Internal Energy also known as Heat Conversions between
Internal Energy (also known as Heat)
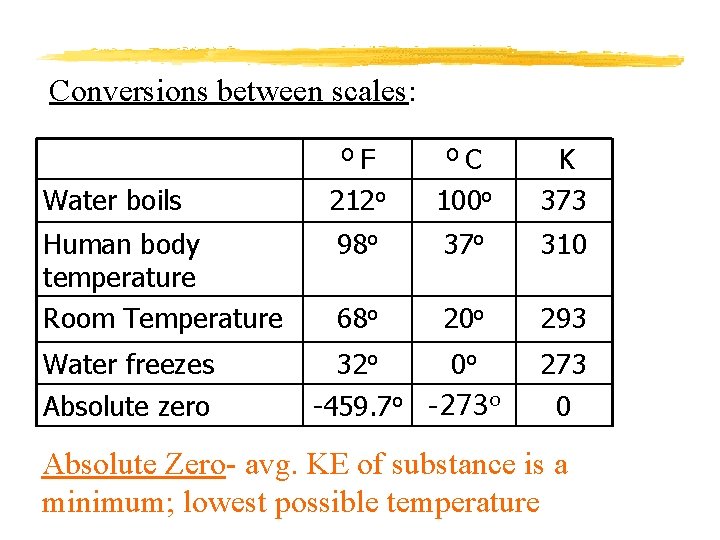
Conversions between scales: F 212 o O C 100 o K 373 98 o 37 o 310 68 o 20 o 293 O Water boils Human body temperature Room Temperature Water freezes Absolute zero 32 o 0 o -459. 7 o -273 o 273 0 Absolute Zero- avg. KE of substance is a minimum; lowest possible temperature
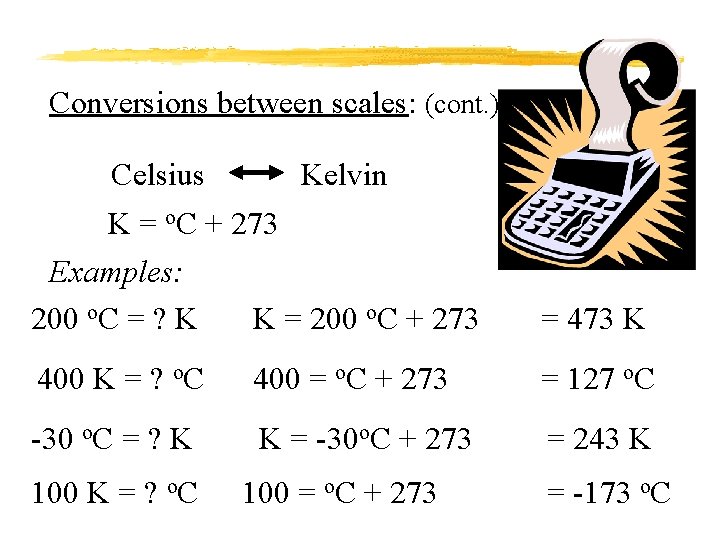
Conversions between scales: (cont. ) Celsius Kelvin K = o. C + 273 Examples: 200 o. C = ? K K = 200 o. C + 273 = 473 K 400 K = ? o. C 400 = o. C + 273 = 127 o. C -30 o. C = ? K K = -30 o. C + 273 = 243 K 100 K = ? o. C 100 = o. C + 273 = -173 o. C
B. Temperature change and its affect on matter 1. Heat is energy, so when it is added to or removed from a substance, the mass of that substance does not change 2. When objects are heated, they expand 3. When objects are cooled, they contract
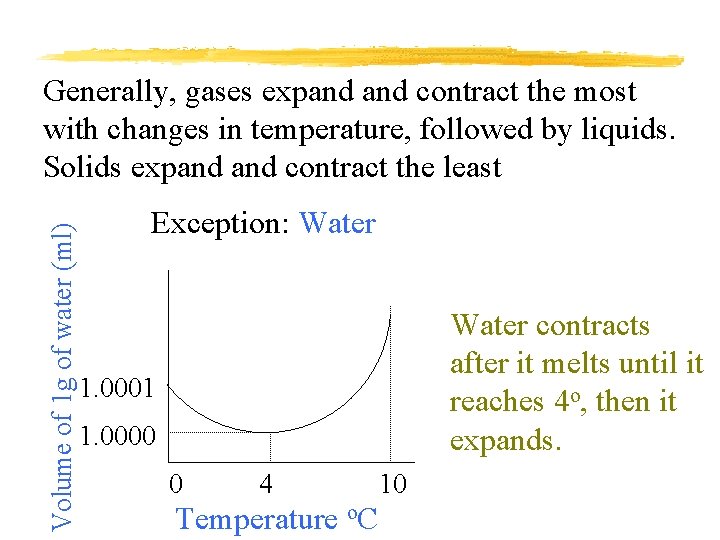
Volume of 1 g of water (ml) Generally, gases expand contract the most with changes in temperature, followed by liquids. Solids expand contract the least Exception: Water contracts after it melts until it reaches 4 o, then it expands. 1. 0001 1. 0000 0 4 Temperature o. C 10
