INTERMOLECULAR FORCES Introduction l The physical properties of
























































- Slides: 138

INTERMOLECULAR FORCES Introduction: l. The physical properties of melting point, boiling point, vapor pressure, evaporation, viscosity, surface tension, and solubility are related to the strength of attractive forces between molecules. l. These attractive forces are called Intermolecular Forces. The amount of "stick togetherness" is important in the interpretation of the various properties listed above. l. There are four types of intermolecular forces. Most of the intermolecular forces are identical to bonding between atoms in a single molecule. Intermolecular forces just extend the thinking to forces between molecules and follows the patterns already set by the bonding within molecules.

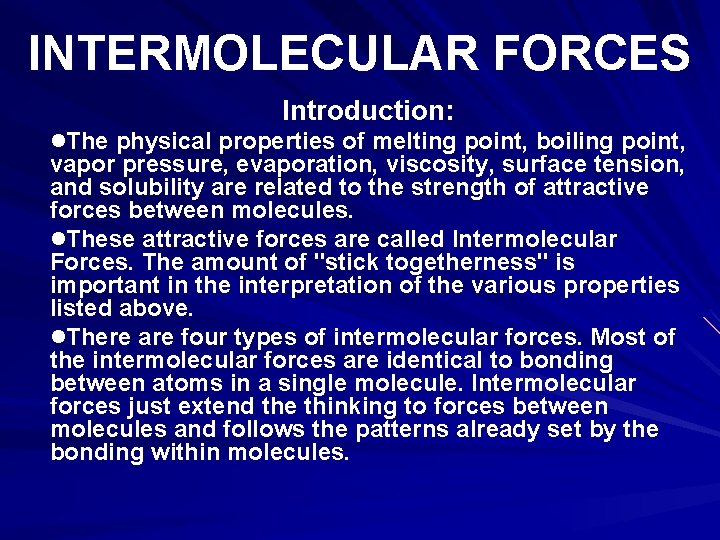
1. IONIC FORCES The forces holding ions together in ionic solids are electrostatic forces. Opposite charges attract each other. These are the strongest intermolecular forces. Ionic forces hold many ions in a crystal lattice structure.

IONIC FORCES

Ion - Ion Interactions §Two oppositely-charged particles flying about in a vacuum will be attracted toward each other, and the force becomes stronger and stronger as they approach until eventually they will stick together and a considerable amount of energy will be required to separate them. §They form an ion-pair, a new particle which has a positively-charged area and a negatively-charged area. § There are fairly strong interactions between these ion pairs and free ions, so that these the clusters tend to grow, and they will eventually fall out of the gas phase as a liquid or solid (depending on the temperature).

Ion - Ion Interactions in the Gas Phase

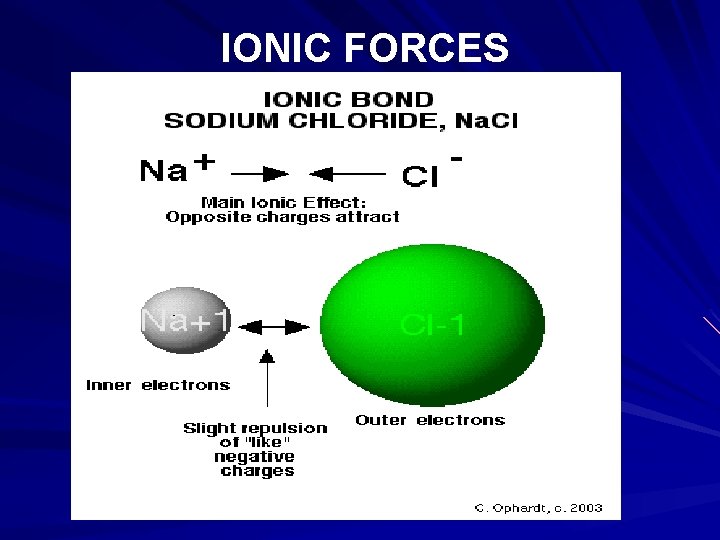
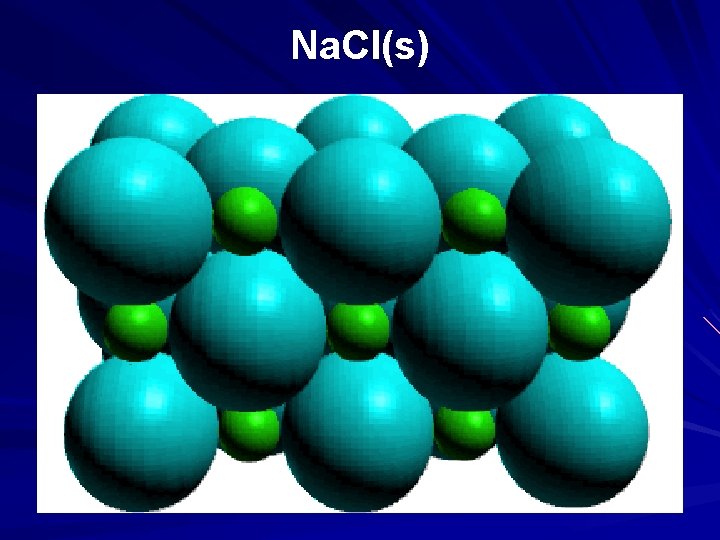
A small representative bit of a sodium chloride lattice

