Intermolecular forces Liquids and Solids Chapter objectives Understand

Intermolecular forces Liquids and Solids

Chapter objectives • Understand the three intermolecular forces in pure liquid in relation to molecular structure/polarity • Understand the physical properties of liquids that is relevant to intermolecular force: vapor pressure and boiling 2

How Gecko can climb upside down on the ceiling? • Gecko are capable of climbing vertical or even upsidedown, utilizing micrometer size hairs to adhere to surfaces: Intermolecular force. • New research showed gecko can turn on and off the “stickiness”. 3
Intermolecular forces affect physical properties of solid and liquid • Stronger intermolecular force in the liquid prevent liquid molecules from escaping into gas state Molecules need more heat energy to escape from the liquid (higher temperature) Leading to ____ (higher, lower) boiling point. 4

Intermolecular forces in Pure liquids • Dipole-dipole force • Dispersion force (aka London force) • Hydrogen bonding 5
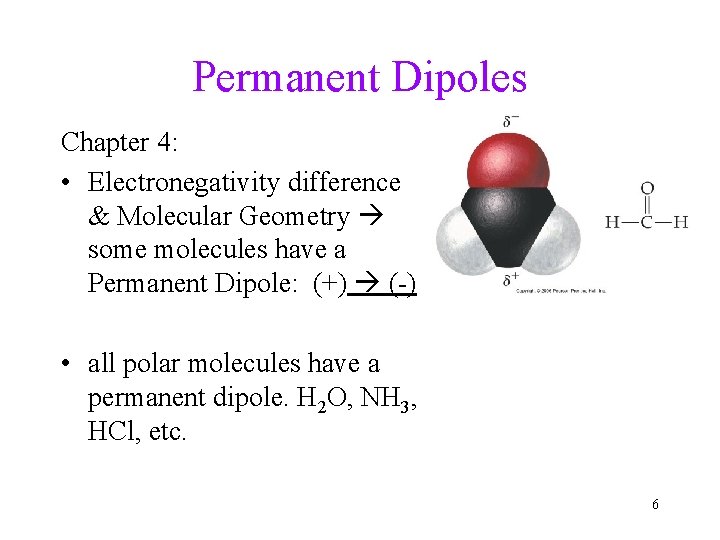
Permanent Dipoles Chapter 4: • Electronegativity difference & Molecular Geometry some molecules have a Permanent Dipole: (+) (-) • all polar molecules have a permanent dipole. H 2 O, NH 3, HCl, etc. 6
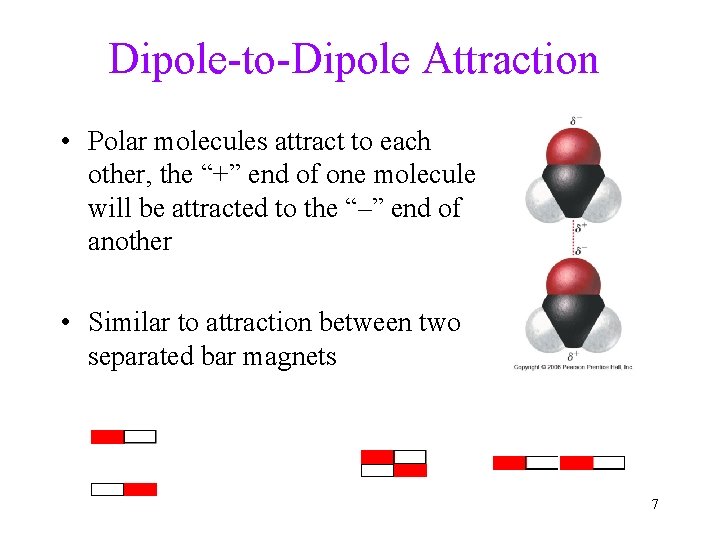
Dipole-to-Dipole Attraction • Polar molecules attract to each other, the “+” end of one molecule will be attracted to the “–” end of another • Similar to attraction between two separated bar magnets 7

