International conference Quality of Active Pharmaceutical Ingredients Hyderabad

International conference Quality of Active Pharmaceutical Ingredients Hyderabad, 5 -6 -7 September 2009 API Inspections: the EDQM experience – 7 September 2009 Florence Benoit-Guyod, EDQM Inspector Certification of substances Division, EDQM
The Certification Procedure • Intended to be applied for the assessment of the quality of pharmaceutical substances with regards to the criteria of the Ph. Eur. monograph(s) • It ensures that all possible impurities and contamination can be fully controlled by the requirements of the monograph(s) Additional benefits: • centralised assessment for APIs, attractive to applicants and National Competent Authorities • Identification of potential divergent practices by national assessors may contribute to more consistent assessment approaches across Europe Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 2

Inspection • An inspection may be part of the Certification Procedure • The inspection is performed either before or after the CEP is granted • The inspection aim is to verify the compliance with the submitted dossier and with the EU GMP Part II • For sterile substances compliance with Annex 1 EU GMP Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 3

Role of the National Competent Authority • The Competent Authority may carry out an inspection of an active substance manufacturer in order to ensure that a manufacturing authorisation holder of a medicinal product has fulfilled its obligations under Article 46 (f) and/or Article 50 (f) of the below mentioned Directives (Article 111 of Directive 2001/83/EC and Article 80 of Directive 2001/82/EC) Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 4

Conditions for an inspection • When requested by a member State, EMEA, European Commission or EDQM (if there are grounds for suspicion of noncompliance, need to verify data submitted) • When requested by the manufacturer itself Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 5

Responsibility of the manufacturer • In the CEP procedure the manufacturer has to declare: - Compliance to Good Manufacturing Practices (GMP) - Willingness to be inspected Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 6

EDQM Inspection Program • In application of Directives 2001/82/EC and 2001/83/EC as amended, the European Commission gave a mandate to the EDQM to establish an annual program for inspections • Inspections are performed inside and outside Europe and involve manufacturing sites and brokers/distributors holding CEP(s) Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 7

EDQM Inspection Program • The draft program is circulated to the Member States for comments and presented to the GMP/GDP Inspectors Working Group at EMEA for discussion. • The program is finally adopted by the CEP Steering Commitee. • The final program is circulated to all EEA Member States Competent Authorities Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 8

Selection of the sites • Done in accordance with the EMEA guidance EMEA/INSP/GMP/313538/ 2006 • According to a risk-based approach: - main criteria: request from the assessors - sterile substances - inspection by equivalent authority - several triggers involved - regulatory environment of the manufacturing site Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 9

How the system works • Inspection performed by team composed of an EDQM inspector and an inspector coming from an EU/EEA or MRA National Competent Authority • The compliance to the submitted dossier and to the EU Good Manufacturing Practices Part II is verified • An inspection report is issued within 6 weeks • Immediate actions are taken in case of major or critical deficiencies Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 10

Inspection Outcome • According to the inspection results the Company is quoted as compliant, borderline or non compliant. • Companies found compliant may be reinspected/re-evaluated within 3 -5 years depending on the numbers and classification of deficiencies found. • Companies found borderline may be reinspected earlier (about 2 years) Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 11

Inspection follow-up • The company must reply to the deficiencies found within one month from the receipt of the inspection report • The replies should be fully documented and reflect actual measures in place • Discrepancies with the certification dossier are specifically addressed and managed by the revision process at DCEP Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 12

Positive Outcome • In case of positive conclusion of the inspection, and if any expected changes for CEP revision have been submitted, an inspection attestation is delivered, stating the compliance with the CEP and with the GMP • A GMP Certificate may be issued by the participating Inspectorate (EMEA/INS/GMP/871/04) Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 13

Negative Outcome • In case of critical/major GMP deficiencies or in case of major deviation compared to the dossier (failure in the declarations and commitments) the corresponding CEP is suspended and/or on-going CEP application is rejected • Suspension is endorsed by an Ad Hoc Committee • All Ph. Eur Member States/Observers/Partners, EMEA, EU Commission and local Inspectorate are informed Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 14