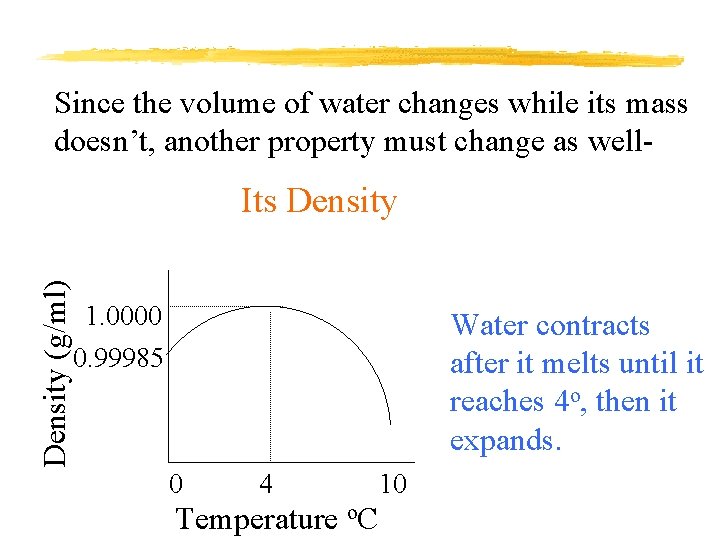
Since the volume of water changes while its mass doesn’t, another property must change as well- Density (g/ml) Its Density 1. 0000 0. 99985 Water contracts after it melts until it reaches 4 o, then it expands. 0 4 Temperature o. C 10
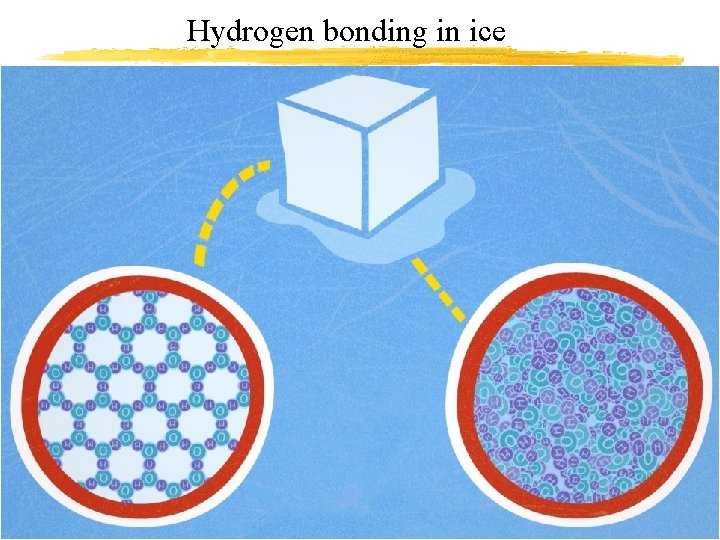
Hydrogen bonding in ice
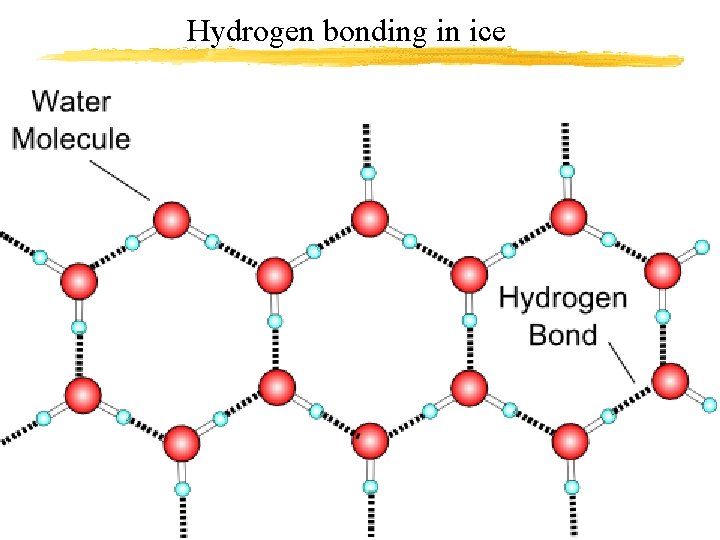
Hydrogen bonding in ice
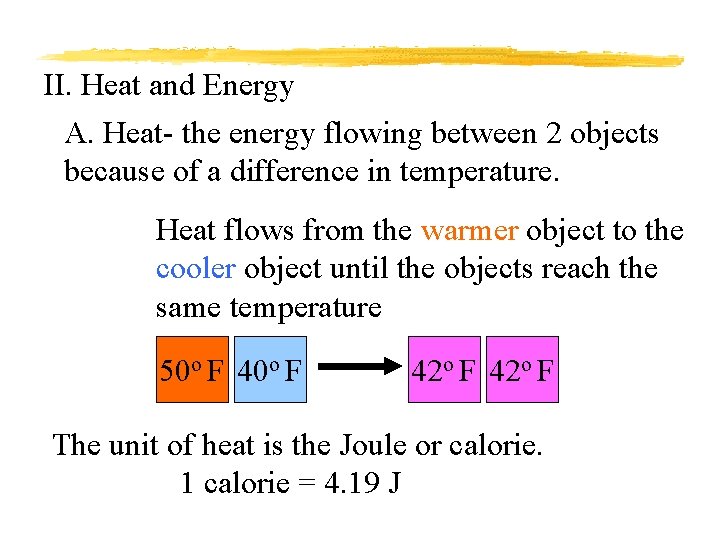
II. Heat and Energy A. Heat- the energy flowing between 2 objects because of a difference in temperature. Heat flows from the warmer object to the cooler object until the objects reach the same temperature 50 o F 42 o F The unit of heat is the Joule or calorie. 1 calorie = 4. 19 J
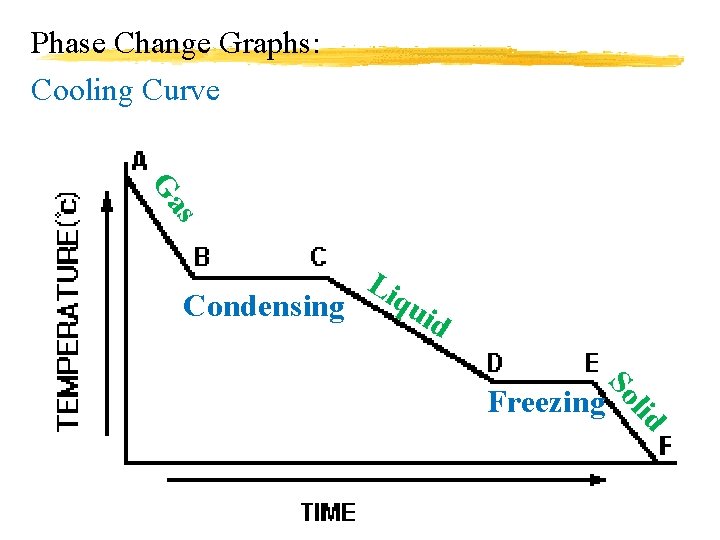
Phase Change Graphs: Cooling Curve s Ga Condensing Li qu id lid So Freezing
1. Melting and Freezing • The temperature of most materials remains constant during melting or freezing (avg. KE remains constant) • When a substance melts, it must gain energy; when a substance freezes, it must lose energy. But wait: If the Avg. KE remains constant, where does the energy go or come from when the object is melting or freezing? The PE of the molecules in the substance