Polar Molecules Can be attracted by Charge • Demo: https: //www. youtube. com/watch? v=jk. Yz 1 Wlp. RSQ • Note: Only works for polar liquid. Nonpolar liquid, such as gasoline, doesn’t work. • When two polar molecules are coming close, there are both attraction and repulsion between them. • Since the Coulombic force depends on the distance between the charge, the attraction between two polar molecules is STRONGER than repulsion, leading to intermolecular attractive force. 8
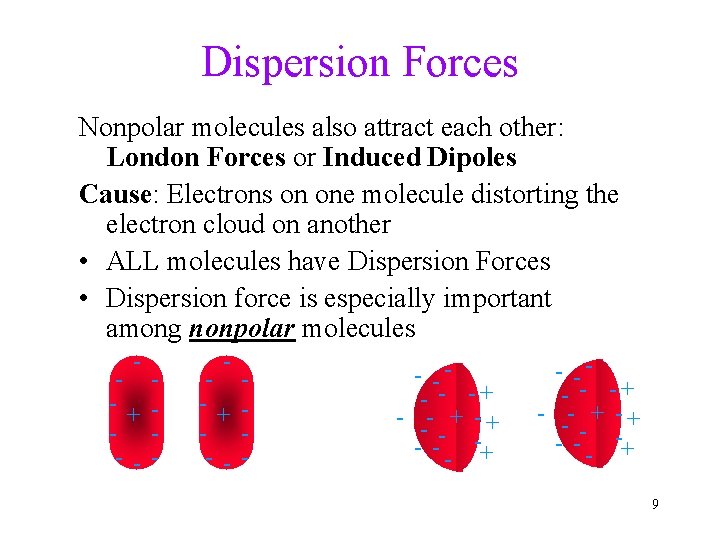
Dispersion Forces Nonpolar molecules also attract each other: London Forces or Induced Dipoles Cause: Electrons on one molecule distorting the electron cloud on another • ALL molecules have Dispersion Forces • Dispersion force is especially important among nonpolar molecules - - + - - - - -- - -+ - --- -+ - -- - -+ - - + -+ - -- + 9
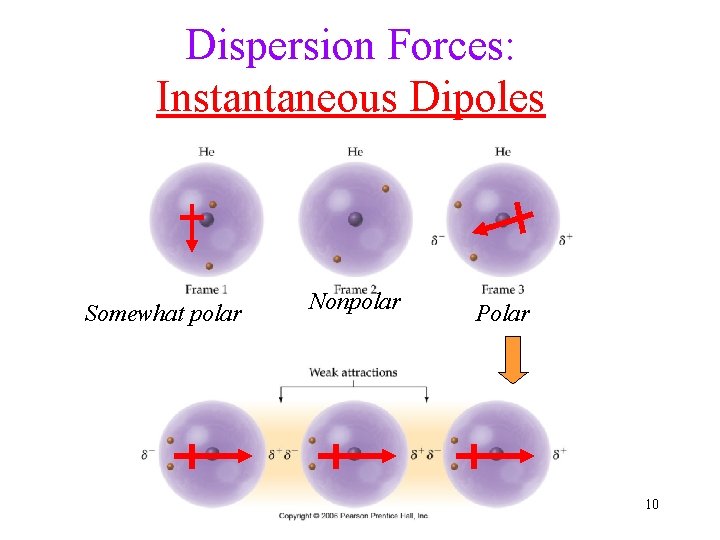
Dispersion Forces: Instantaneous Dipoles Somewhat polar Nonpolar Polar 10

