INTERMOLECULAR FORCES What Holds Molecules to Each Other



















- Slides: 34

INTERMOLECULAR FORCES What Holds Molecules to Each Other

Types of Bonds l Recall that there are three fundamental types of bonding. 1) Ionic bonding 2) Covalent bonding 3) Metallic bonding

Types of Bonds Because ionic and covalent bonding uses electrostatic attractions between areas of full charge, the resulting force of attraction is strong. l Ionic bonds are held together by attractions between cations and anions. l

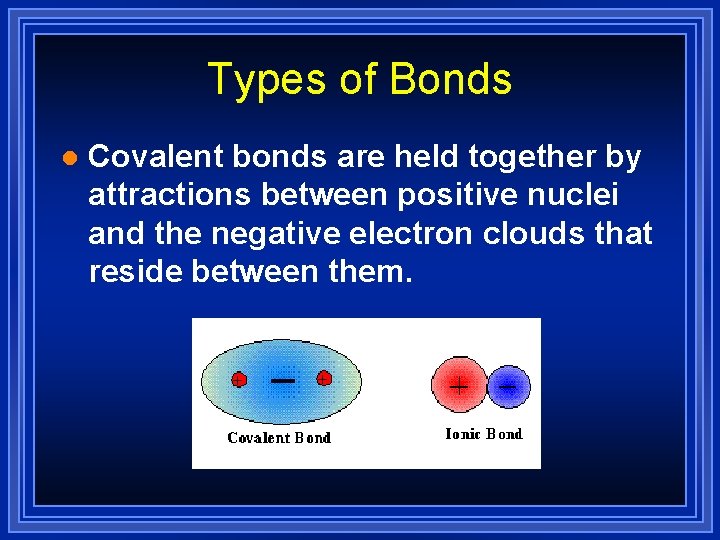
Types of Bonds l Covalent bonds are held together by attractions between positive nuclei and the negative electron clouds that reside between them.

Types of Bonds l The results of these bonding processes are the strongest, commonly used, mechanisms for attaching atoms to one another.

Intermolecular Forces Intermolecular forces are a secondary method of holding a structure together. l As the name implies, these are forces that exist BETWEEN molecules. Bonds exist WITHIN molecules. l

Intermolecular Forces Intermolecular forces are only associated with systems that use covalent bonding within the molecules. l Intermolecular forces are not encountered in systems that employ ionic bonding. l

Intermolecular Forces Some elements, such as the Noble Gases, exist with intermolecular forces and no bonding at all. l Intermolecular forces are what make solid and liquid molecular compounds possible. l

Intermolecular Forces Intermolecular forces exist in three different levels of strength. l The three intermolecular forces (from strongest to weakest) are hydrogen bonding, dipole–dipole forces and London dispersion forces. l

Intermolecular Forces Polar molecules will have a partially positive side and a partially negative side, or a dipole. l The partial positive on one molecule will be attracted to the partial negative on a second molecule. l This attraction is an intermolecular force. l

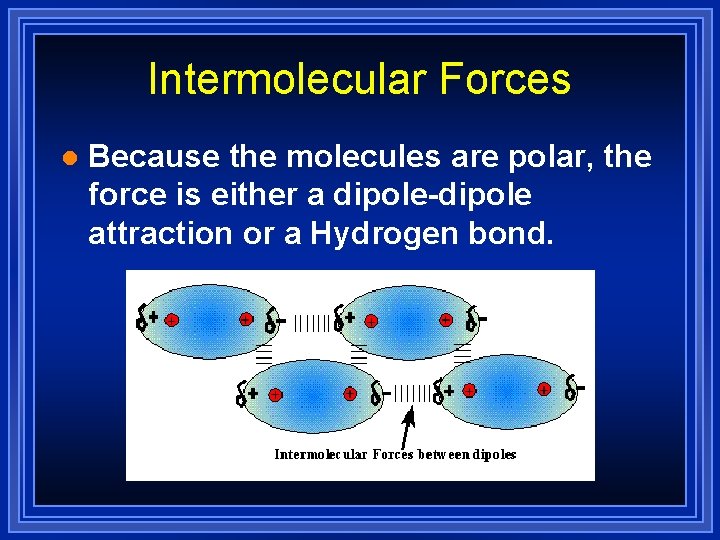
Intermolecular Forces l Because the molecules are polar, the force is either a dipole-dipole attraction or a Hydrogen bond.