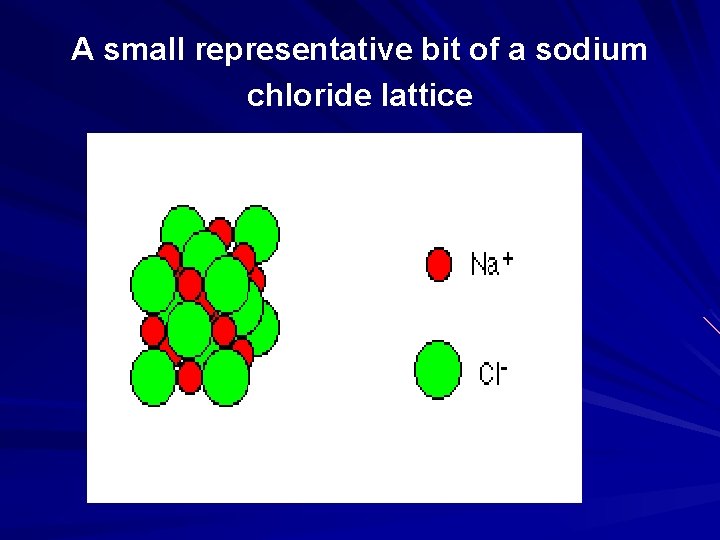
exploded version of a sodium chloride lattice

Na. Cl(s)

Ionic Bonding Ionic bonding is best treated using a simple electrostatic model. The electrostatic model is simply an application of the charge principles that opposite charges attract and similar charges repel. An ionic compound results from the interaction of a positive and negative ion, such as sodium and chloride in common salt. The IONIC BOND results as a balance between the force of attraction between opposite plus and minus charges of the ions and the force of repulsion between similar negative charges in the electron clouds. In crystalline compounds this net balance of forces is called the LATTICE ENERGY. Lattice energy is the energy released in the formation of an ionic compound.

Ionic Bonding DEFINITION: The formation of an IONIC BOND is the result of the transfer of one or more electrons from a metal onto a non-metal. Metals, with only a few electrons in the outer energy level, tend to lose electrons most readily. The energy required to remove an electron from a neutral atom is called the IONIZATION POTENTIAL. Energy + Metal Atom ---> Metal (+) ion + e- Non-metals, which lack only one or two electrons in the outer energy level have little tendency to lose electrons - the ionization potential would be very high. Instead non-metals have a tendency to gain electrons. The ELECTRON AFFINITY is the energy given off by an atom when it gains electrons. Non-metal Atom + e- --- Non-metal (-) ion + energy The energy required to produce positive ions (ionization potential) is roughly balanced by the energy given off to produce negative ions (electron affinity). The energy released by the net force of attraction by the ions provides the overall stabilizing energy of the compound.

METALLIC STRUCTURES What is a metallic bond? Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Even a metal like sodium (melting point 97. 8°C) melts at a considerably higher temperature than the element (neon) which precedes it in the Periodic Table.