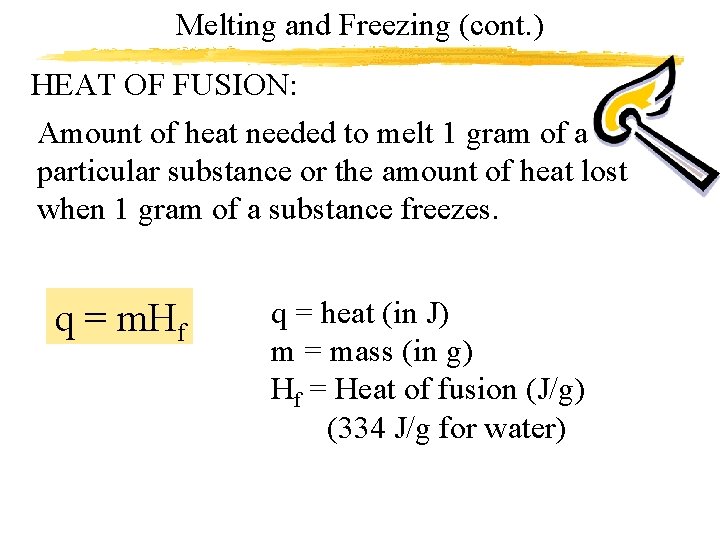
Melting and Freezing (cont. ) HEAT OF FUSION: Amount of heat needed to melt 1 gram of a particular substance or the amount of heat lost when 1 gram of a substance freezes. q = m. Hf q = heat (in J) m = mass (in g) Hf = Heat of fusion (J/g) (334 J/g for water)
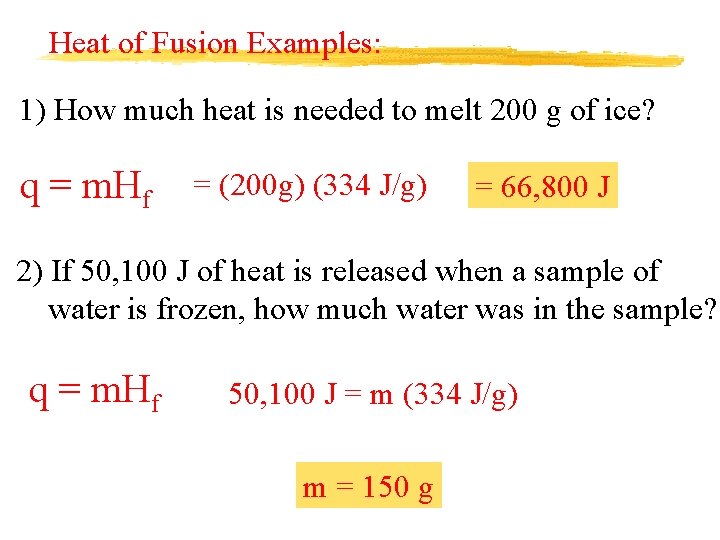
Heat of Fusion Examples: 1) How much heat is needed to melt 200 g of ice? q = m. Hf = (200 g) (334 J/g) = 66, 800 J 2) If 50, 100 J of heat is released when a sample of water is frozen, how much water was in the sample? q = m. Hf 50, 100 J = m (334 J/g) m = 150 g

Melting and Freezing (cont. ) Factors affecting melting/freezing points 1. A substance dissolved in a liquid decreases its freezing/melting point. Salt “melts” ice by lowering its freezing temperature. Antifreeze prevents water from freezing

Melting and Freezing (cont. ) 2. Pressure Applying pressure on the surface of ice lowers the melting point of the ice The pressure of a skate blade on the ice melts the ice directly beneath it. The ice refreezes after the blade is removed.