Intermolecular Forces Because these attractions are between areas of partial charge, they will produce weak forces of attraction. l A system that has this mechanism holding the structure together will break up relatively easily. l

Intermolecular Forces It will always break at the weak links – the dipole-dipole forces or Hydrogen bonds. l The covalent bonds will remain intact. l

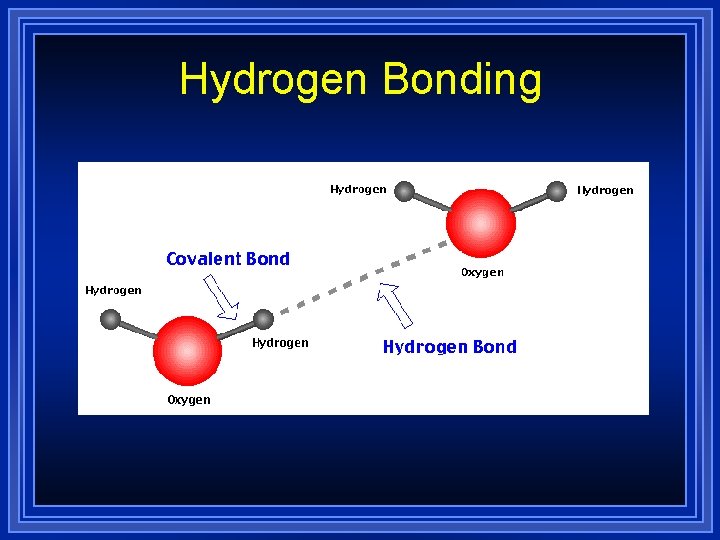
Hydrogen Bonding The difference between dipole-dipole forces and Hydrogen bonding is subtle. l When hydrogen is directly bonded to nitrogen, oxygen or fluorine, then the system will be capable of Hydrogen bonding. l

Hydrogen Bonding In these systems, the difference between the electronegativity values of the bonded atoms will produce fairly large partial charges. l As a result, the resulting intermolecular forces will be strong. They will still not be as strong as a true bond, however. l

Hydrogen Bonding


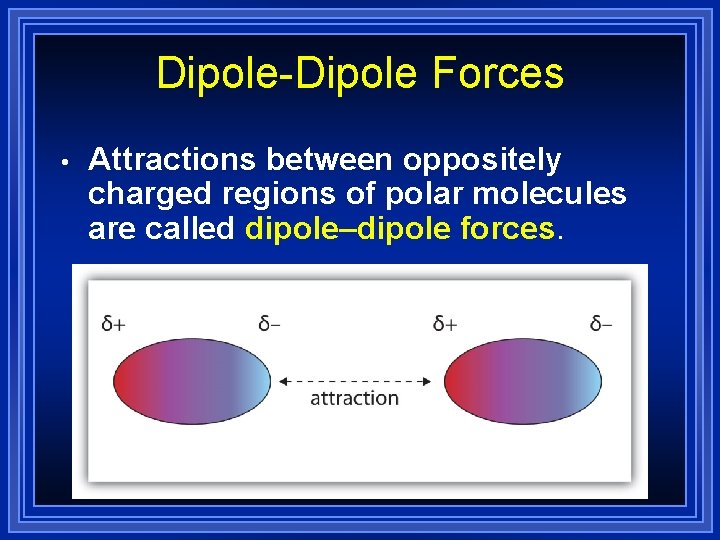
Dipole-Dipole Forces • Attractions between oppositely charged regions of polar molecules are called dipole–dipole forces.

Dipole-Dipole Forces • Dipole-dipole forces exclude those molecules covered by Hydrogen bonding. • Hydrogen bonding intermolecular forces are about 10 times stronger than dipole-dipole forces because they involve large differences in electronegativity, thus creating a large electric dipole.

Dipole-Dipole Forces Dipole-dipole forces depend on the number of electrons. l Bigger molecules result in more electrons, and more electrons mean stronger forces. l

London Dispersion Forces l Dispersion forces are weak forces that result from temporary shifts in the density of electrons in electron clouds.