Dispersion Force Depends on Structure and #electrons • Strength of the dispersion force gets Larger with larger molecules • Electron mobility: how easily the electrons can move within a molecule, or be polarized. =O < =S -F < -Cl < -I • more electrons + electron farther from the nuclei the larger the dipole that can be induced 11
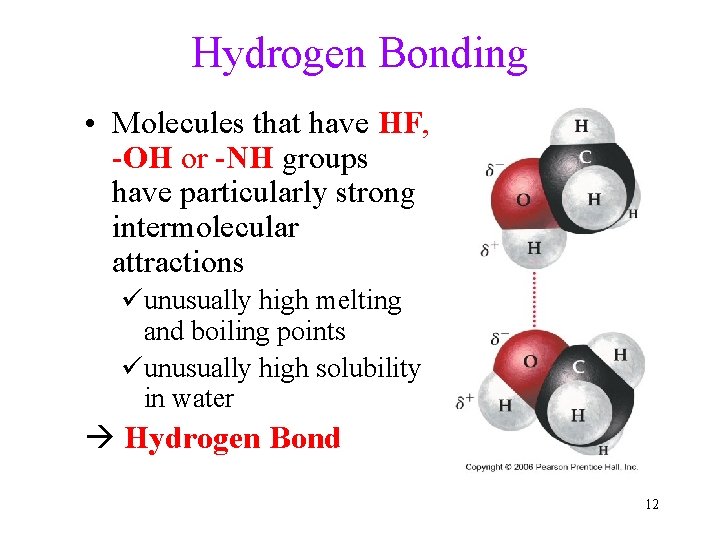
Hydrogen Bonding • Molecules that have HF, -OH or -NH groups have particularly strong intermolecular attractions üunusually high melting and boiling points üunusually high solubility in water Hydrogen Bond 12
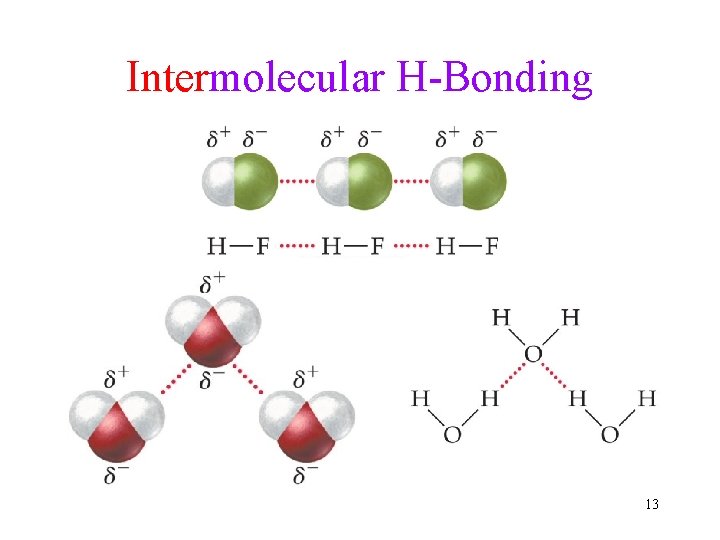
Intermolecular H-Bonding 13
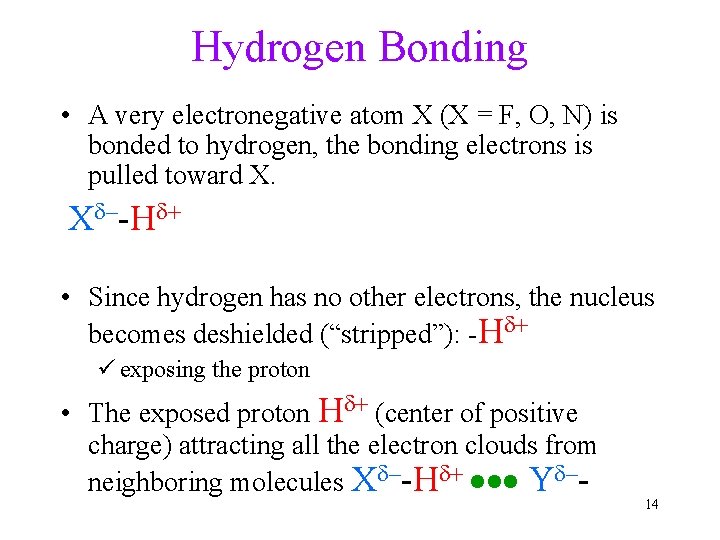
Hydrogen Bonding • A very electronegative atom X (X = F, O, N) is bonded to hydrogen, the bonding electrons is pulled toward X. Xd–-Hd+ • Since