2. Boiling (or Vaporizing) and Condensing • The temperature of most materials remains constant during boiling or condensing (avg. KE remains constant) • When a substance vaporizes, it must gain energy; when a substance condenses, it must lose energy. • While a substance boils or condenses, there is a change in the PE of the molecules
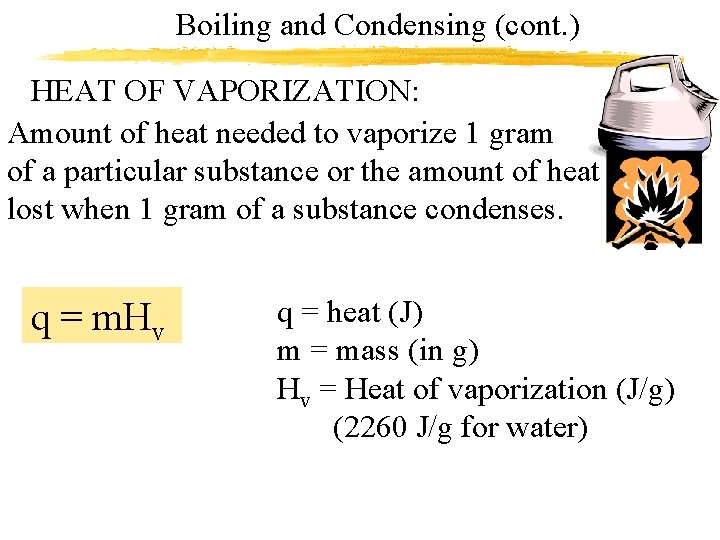
Boiling and Condensing (cont. ) HEAT OF VAPORIZATION: Amount of heat needed to vaporize 1 gram of a particular substance or the amount of heat lost when 1 gram of a substance condenses. q = m. Hv q = heat (J) m = mass (in g) Hv = Heat of vaporization (J/g) (2260 J/g for water)
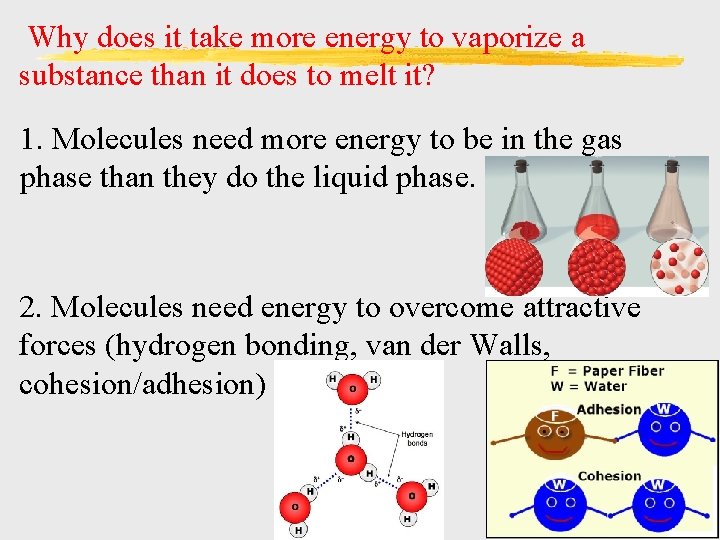
Why does it take more energy to vaporize a substance than it does to melt it? 1. Molecules need more energy to be in the gas phase than they do the liquid phase. 2. Molecules need energy to overcome attractive forces (hydrogen bonding, van der Walls, cohesion/adhesion)
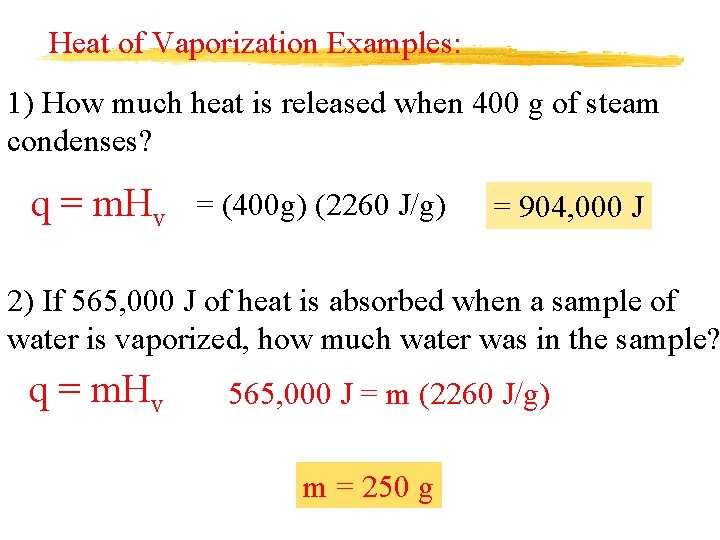
Heat of Vaporization Examples: 1) How much heat is released when 400 g of steam condenses? q = m. Hv = (400 g) (2260 J/g) = 904, 000 J 2) If 565, 000 J of heat is absorbed when a sample of water is vaporized, how much water was in the sample? q = m. Hv 565, 000 J = m (2260 J/g) m = 250 g

Boiling and Condensing (cont. ) Factors affecting boiling/condensation points 1. A substance dissolved in a liquid increases its boiling/condensation point. Antifreeze not only prevents freezing but also prevents water in the cooling system from boiling away.
