International Liver Surgery Outcomes Study Liver Group org





- Slides: 7

International Liver Surgery Outcomes Study Liver. Group. org Collaborative Headquarters | Royal Free London NHS Foundation Trust | UK

International Liver Surgery Outcomes Study Mortality after major liver resection is claimed to have improved (1 -2%). However, epidemiological studies show mortality rates of up to 6%. Outcome in liver surgery is influenced by: • Indications ranging from colorectal liver metastases to cholangiocarcinoma. • Complexity ranging from wedge resections to two-stage hepatectomy and trisectionectomy with biliary reconstruction and reopertive surgery. • Skills/equipment ranging from single general surgeon private practice to hepatobiliary/transplant centers. Headquarters | Royal Free London NHS Foundation Trust | UK

International Liver Surgery Outcomes Study Outcomes of liver surgery are not reported in a uniform way: • Morbidity and mortality reported at hospital discharge, 30 or 90 days • Morbidity reported as minor vs. major, severe, Clavien-Dindo, CCI • Underreported in registries and retrospective studies • Patient selection in RCT Headquarters | Royal Free London NHS Foundation Trust | UK

International Liver Surgery Outcomes Study Primary objective: Provide a verified record of the true perioperative morbidity and mortality rates of a representative set of liver surgeons in 2019 worldwide. Secondary objectives: Identify modifiable risk factors for morbidity and mortality using multivariable models. Headquarters | Royal Free London NHS Foundation Trust | UK

International Liver Surgery Outcomes Study ”Snapshot” collaborative study: • 3 month data collection • 3 month postoperative follow up • within a 1 year frame period (2019) Registration open to any individual surgeon performing liver resections May require local ethics approval (country- or center-dependent) Data entry requires approximately 15 minutes per patient Several tools regarding classifications, volume assessment, etc. available on the website Headquarters | Royal Free London NHS Foundation Trust | UK

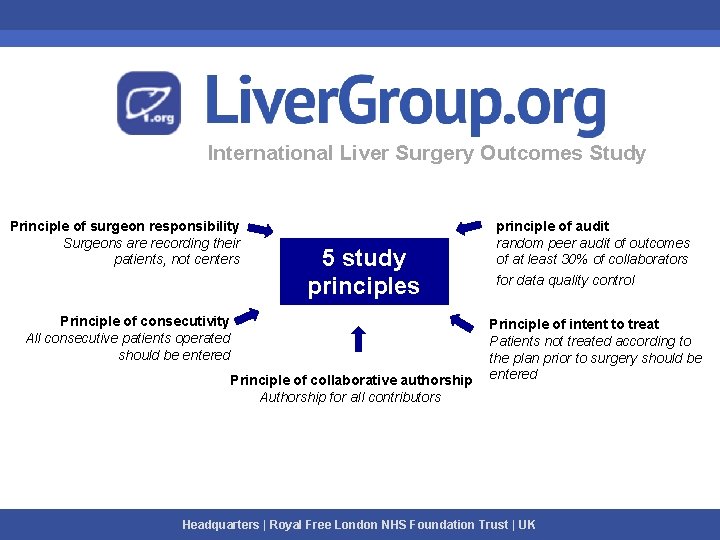
International Liver Surgery Outcomes Study Principle of surgeon responsibility Surgeons are recording their patients, not centers 5 study principles Principle of consecutivity All consecutive patients operated should be entered Principle of collaborative authorship Authorship for all contributors principle of audit random peer audit of outcomes of at least 30% of collaborators for data quality control Principle of intent to treat Patients not treated according to the plan prior to surgery should be entered Headquarters | Royal Free London NHS Foundation Trust | UK

International Liver Surgery Outcomes Study ”Snapshot" study on liver surgery worldwide over 3 months Provide a verified record of true morbidity and mortality Identify risk factors of outcome after lifer surgery Study period January to December 2019 Liver. Group. org Collaborative Headquarters | Royal Free London NHS Foundation Trust | UK