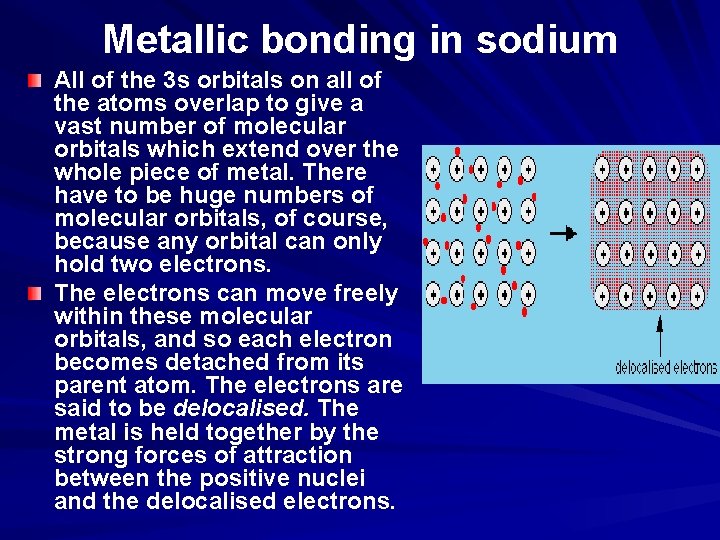
Metallic bonding in sodium Sodium has the electronic structure 1 s 22 p 63 s 1. When sodium atoms come together, the electron in the 3 s atomic orbital of one sodium atom shares space with the corresponding electron on a neighbouring atom to form a molecular orbital - in much the same sort of way that a covalent bond is formed. The difference, however, is that each sodium atom is being touched by eight other sodium atoms - and the sharing occurs between the central atom and the 3 s orbitals on all of the eight other atoms. And each of these eight is in turn being touched by eight sodium atoms, which in turn are touched by eight atoms - and so on, until you have taken in all the atoms in that lump of sodium.

Metallic bonding in sodium All of the 3 s orbitals on all of the atoms overlap to give a vast number of molecular orbitals which extend over the whole piece of metal. There have to be huge numbers of molecular orbitals, of course, because any orbital can only hold two electrons. The electrons can move freely within these molecular orbitals, and so each electron becomes detached from its parent atom. The electrons are said to be delocalised. The metal is held together by the strong forces of attraction between the positive nuclei and the delocalised electrons.


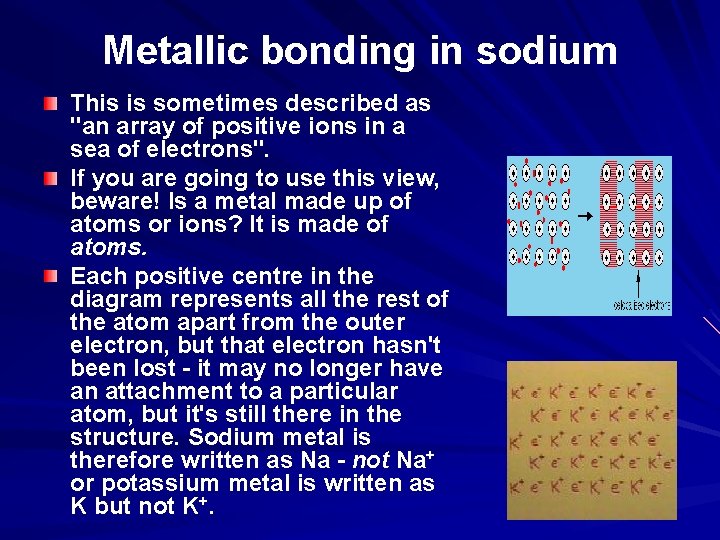
Metallic bonding in sodium This is sometimes described as "an array of positive ions in a sea of electrons". If you are going to use this view, beware! Is a metal made up of atoms or ions? It is made of atoms. Each positive centre in the diagram represents all the rest of the atom apart from the outer electron, but that electron hasn't been lost - it may no longer have an attachment to a particular atom, but it's still there in the structure. Sodium metal is therefore written as Na - not Na+ or potassium metal is written as K but not K+.

