Intermolecular Forces Liquids and Solids Chapter 11 Brown

Intermolecular Forces, Liquids, and Solids Chapter 11 – Brown & Le. May

Temperature Review Measure of kinetic energy n What can you say about the KE of salt particles, water molecules, and oxygen particles at room temperature? n State determined by strength of forces that keep particles together n

Strength Compare energy needed for phase change vs. decomposition in HCl(l) n Intermolecular (called weak) because they are weaker than ionic or covalent n Boiling point reflects strength of bonds in liquid n Melting point reflects strength of bonds in solids n

Kinds of Intermolecular Forces Three major kinds: dipole-dipole, London dispersion, and hydrogen bonding n In solutions, ion-dipole n All are electrostatic in nature n Approximately 15% of covalent or ionic strength n

Ion - dipole When? n Ionic solid + polar liquid n Increases with increasing charge of ion or polarity of solvent n Determines solubility n

Dipole-Dipole forces Weaker than previous n + end of one attracts – end of another n If size is equal, more polar has stronger dipole attractions. (NH 3 vs H 2 O) n If polarity is the same but masses differ, than smallest is stronger. (Able to orient better) n

London Dispersion Forces n n n n All molecules have this Only attraction in nonpolar molecules How can Iodine be a solid? Temporary lopsided charge builds up from random motion of electrons - 1930 Increases with mass – we say it has greater polarizability Straight molecule is more polarizable than a curled up molecule – why? Halogen Family is a great essay

Hydrogen Bond Strongest of all “weak” forces n Is caused when H is bonded to F, O, or N n These are so electronegative that the H is a “naked nucleus” or bare proton n Very attractive! n Will bond to nearby electron pairs n
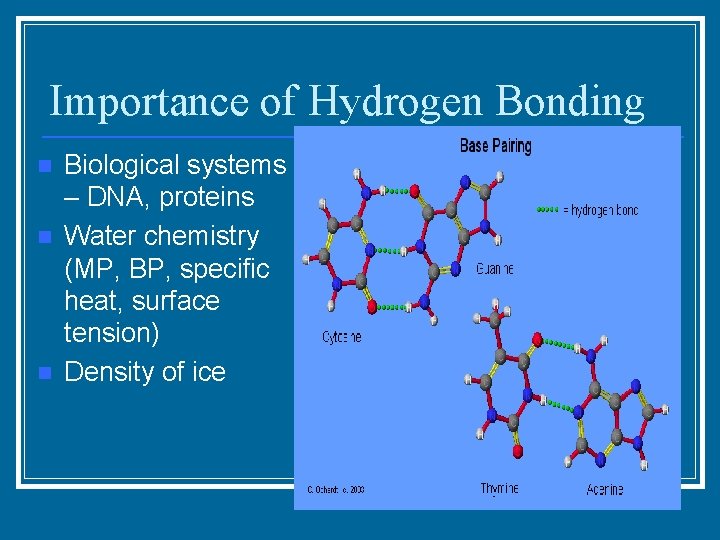
Importance of Hydrogen Bonding n n n Biological systems – DNA, proteins Water chemistry (MP, BP, specific heat, surface tension) Density of ice
Density n n n Most solids are more dense than liquid Water is less dense because of hydrogen bonding At 4°C, water becomes less dense Important for life in winter Causes lake turnover Alum example

Practice n Look at Flow Chart

Properties of Liquids n n n Viscosity “Slower than…. . Resistance of a liquid to flow Time it as it goes through a small tube with gravity acting upon it. Poise – 1 g/cm-s Trends – same substance – decreases with increasing temperature series (same structure) – increases with increasing mass

Surface Tension n n How many drops on a penny? Uneven forces at surface Acts like pond scum Definition – energy needed to increase the surface area of a liquid by a certain amount § Water is high – why? n n Called “cohesive” force – together Water moving up a stem – adhesive force Capillary acion – rise up a thin tube Meniscus!

