International Max Planck Research School for Competition and






- Slides: 14

International Max Planck Research School for Competition and Innovation Owais Hassan Shaikh IMPRS-CI 2010 Data Exclusivity in Free Trade Agreements and Access to Originator (R&D Pharma) Medicine WIPO – 31. 05. 2012

Owais H. Shaikh IMPRS-CI 2010 Preliminaries • What is data exclusivity. . . • Protection of clinical trial data, submitted with a new drug application, against usage (reliance) by either drug regulatory authorities or generic companies for approving subsequent generic applications. • Data exclusivity through FTAs • Current debate focuses on availability of affordable medicines • However, data exclusivity provisions in specific FTAs (mainly US) may restrict total access (originator + generic) How specific FTA provisions relating to data exclusivity affect access to originator company’s medicine? 22

Owais H. Shaikh Data Exclusivity Protection IMPRS-CI 2010 Reliance on Clinical Trial Data is not allowed Reliance on foreign marketing approval is not allowed EU FTAs US FTAs 3

Owais H. Shaikh IMPRS-CI 2010 Reliance on prior foreign marketing approval not allowed If a Party requires or permits, . . . the submission of evidence of prior marketing approval (of originator’s medicine) in the other territory, the Party shall not, without the consent of a person that previously submitted the safety or efficacy information to obtain marketing approval in the other territory, authorize another to market a same or a similar product based on: . . . (ii) evidence of prior marketing approval in the other territory for at least five years for pharmaceutical products from the date of marketing approval of the new product in the Party. (Art. 14. 9(b) US-Bahrain FTA) Art. 17. 10(c) US-Australia FTA; Art 15. 10. 1(b) CAFTA-DR; Art 18. 9. 1(b) US-Korea; Art 15. 10. 1 US-Morocco; Article 15. 9. 1(b) US-Oman and Article 16. 8. 2 US-Singapore. Reduced or no access to originator medicine (as well as no or delayed access to generic medicine) 4

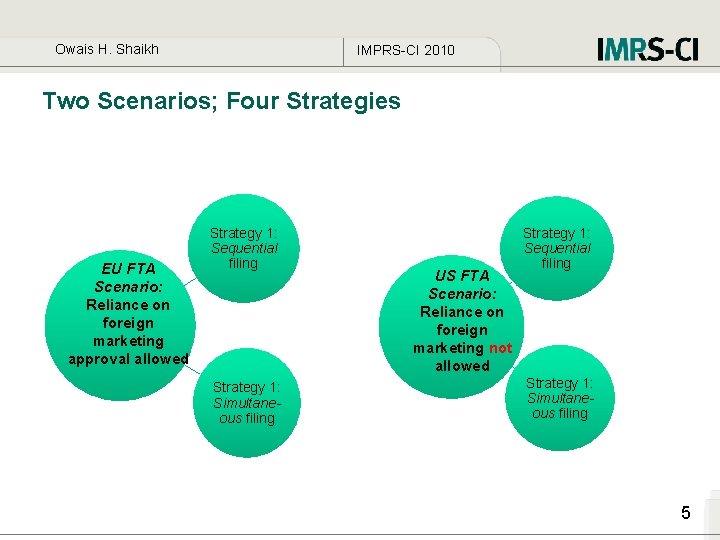
Owais H. Shaikh IMPRS-CI 2010 Two Scenarios; Four Strategies EU FTA Scenario: Reliance on foreign marketing approval allowed Strategy 1: Sequential filing Strategy 1: Simultaneous filing US FTA Scenario: Reliance on foreign marketing not allowed Strategy 1: Sequential filing Strategy 1: Simultaneous filing 5

Owais H. Shaikh IMPRS-CI 2010 Hypothetical FTA between countries A, B and US/EU Assumptions: 1. Other that data exclusivity no exclusivity (patent, orphan drug or pediatric) remaining for the originator medicine. 2. Parallel importation is not allowed (National exhaustion); 3. Drug authorities take 1 year to approve (originator or generic) medicine; 4. Generic companies take 1 year to develop a bioequivalent generic drug; 5. Term of data exclusivity is 5 years. 6