Boiling and Condensing (cont. ) 2. Pressure Increasing the pressure on a liquid raises the boiling point of that liquid Example: Pressure cooker We say that water boils at 100 o. C (32 o. F, 373 K). This (and other temp’s on a reference chart) is known as the normal boiling point. The normal boiling point occurs at Standard Pressure (101. 3 k. Pa, 1 atm, 760 mm. Hg, or sea level pressure)
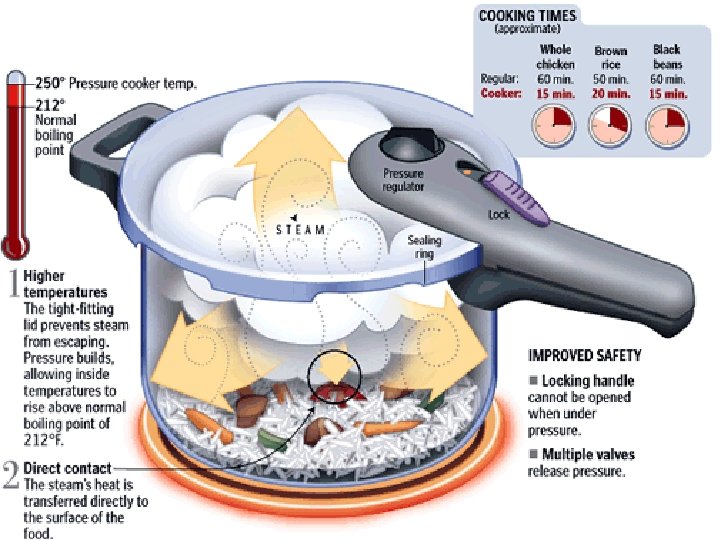
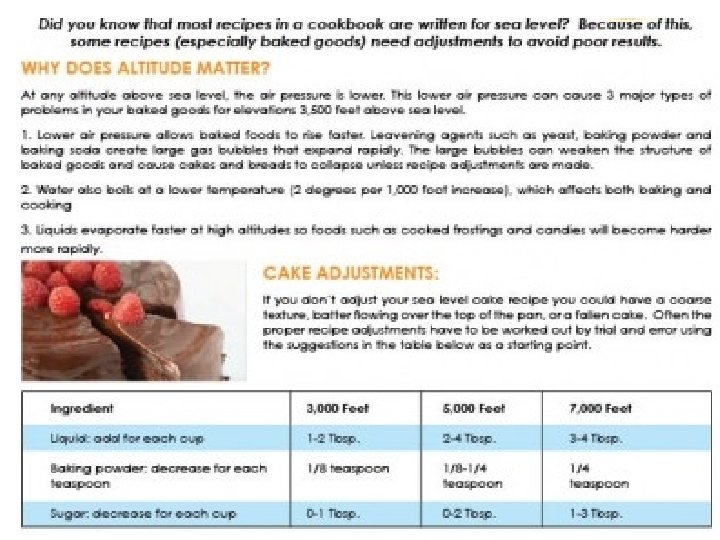
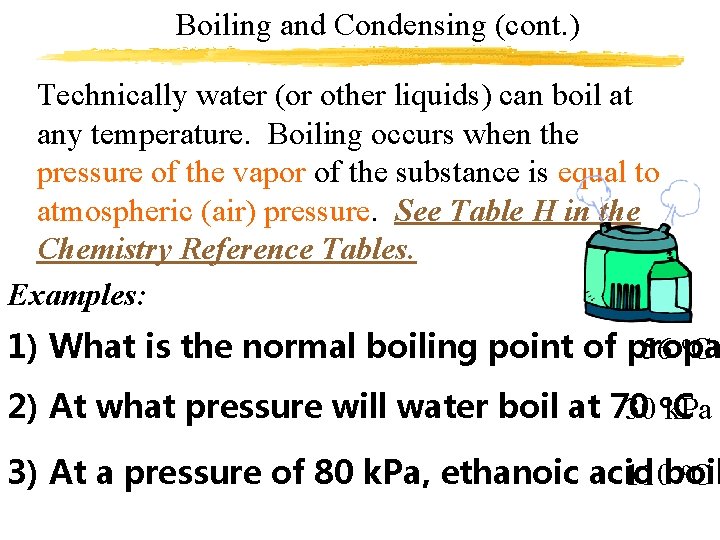
Boiling and Condensing (cont. ) Technically water (or other liquids) can boil at any temperature. Boiling occurs when the pressure of the vapor of the substance is equal to atmospheric (air) pressure. See Table H in the Chemistry Reference Tables. Examples: 1) What is the normal boiling point of propa 56 o. C 2) At what pressure will water boil at 70 C 30 ok. Pa o. C 3) At a pressure of 80 k. Pa, ethanoic acid 110 boil
3. Evaporation: Process where the faster moving (more KE) molecules at a liquid’s surface escape into the gas phase. When this happens, the temperature of the substance decreases. Perspiration Examples: Dog panting
C. Specific Heat Capacity (or Specific Heat, or Heat Capacity) The amount of heat needed to raise 1 gram of a substance 1 o. C ( or how much needs to be lost to lower the temperature 1 o. C) 40 o. C 41 o. C Amount of heat equal to specific heat added to substance **The calorie is defined by how much heat is needed to raise the temperature of 1 g of water 1 o. C