Phase Changes Solid to Liquid is called Heat of Fusion Hfus For water, 6 k. J/mol Liquid to Gas is called Heat of Vaporization Hvap For water, 40. 7 k. J/mol Hsub is sum of each n

Heating Curve Try a problem n Remember - flat during phase change, temperature change when heating a single phase n Cooling is opposite n

Supercooling Happens with some liquids - remove heat and it doesn’t freeze when it should n Very unstable n May happen during hibernation n
Critical Temperature Highest temperature at which a liquid can form from a gas when pressure is applied. n Above this, the substance is called a supercritical fluid. n Gas just becomes more compressed. n Critical pressure - pressure at the critical temperature n

Vapor pressure forms above any liquid if container is closed – why? n Equilibrium is reached n This is vapor pressure n Higher if forces holding liquid together are weak - called a volatile (fleeing) liquid n
Boiling Point Temperature at which the VP equals atmospheric pressure n Normal BP - boiling point at 1 atm n Everest? Autoclave? n
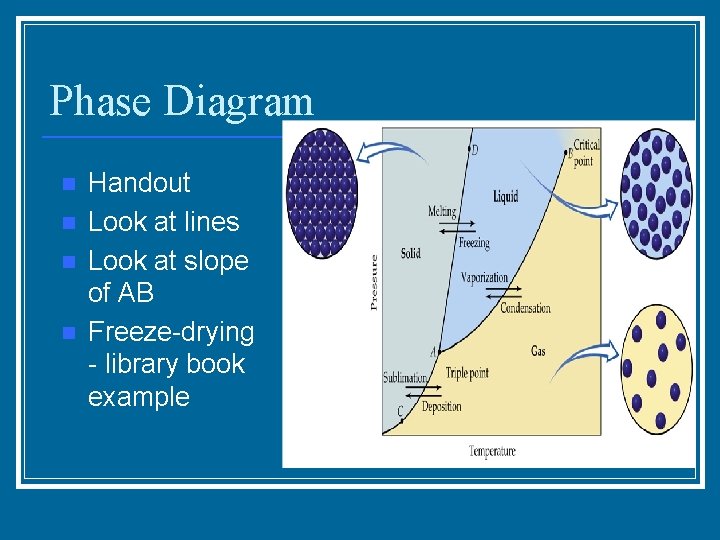
Phase Diagram n n Handout Look at lines Look at slope of AB Freeze-drying - library book example
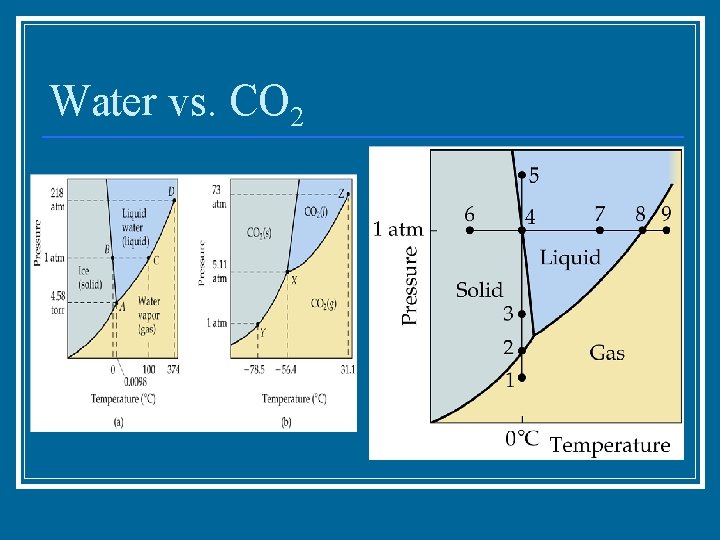
Water vs. CO 2