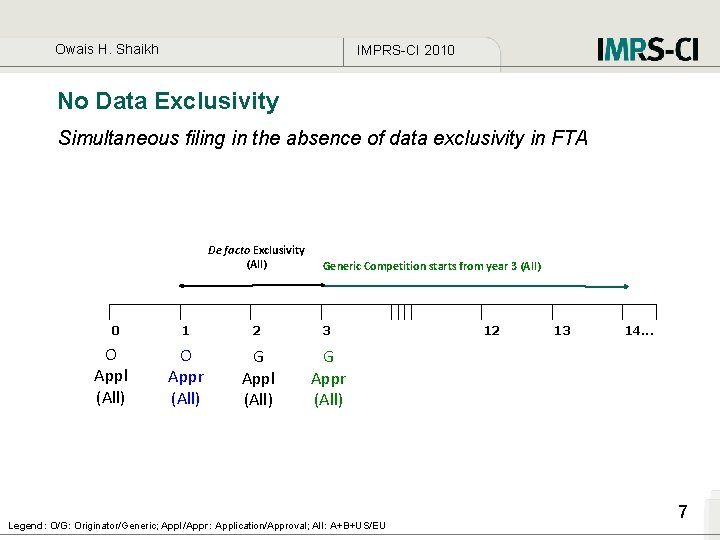
Owais H. Shaikh IMPRS-CI 2010 No Data Exclusivity Simultaneous filing in the absence of data exclusivity in FTA De facto Exclusivity (All) 0 O Appl (All) Generic Competition starts from year 3 (All) 1 2 3 O Appr (All) G Appl (All) G Appr (All) Legend: O/G: Originator/Generic; Appl/Appr: Application/Approval; All: A+B+US/EU 12 13 14. . . 7

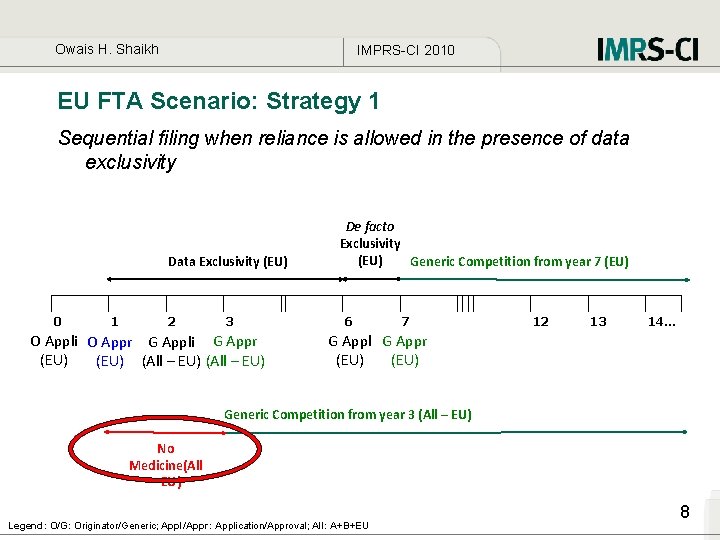
Owais H. Shaikh IMPRS-CI 2010 EU FTA Scenario: Strategy 1 Sequential filing when reliance is allowed in the presence of data exclusivity Data Exclusivity (EU) 0 1 2 3 O Appli O Appr G Appli G Appr (EU) (All – EU) De facto Exclusivity (EU) Generic Competition from year 7 (EU) 6 7 12 13 14. . . G Appl G Appr (EU) Generic Competition from year 3 (All – EU) No Medicine(All – EU) Legend: O/G: Originator/Generic; Appl/Appr: Application/Approval; All: A+B+EU 8