hydrogen has no other electrons, the nucleus becomes deshielded (“stripped”): -Hd+ ü exposing the proton • The exposed proton Hd+ (center of positive charge) attracting all the electron clouds from neighboring molecules Xd–-Hd+ Yd–- 14
H-Bonds vs. Chemical Bonds • Hydrogen bonds are not chemical bonds • Hydrogen bonds are attractive forces between molecules • Chemical bonds are attractive forces that make molecules 15
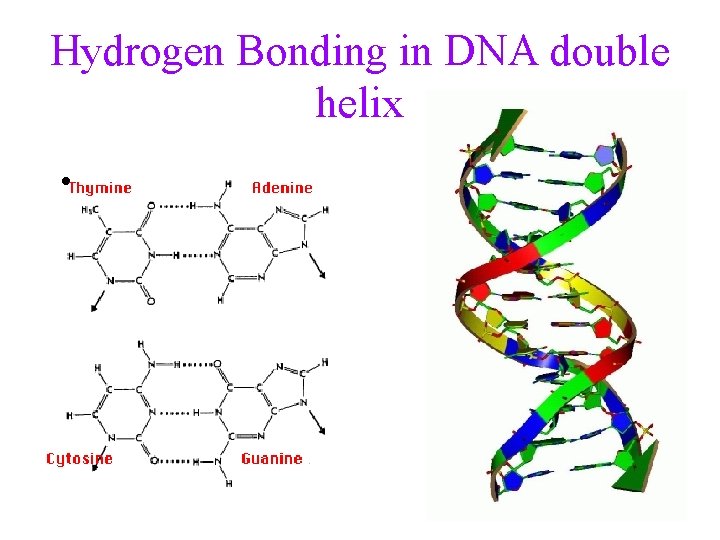
Hydrogen Bonding in DNA double helix • 16
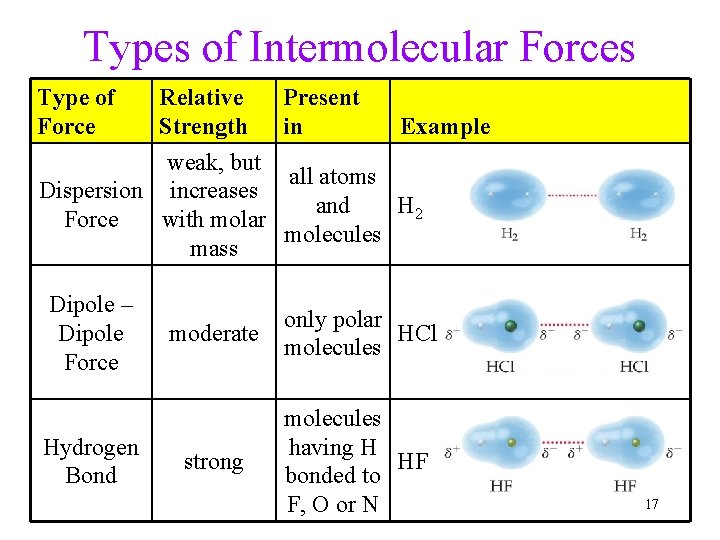
Types of Intermolecular Forces Type of Force Relative Strength weak, but Dispersion increases Force with molar mass Dipole – Dipole Force Hydrogen Bond Present in Example all atoms and H 2 molecules moderate only polar HCl molecules strong molecules having H HF bonded to F, O or N 17