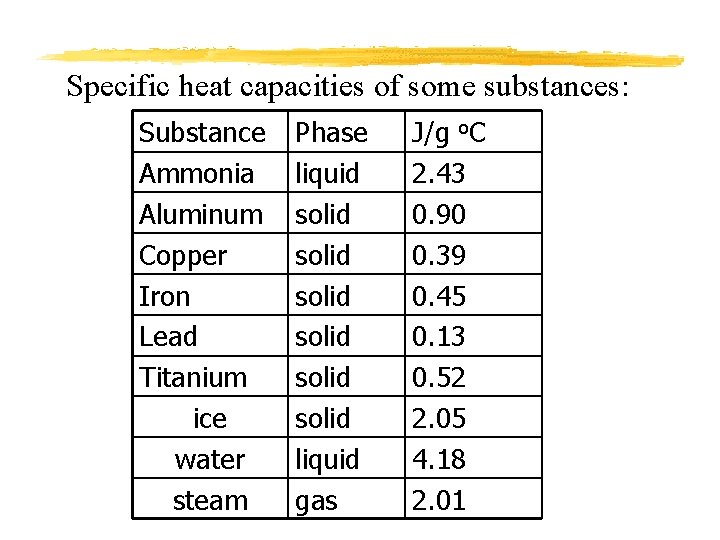
Specific heat capacities of some substances: Substance Ammonia Aluminum Copper Iron Lead Titanium ice water steam Phase liquid solid solid liquid gas J/g o. C 2. 43 0. 90 0. 39 0. 45 0. 13 0. 52 2. 05 4. 18 2. 01

Understanding Specific Heat Capacity Assuming that each of the following substances have equal masses, start at the same temperature, and have the same amount of heat added to them: Iron, copper, aluminum, lead 1. Which substance will heat up the quickest? Lead- smallest specific heat capacity 2. Which substance will heat up the slowest? Aluminum- largest specific heat capacity 3. Which substance will cool off the quickest? Lead- smallest specific heat capacity
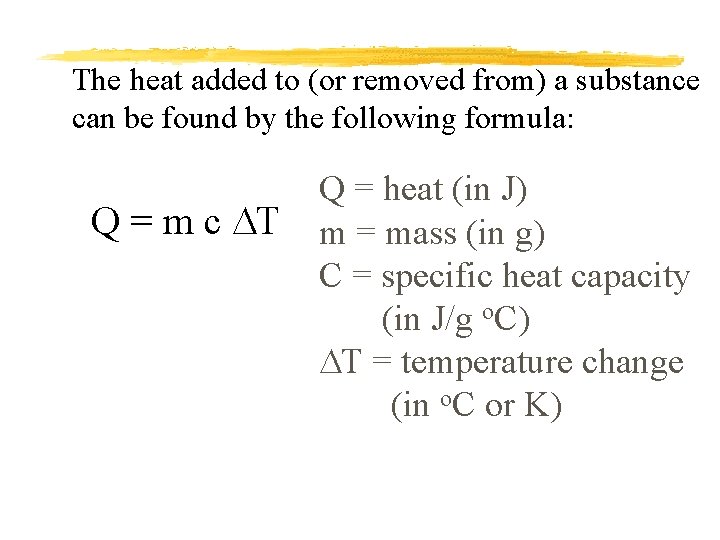
The heat added to (or removed from) a substance can be found by the following formula: Q = m c DT Q = heat (in J) m = mass (in g) C = specific heat capacity (in J/g o. C) DT = temperature change (in o. C or K)
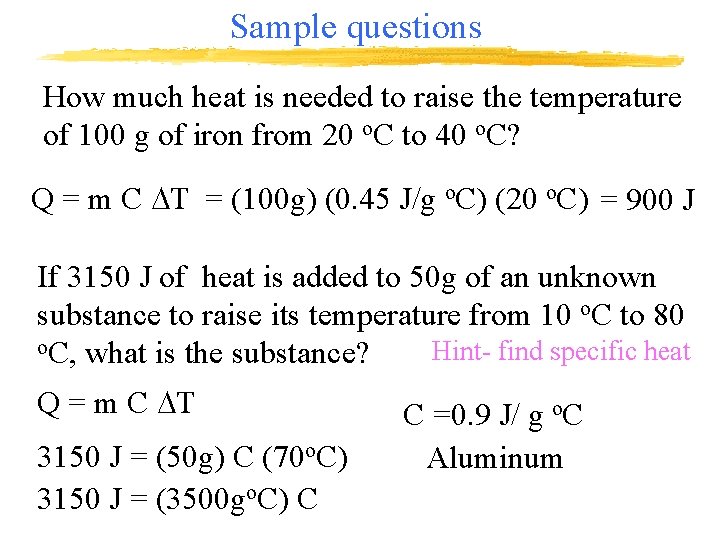
Sample questions How much heat is needed to raise the temperature of 100 g of iron from 20 o. C to 40 o. C? Q = m C DT = (100 g) (0. 45 J/g o. C) (20 o. C) = 900 J If 3150 J of heat is added to 50 g of an unknown substance to raise its temperature from 10 o. C to 80 o. C, what is the substance? Hint- find specific heat Q = m C DT 3150 J = (50 g) C (70 o. C) 3150 J = (3500 go. C) C C =0. 9 J/ g o. C Aluminum