Negative Outcome • Information published on the EDQM website (CEP database and Certification webpages) • Holder and manufacturer are informed and a possibility of hearing is given • Statement of GMP non-compliance is issued by the EEA Inspectorate Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 15

Suspension of the CEP • With effect of 2009 the CEPs are suspended for a period of two years • Company is requested to apply within this timeframe for a re-inspection • Based on a valid justification, the company may ask for an extension of this period • Lifting the suspension can only be done after an inspection with positive outcome Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 16
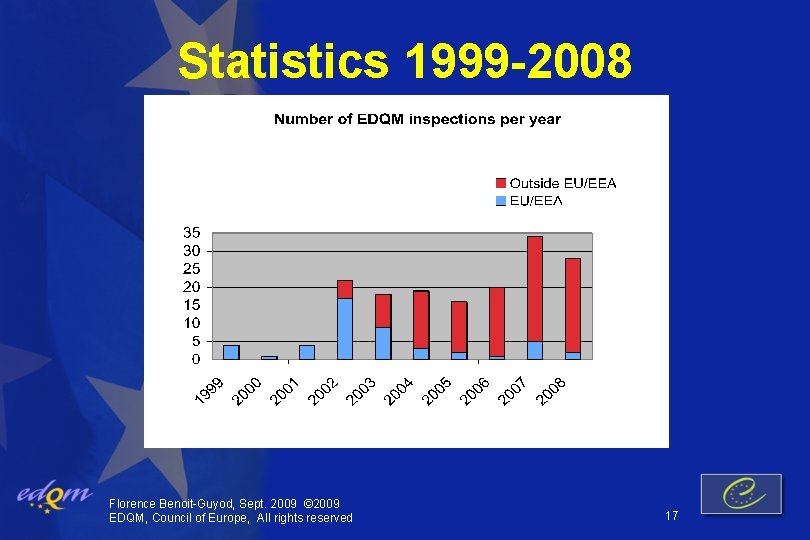
Statistics 1999 -2008 Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 17
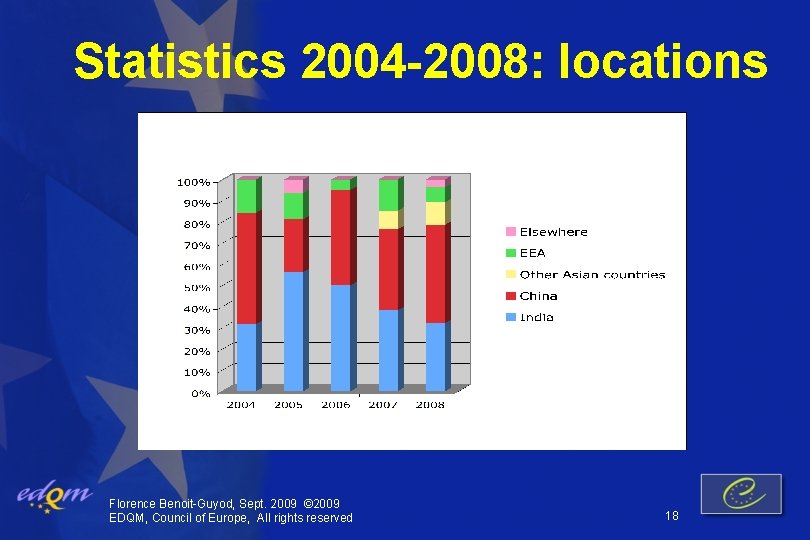
Statistics 2004 -2008: locations Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 18
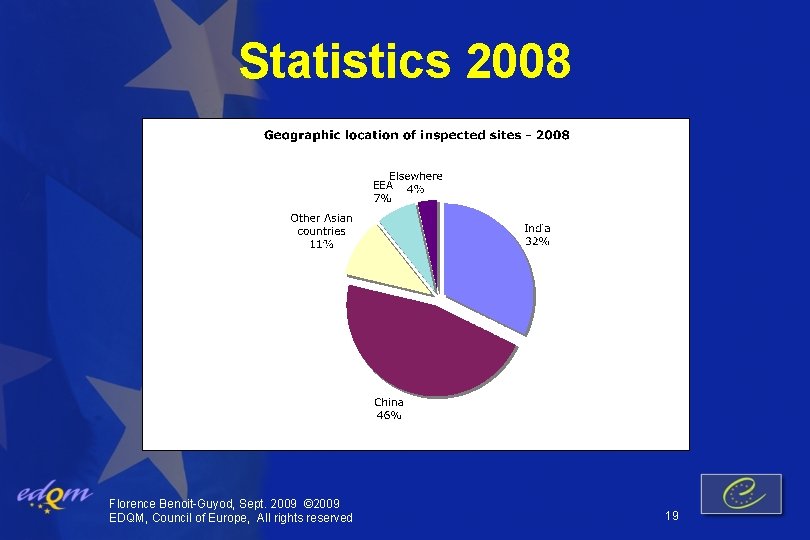
Statistics 2008 Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 19
General compliance trends Non compliant sites: • 2007: 18% • 2008: 21% • 2009: 35% (updated to June) This is the result of the ability of EDQM to identify sites with higher risk of noncompliance and to focus on them Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 20

