But not everyone speaks English!

Translations � Bulgarian � Dutch � French � German � Hebrew � Japanese � Spanish � Swedish

Using the guidelines � We want these guidelines to be widely used as possible. � We know they may need to be adapted to meet local needs � However, we would expect that the International Guidelines would be acknowledged. � Guidance will be provided at the front of the document on the precise wording etc

Implementation � The next challenge is get these guidelines implemented into clinical practice � That � It is probably a harder task to achieve! will be a good point for future discussion

What’s new?

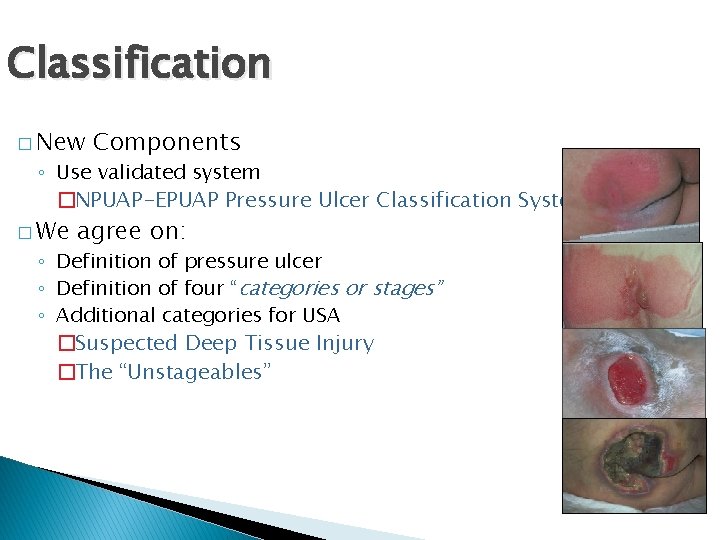
Classification � New Components ◦ Use validated system �NPUAP-EPUAP Pressure Ulcer Classification System � We agree on: ◦ Definition of pressure ulcer ◦ Definition of four “categories or stages” ◦ Additional categories for USA �Suspected Deep Tissue Injury �The “Unstageables”

Pressure Ulcer • A pressure ulcer is localized injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure, or pressure in combination with shear. A number of contributing or confounding factors are also associated with pressure ulcers; the significance of these factors is yet to be elucidated.

Category I: Non-blanchable redness of intact skin � Intact skin with nonblanchable redness of a localized area usually over a bony prominence. Discoloration of the skin, warmth, edema, hardness or pain may also be present. Darkly pigmented skin may not have visible blanching.

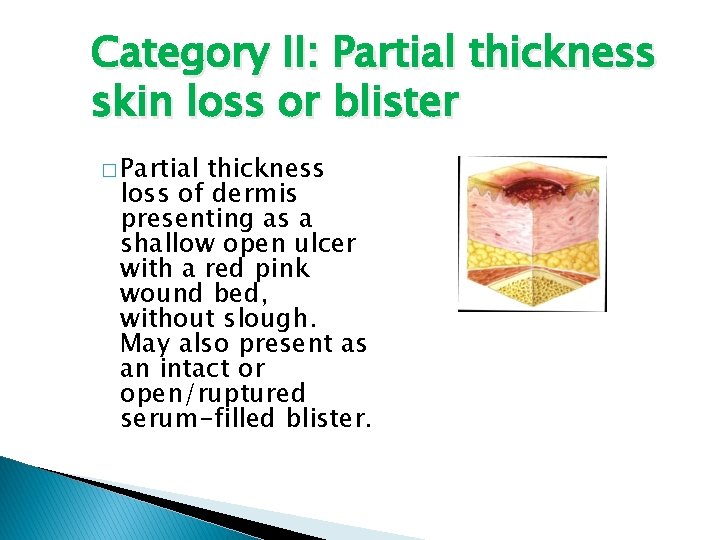
Category II: Partial thickness skin loss or blister � Partial thickness loss of dermis presenting as a shallow open ulcer with a red pink wound bed, without slough. May also present as an intact or open/ruptured serum-filled blister.