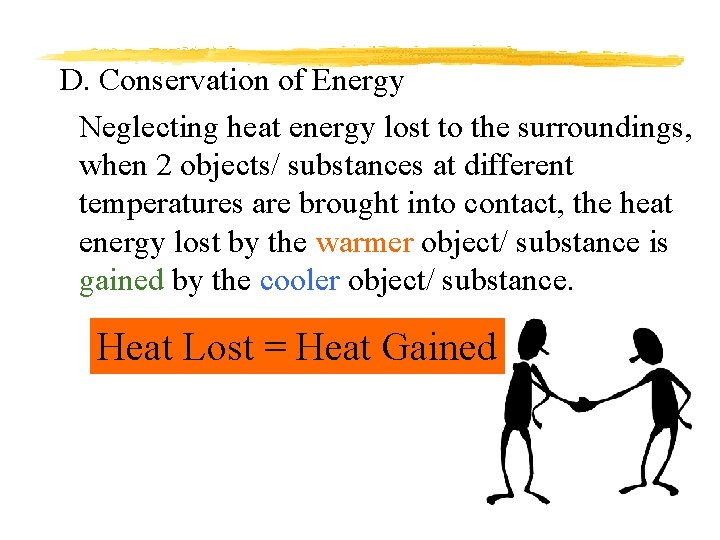
D. Conservation of Energy Neglecting heat energy lost to the surroundings, when 2 objects/ substances at different temperatures are brought into contact, the heat energy lost by the warmer object/ substance is gained by the cooler object/ substance. Heat Lost = Heat Gained
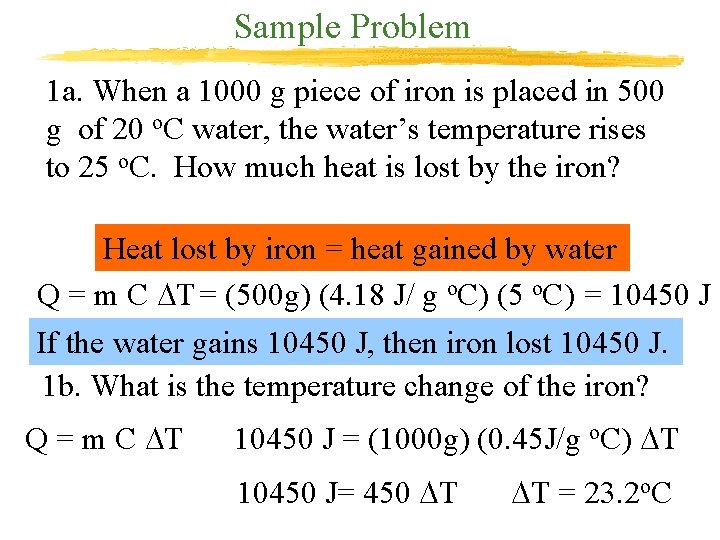
Sample Problem 1 a. When a 1000 g piece of iron is placed in 500 g of 20 o. C water, the water’s temperature rises to 25 o. C. How much heat is lost by the iron? Heat lost by iron = heat gained by water Q = m C DT = (500 g) (4. 18 J/ g o. C) (5 o. C) = 10450 J If the water gains 10450 J, then iron lost 10450 J. 1 b. What is the temperature change of the iron? Q = m C DT 10450 J = (1000 g) (0. 45 J/g o. C) DT 10450 J= 450 DT DT = 23. 2 o. C
E. Calorimetry Every substance has a unique set of characteristics that can help us identify it. One of those characteristics is a substance’s specific heat capacity. If we can determine how much heat an unknown substance absorbs, we can identify it by looking up its specific heat.
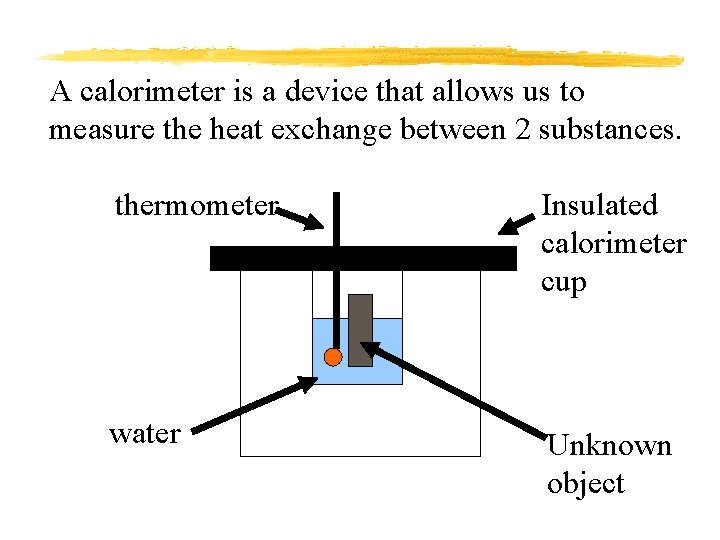