Surface Tension Surface tension: Tendency of liquid to minimize total surface. • Cause: Intermolecular forces between molecules on the surface • Results: Objects denser than water can float on water. 18

Viscosity: Internal friction within the liquid. • Strong intermolecular forces slows down molecular movement. • Viscous liquid takes longer to flow. • Honey has higher viscosity than water • Used motor oil has less viscosity 19
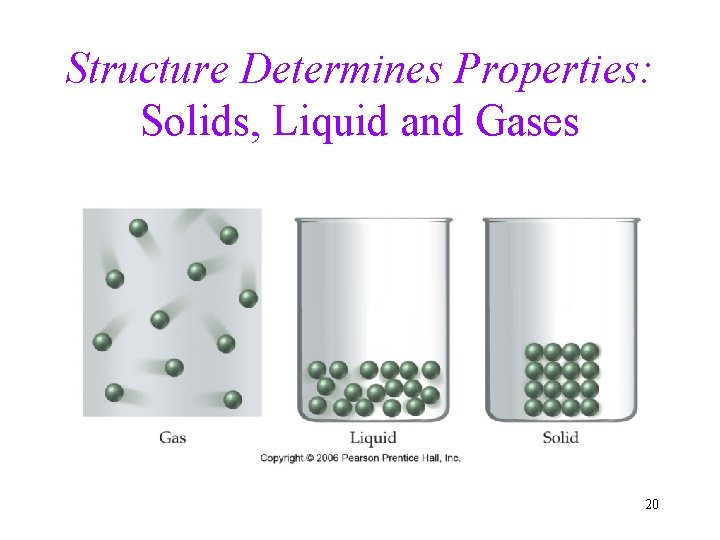
Structure Determines Properties: Solids, Liquid and Gases 20
Intermolecular forces Affect the Physical States • The state a material exists in depends on the attraction between molecules and their ability to overcome the attraction • The attractive forces between Ions or Molecules Their structure ü the attractions are electrostatic ü depend on shape, polarity, etc. • The ability of the molecules to overcome the attraction Kinetic energy they possess 21

Evaporation: Escaping from the Surface • Evaporation : molecules of a liquid breaking free from the surface: Liquid Gas üalso known as vaporization • Physical change • a substance is converted from its liquid form to its gaseous form üthe gaseous form is called a vapor 22

Evaporation: Liquid Gas • Molecules of the liquid mix with and dissolve in the air • happens at the surface • molecules on the Surface experience a smaller net attractive force than molecules in the Interior • but all the surface molecules do not escape at once, only the ones with sufficient kinetic energy to overcome the attractions will escape 23