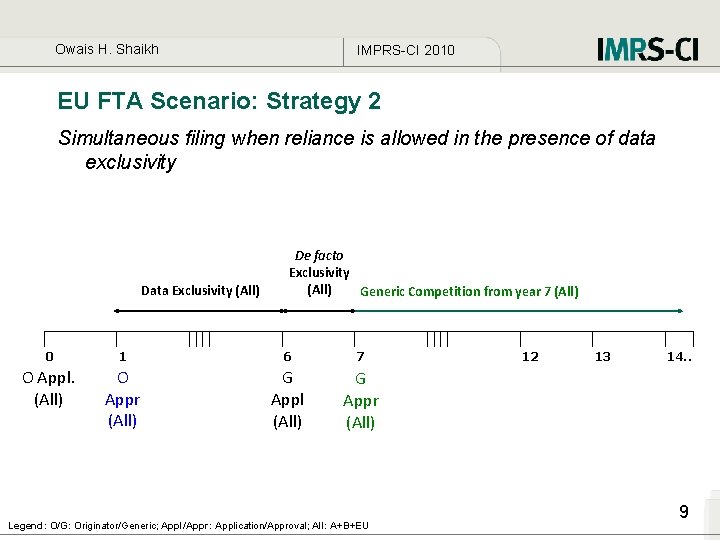
Owais H. Shaikh IMPRS-CI 2010 EU FTA Scenario: Strategy 2 Simultaneous filing when reliance is allowed in the presence of data exclusivity Data Exclusivity (All) De facto Exclusivity (All) Generic Competition from year 7 (All) 0 1 6 7 O Appl. (All) O Appr (All) G Appl (All) G Appr (All) Legend: O/G: Originator/Generic; Appl/Appr: Application/Approval; All: A+B+EU 12 13 14. . 9

Owais H. Shaikh IMPRS-CI 2010 US FTA Scenario: Strategy 1 Sequential filing when reliance is not allowed in the presence of data exclusivity De facto Exclusivity (US) Data Exclusivity (US) 0 O Appli (US) 1 O Appr (US) 6 Data Exclusivity (A) 7 G Appl G Appr (A) O Appl O appr (A) No Medicine (All – US) De facto Exclusivity Data Exclusivity (B) (A) 12 G Appl (A) O Appl (B) Generic Competition from year 7 (US) 13 14. . . G Appr (A) O appr (B) Generic Competition from year 13 (A) No Medicine (B)! Legend: O/G: Originator/Generic; Appl/Appr: Application/Approval; All: A+B+US 10

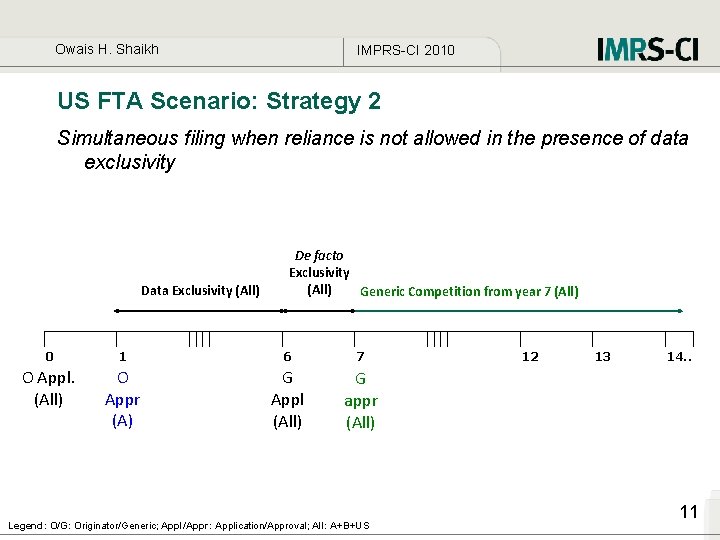
Owais H. Shaikh IMPRS-CI 2010 US FTA Scenario: Strategy 2 Simultaneous filing when reliance is not allowed in the presence of data exclusivity Data Exclusivity (All) De facto Exclusivity (All) Generic Competition from year 7 (All) 0 1 6 7 O Appl. (All) O Appr (A) G Appl (All) G appr (All) Legend: O/G: Originator/Generic; Appl/Appr: Application/Approval; All: A+B+US 12 13 14. . 11