Category III: Full Thickness (fat visible) � Full thickness tissue loss. Subcutaneous fat may be visible but bone, tendon or muscle are not exposed. Some slough may be present. May include undermining and tunneling.

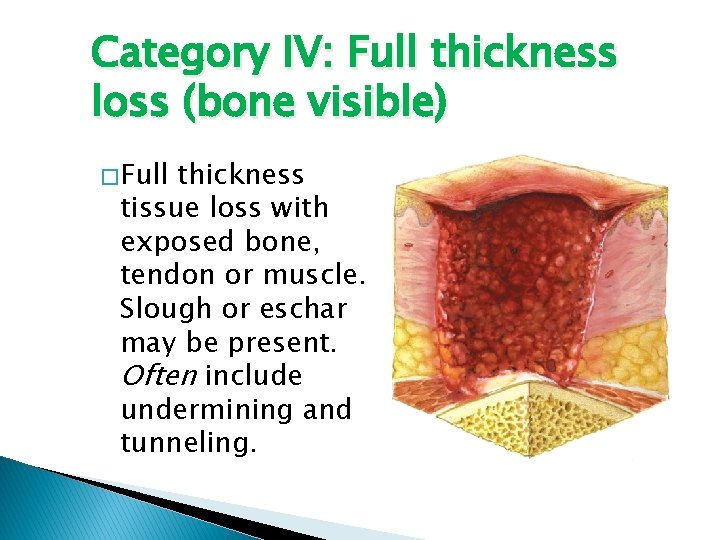
Category IV: Full thickness loss (bone visible) � Full thickness tissue loss with exposed bone, tendon or muscle. Slough or eschar may be present. Often include undermining and tunneling.

What’s new � Focus upon assessment of healing (PUSH tool) � Pain in pressure ulcer, as categories change pain increases � Use of dc electrotherapies?

Guideline topics

Topics � Etiology � Risk Assessment � Skin Assessment � Nutrition � Repositioning � Support Surfaces � Special Population: Patients in operating room

Topics � Classification of PU � Assessment & Monitoring Healing � Nutrition for Healing � Pain Assessment & Management � Support Surfaces � Principles of Wound Bed Preparation & Biofilms (Scientific Explanation to Guide Treatment Decisions) � Wound Cleansing

Topics � Debridement � Dressings � Assessment and Treatment of Infection � Biophysical Agents � Growth Factors & Biological Dressings � Operative Repair � Palliative Care

‘A’ level recommendations

A level recommendations � Offer high-protein mixed oral nutritional supplements and/or tube feeding, in addition to the usual diet, to individuals with nutritional risk and pressure ulcer risk because of acute or chronic diseases, or following a surgical intervention

A level recommendations � Repositioning should be undertaken to reduce the duration and magnitude of pressure over vulnerable areas of the body � Frequency of repositioning will be influenced by variables concerning the individual (Strength of Evidence = C) and the support surface in use

A level recommendations � Use higher-specification foam mattresses rather than standard hospital foam mattresses for all individuals assessed as at risk for pressure ulcer development � There is no evidence of the superiority of one higher-specification foam mattress over alternative higher-specification foam mattresses

A level recommendations � Both alternating pressure active support overlays and replacement mattresses have a similar efficacy in terms of pressure ulcer incidence � That’s all the ‘A’ level recommendations covering prevention!

A level recommendations � Consider the use of direct contact (capacitative) electrical stimulation (ES) in the management of recalcitrant Category/Stage II, III, and IV pressure ulcers to facilitate wound healing � Only ‘A’ level recommendation in treatment of pressure ulcers!

Oops! � Lots of pressure ulcer activity but few strong recommendations � Guidance for future research?

The other recommendations? � Prevention �B level � C level � No SOE � Treatment � B level � C level 12 50 5 44 294

What’s next