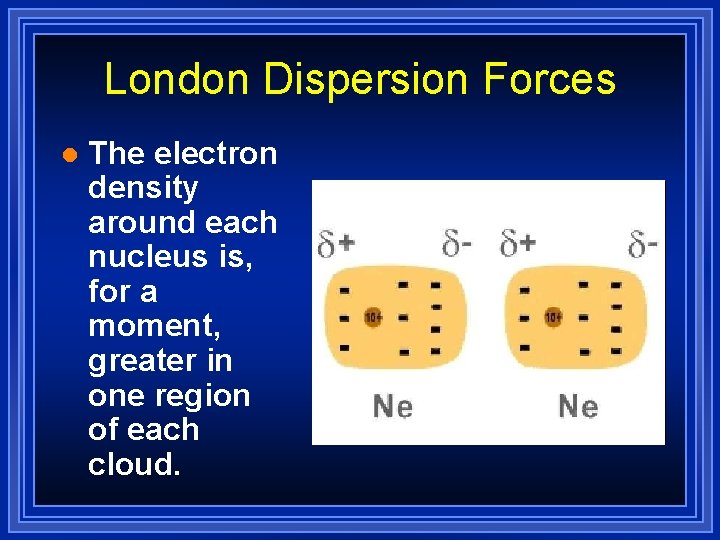
London Dispersion Forces l The electron density around each nucleus is, for a moment, greater in one region of each cloud.

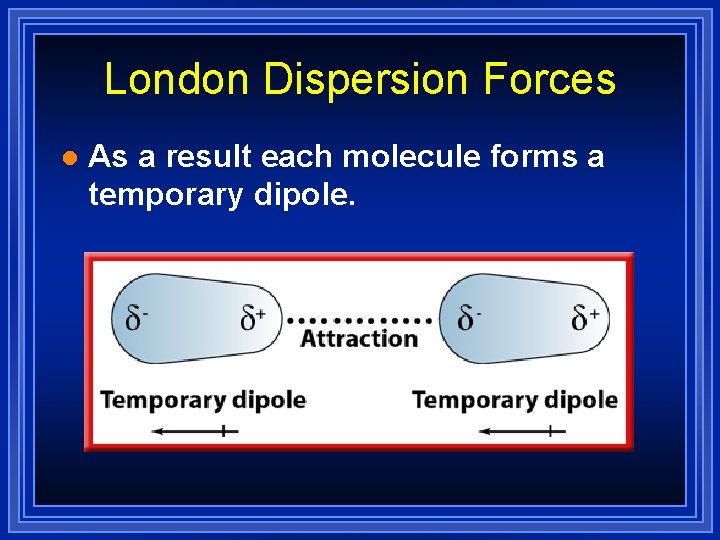
London Dispersion Forces l As a result each molecule forms a temporary dipole.

London Dispersion Forces When temporary dipoles are close together, a weak dispersion force exists between oppositely charged regions of the dipoles. l Due to the temporary nature of the dipoles, dispersion forces are the weakest intermolecular force. l

London Dispersion Forces l Dispersion forces exist between noble gases and compounds that are nonpolar.

London Dispersion Forces Dispersion forces increase as the mass of the molecule increases. l C 2 H 6 (MW = 30. 0 g/mol) has stronger dispersion forces than CH 4 (MW = 16. 0 g/mol). l

Intermolecular Forces l To determine what type of intermolecular force a compound has, ask yourself the following questions. 1) Does the compound contain hydrogen attached to N, O, or F? Ø If yes, the force is hydrogen bonding.

Intermolecular Forces Determine the number of bonds from the Wetter Way and draw the dashdot diagram.

Intermolecular Forces 2) Does the central element of the compound contain any lone pairs of electrons or are different elements attached to the central atom? Ø If yes, the force is dipole. Ø If no, the force is London dispersion.

Problem 1) Determine the type of intermolecular force in each of the following compounds. a) BCl 3 dispersion b) Xe dispersion

Problem l Determine the type of intermolecular force in each of the following compounds. c) NH 3 hydrogen bonding d) CH 4 dispersion

Problem l Determine the type of intermolecular force in each of the following compounds. e) SO 2 dipole-dipole f) H 2 dispersion

Problem l Determine the type of intermolecular force in each of the following compounds. g) SO 3 dispersion h) CH 3 Cl dipole-dipole

Problem l Determine the type of intermolecular force in each of the following compounds. i) HF hydrogen bonding j) HBr dipole-dipole