Structure of Solids Amorphous (rubber, plastics) - large or mixtures - no true structure n Crystalline - highly ordered structure n Crystalline solids have true melting points n
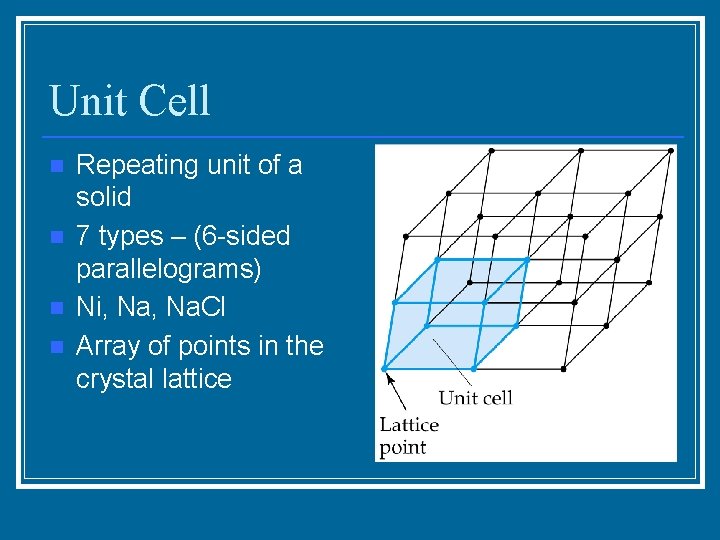
Unit Cell n n Repeating unit of a solid 7 types – (6 -sided parallelograms) Ni, Na. Cl Array of points in the crystal lattice
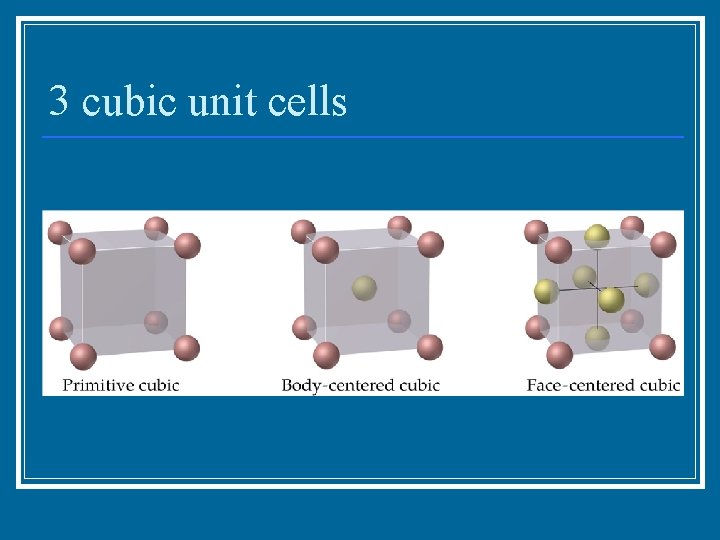
3 cubic unit cells
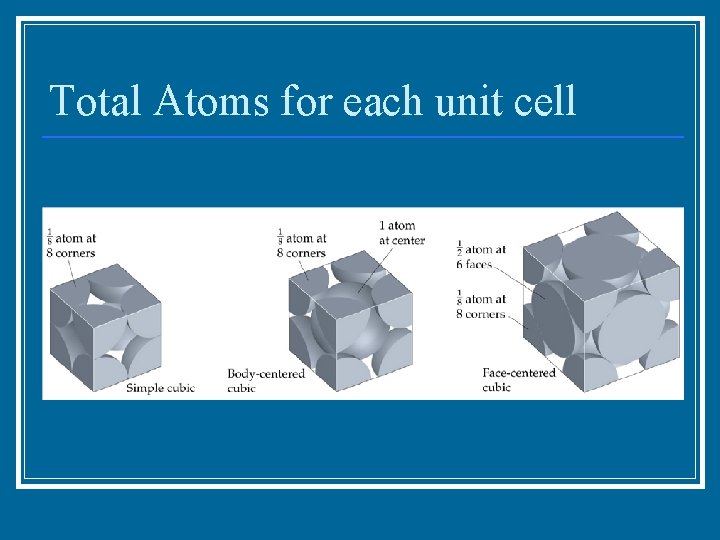
Total Atoms for each unit cell
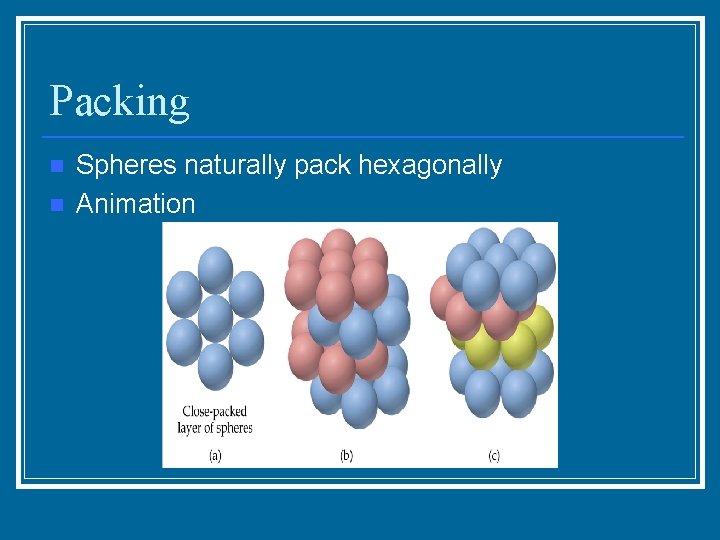
Packing n n Spheres naturally pack hexagonally Animation

Bonding n n n n Shown by x-ray diffraction Molecular - low MP If unit packs well, mp can be high Covalent Network Solid - very strong Many covalent bonds in 3 -D Diamond, graphite, Si. O 2, Si. C, BN Ionic - greater charge, greater MP Metallic solids - hexagonal close packed, mp varies
- Slides: 27