2008 Review • • 28 inspections performed 2 refusals of inspection 6 sites non GMP compliant Results: 16 CEPs were suspended and 9 dossiers blocked (the current policy is now to withdraw the application) Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 21

2009 Review (end June) • • • 17 sites inspected (9 China, 7 India, 1 Hungary) 6 sites non compliant 4 sites borderline (follow-up on going) CEPs suspended: 8 Dossier closed: 2 CEPs withdrawn after suspension due to negative re-inspection: 2 • Outcome: 35% of the sites were found non compliant; an additional 18% has a borderline level of compliance Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 22
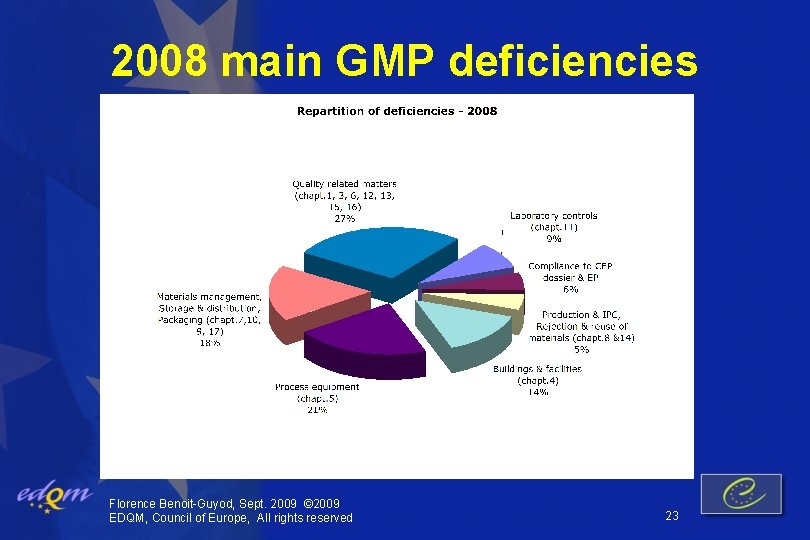
2008 main GMP deficiencies Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 23
2008 main GMP deficiencies v. Quality related matters Validation of processes, qualification of equipment, quality review, change control v. Process equipment, buildings and facilities Cleanliness, maintenance v. Materials management Traceability, key starting material vendor approval, storage Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 24

Conclusions • Experience shows that the inspection remains a powerful tool to detect non compliant manufacturers • Finished product manufacturers must improve their ability to select API manufacturers who comply to the EU GMP Part II Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 25

Perspectives • Further develop the risk-based approach when elaborating the programme • Reinforce collaboration and sharing of information with EU and International Inspectorates Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 26

THANK YOU FOR YOUR ATTENTION Florence Benoit-Guyod, Sept. 2009 © 2009 EDQM, Council of Europe, All rights reserved 27
- Slides: 27