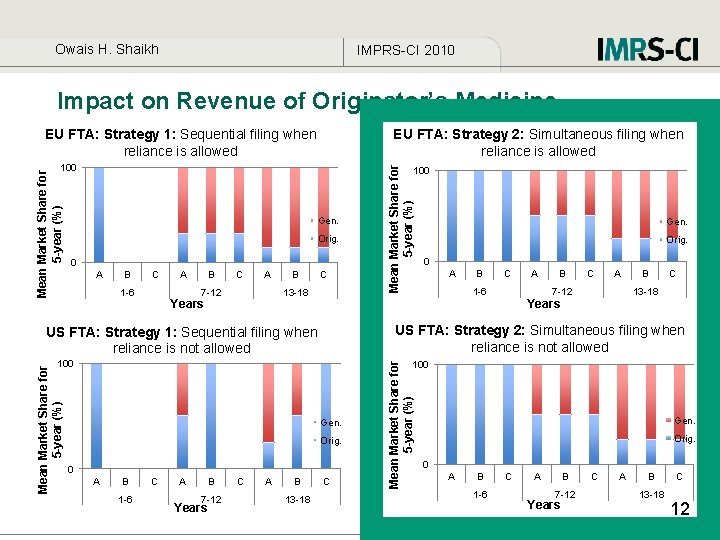
Owais H. Shaikh IMPRS-CI 2010 Impact on Revenue of Originator’s Medicine EU FTA: Strategy 1: Sequential filing when reliance is allowed 100 Gen. Orig. 0 A B C 1 -6 A B C A 7 -12 B C 13 -18 Years Orig. 0 A B C A 1 -6 B C A 7 -12 B C 13 -18 US FTA: Strategy 2: Simultaneous filing when reliance is not allowed US FTA: Strategy 1: Sequential filing when reliance is not allowed 100 Mean Market Share for 5 -year (%) Gen. Years 100 Gen. Orig. 0 A B 1 -6 C A B 7 -12 Years C A B 13 -18 C Mean Market Share for 5 -year (%) 100 Mean Market Share for 5 -year (%) EU FTA: Strategy 2: Simultaneous filing when reliance is allowed Gen. Orig. 0 A B 1 -6 C A B 7 -12 Years C A B 13 -18 C 12

Owais H. Shaikh IMPRS-CI 2010 Insights • In the presence of data exclusivity protection reliance on prior foreign marketing approval (EU FTAs) increases access to medicine in parties to an FTA. • Access to medicine is most enhanced when originator simultaneously applies under EU and US FTA and most restricted when sequentially applies under US FTA. • Originator company earns relatively more revenue when it applies simultaneously under both EU and US FTAs. • A binding provision should be included in FTAs to ensure that Originator files simultaneously in under both EU and US FTAs. • A centralized approval mechanism may be adopted in an FTA (EMEA). 13

Thank you! 1414
 1900 max planck
1900 max planck La historia del átomo
La historia del átomo Max planck encyclopedia of comparative constitutional law
Max planck encyclopedia of comparative constitutional law Biophysics
Biophysics Max planck
Max planck Max planck institut rechtsgeschichte
Max planck institut rechtsgeschichte Bert l. de groot
Bert l. de groot ;,dk jhd'v
;,dk jhd'v Wendelstein 7-x
Wendelstein 7-x Jelle bruineberg
Jelle bruineberg Characteristics of a monopoly
Characteristics of a monopoly Monopoly vs monopolistic competition
Monopoly vs monopolistic competition Monopoly vs oligopoly venn diagram
Monopoly vs oligopoly venn diagram Competition refers to
Competition refers to Absolute min and max
Absolute min and max