Condensation: Gas Liquid • in a closed container, after a liquid evaporates, the vapor molecules are trapped and may eventually turn into liquid • Condensation : the vapor molecules may eventually bump into and stick to the surface of the container or get recaptured by the liquid. ü Physical change : Gas Liquid 24
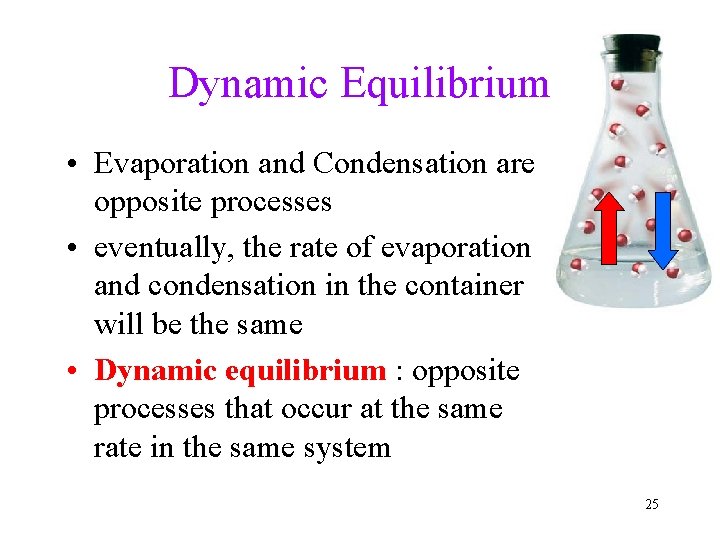
Dynamic Equilibrium • Evaporation and Condensation are opposite processes • eventually, the rate of evaporation and condensation in the container will be the same • Dynamic equilibrium : opposite processes that occur at the same rate in the same system 25
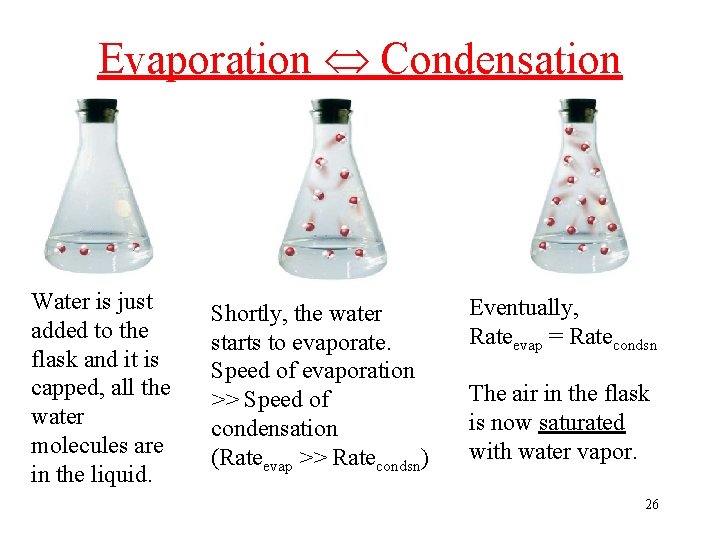
Evaporation Condensation Water is just added to the flask and it is capped, all the water molecules are in the liquid. Shortly, the water starts to evaporate. Speed of evaporation >> Speed of condensation (Rateevap >> Ratecondsn) Eventually, Rateevap = Ratecondsn The air in the flask is now saturated with water vapor. 26
Vapor Pressure Pvap • once equilibrium is reached, then the amount of vapor (mole nvap) in the container will remain the same • Vapor pressure: Pressure exerted by the vapor of the liquid when equilibrium is reached between liquid and gas states. • Depending on the temperature and strength of intermolecular forces (IMF): Stronger IMF, more ______ for liquid to become vapor, ______ (higher, lower) Pvap. 27
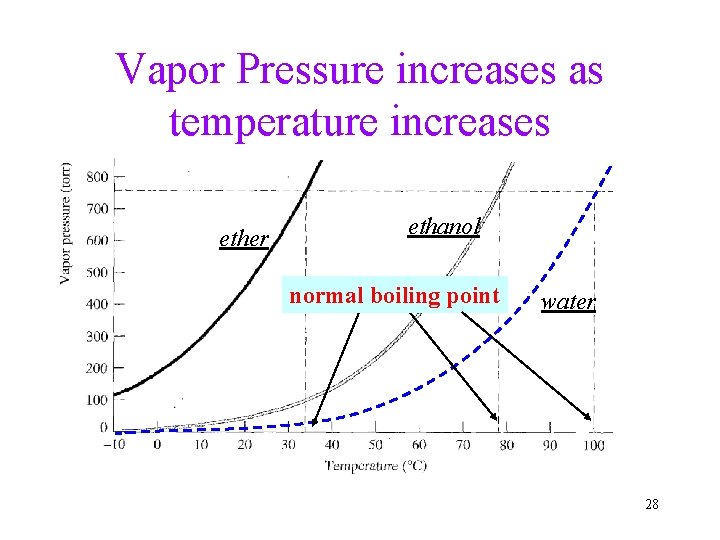
Vapor Pressure increases as temperature increases ether ethanol normal boiling point water 28
Boiling and Boiling Point (b. p. ) • Boiling: vapor pressure of the liquid is the same as the atmospheric pressure. Liquid Gas. Pvap = Pair • Boiling point: the temperature for boiling process ü normal boiling point: temperature when Pair = 1 atm b. p. of water is 100°C • b. p. depends on Pair ü the temperature of boiling water on the top of a mountain will be cooler than boiling water at sea level ü On top of Mount Whitney, b. p. of water is about 84°C 29

Vapor pressure at given temperature vs. Normal Boiling point • At the same temperature, different liquids have different vapor pressure (volatility) • Liquids having higher vapor pressure are normally called “more volatile” • Liquids having higher vapor pressure will have lower normal boiling points 30