A calorimeter is a device that allows us to measure the heat exchange between 2 substances. thermometer Insulated calorimeter cup water Unknown object
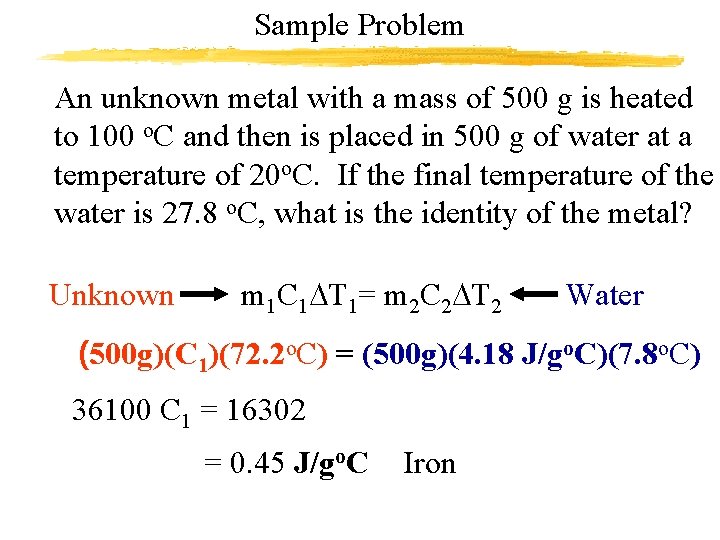
Sample Problem An unknown metal with a mass of 500 g is heated to 100 o. C and then is placed in 500 g of water at a temperature of 20 o. C. If the final temperature of the water is 27. 8 o. C, what is the identity of the metal? Unknown m 1 C 1 DT 1= m 2 C 2 DT 2 Water (500 g)(C 1)(72. 2 o. C) = (500 g)(4. 18 J/go. C)(7. 8 o. C) 36100 C 1 = 16302 = 0. 45 J/go. C Iron
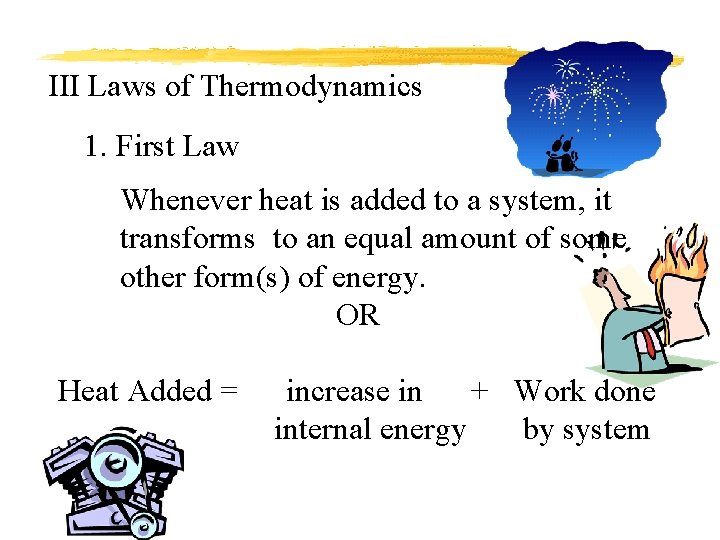
III Laws of Thermodynamics 1. First Law Whenever heat is added to a system, it transforms to an equal amount of some other form(s) of energy. OR Heat Added = increase in + Work done internal energy by system
2. Second Law of Thermodynamics An object or system will naturally proceed from a state of order to disorder (will break down, become unorganized). Disorder is also known as ENTROPY
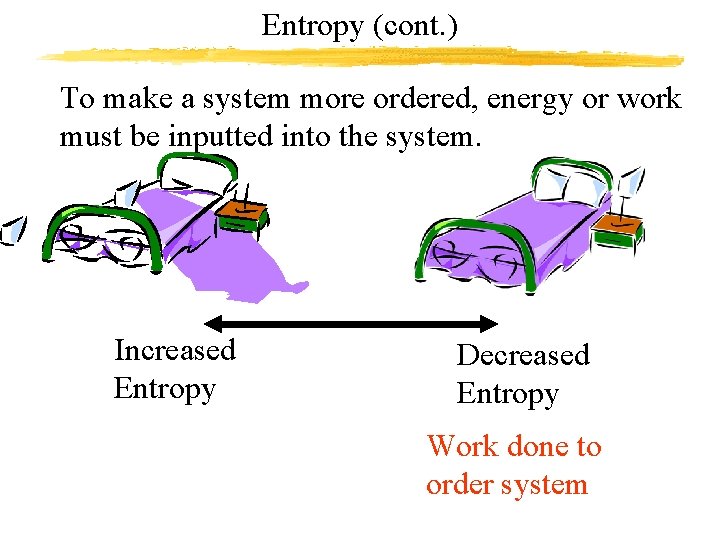
Entropy (cont. ) To make a system more ordered, energy or work must be inputted into the system. Increased Entropy Decreased Entropy Work done to order system
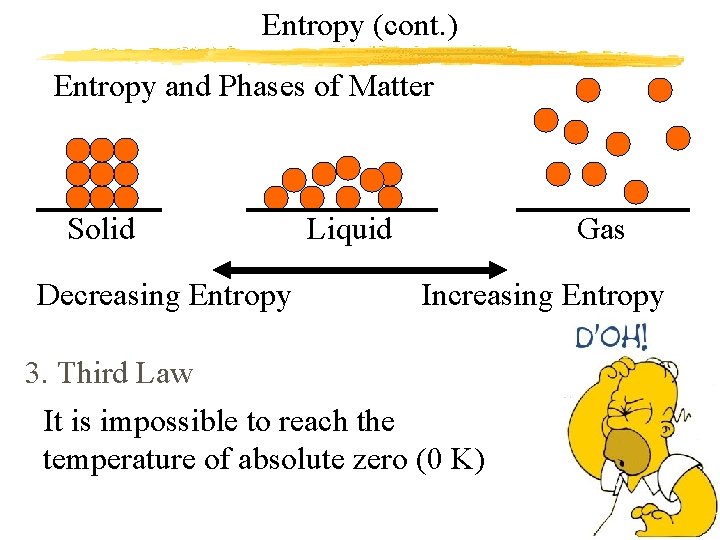
Entropy (cont. ) Entropy and Phases of Matter Solid Decreasing Entropy Liquid Gas Increasing Entropy 3. Third Law It is impossible to reach the temperature of absolute zero (0 K)
- Slides: 41