Energy flow: Evaporation vs. Condensation Evaporation: Liquid absorbs heat from its surroundings to evaporate The surroundings cool off • Endothermic: heat flows into a system from the surroundings ü as alcohol evaporates off your skin, it causes your skin to cool Condensation: Gas releases heat to its surroundings to reduce its temperature The surroundings warms up • Exothermic: heat flows out of a system into the surroundings 31
Sublimation vs. Deposition • Sublimation: the Solid form changes directly to the Gaseous form. Solid Gas ü without going through the liquid form ü Dry ice (solid CO 2) gas CO 2 • like melting, sublimation is endothermic • Deposition is the reverse of Sublimation, exothermic. 32
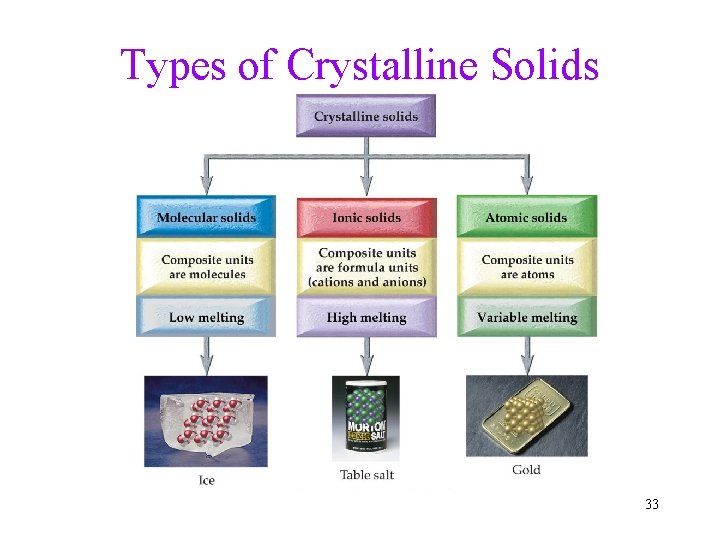
Types of Crystalline Solids 33

Molecular Crystalline Solids • Molecular solid: composite units are molecules. CO 2 H 2 O CO 2 • Held together by intermolecular attractive forces ü dispersion, dipole-dipole, or H -bonding • generally low melting points and DHfusion 34

Ionic Crystalline Solids • Ionic solids: composite units are formula units. Na. Cl Na+ Cl– • Held together by Electrostatic forces between Cation+ and Anion– ü arranged in a geometric pattern called a crystal lattice to maximize attractions • generally higher melting points and DHfusion than molecular solids ü because ionic bonds are stronger than intermolecular forces 35

Atomic Crystalline Solids • Atomic solids: composite units are individual atoms Xe Xe • Held together by either covalent bonds, dispersion forces or metallic bonds • melting points and DHfusion vary depending on the attractive forces between the atoms 36
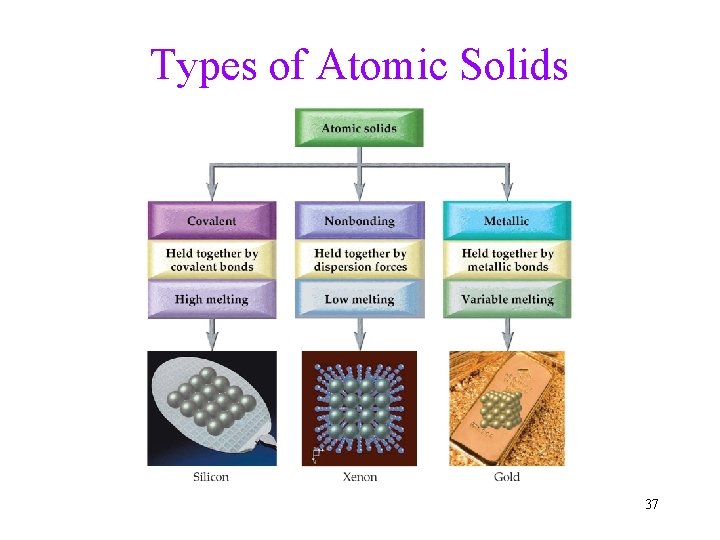
Types of Atomic Solids 37
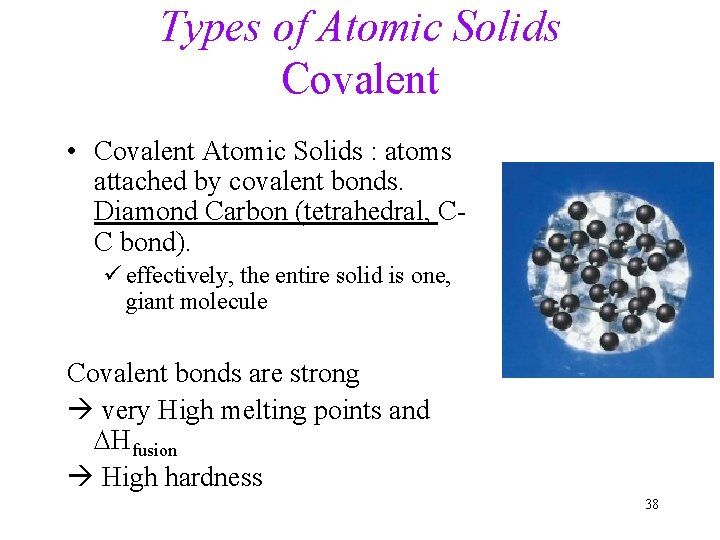
Types of Atomic Solids Covalent • Covalent Atomic Solids : atoms attached by covalent bonds. Diamond Carbon (tetrahedral, CC bond). ü effectively, the entire solid is one, giant molecule Covalent bonds are strong very High melting points and DHfusion High hardness 38
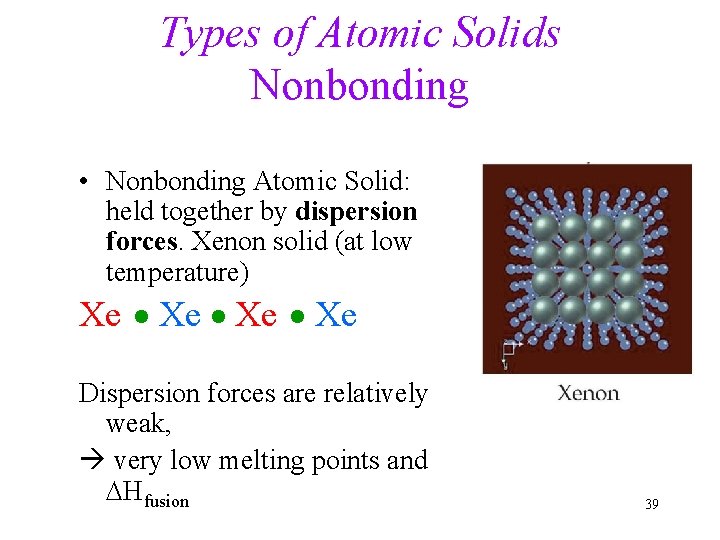
Types of Atomic Solids Nonbonding • Nonbonding Atomic Solid: held together by dispersion forces. Xenon solid (at low temperature) Xe Xe Dispersion forces are relatively weak, very low melting points and DHfusion 39

Types of Atomic Solids Metallic • Metallic solids: held together by metallic bonds • How: metal atoms release some of their electrons to be shared by all the other atoms in the crystal • Metallic bond: the attraction of the metal Cations M+ for the mobile electrons eü often described as “islands of cations in a sea of electrons” 40

Water: A Unique and Important Substance • found in all 3 states on the Earth: Ice, Liquid, Vapor • the most common solvent (liquid) found in nature • without water, life as we know it could not exist ü the search for extraterrestrial life starts with the search for water • relatively high boiling point • expands as it freezes ü most substances contract as they freeze ü causes ice to be less dense than liquid water 41
- Slides: 41