Metallic bonding in magnesium If you work through the same argument with magnesium, you end up with stronger bonds and so a higher melting point. Magnesium has the outer electronic structure 3 s 2. Both of these electrons become delocalised, so the "sea" has twice the electron density as it does in sodium. The remaining "ions" also have twice the charge (if you are going to use this particular view of the metal bond) and so there will be more attraction between "ions" and "sea". More realistically, each magnesium atom has one more proton in the nucleus than a sodium atom has, and so not only will there be a greater number of delocalised electrons, but there will also be a greater attraction for them. Magnesium atoms have a slightly smaller radius than sodium atoms, and so the delocalised electrons are closer to the nuclei. Each magnesium atom also has twelve near neighbours rather than sodium's eight. Both of these factors increase the strength of the bond still further.

Metallic bonding in transition elements Transition metals tend to have particularly high melting points and boiling points. The reason is that they can involve the 3 d electrons in the delocalisation as well as the 4 s. The more electrons you can involve, the stronger the attractions tend to be.

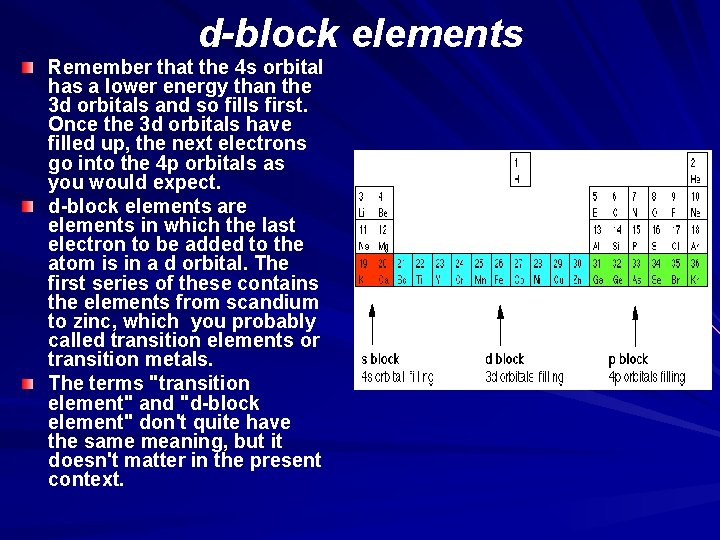
d-block elements Remember that the 4 s orbital has a lower energy than the 3 d orbitals and so fills first. Once the 3 d orbitals have filled up, the next electrons go into the 4 p orbitals as you would expect. d-block elements are elements in which the last electron to be added to the atom is in a d orbital. The first series of these contains the elements from scandium to zinc, which you probably called transition elements or transition metals. The terms "transition element" and "d-block element" don't quite have the same meaning, but it doesn't matter in the present context.

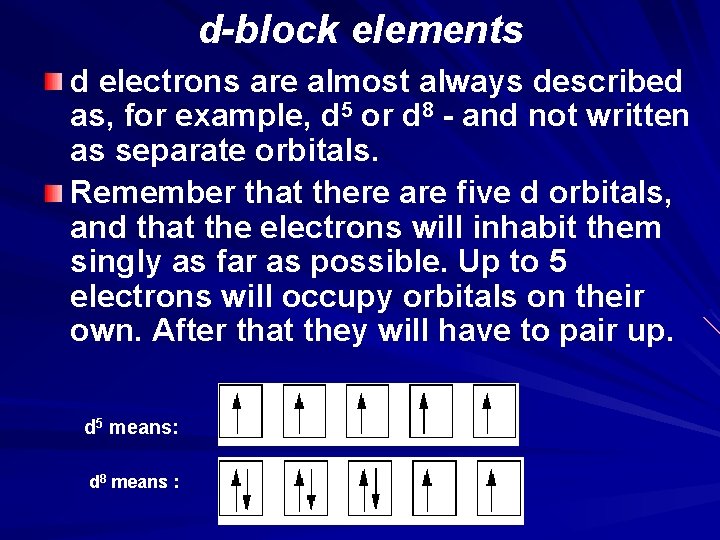
d-block elements d electrons are almost always described as, for example, d 5 or d 8 - and not written as separate orbitals. Remember that there are five d orbitals, and that the electrons will inhabit them singly as far as possible. Up to 5 electrons will occupy orbitals on their own. After that they will have to pair up. d 5 means: d 8 means :

d-block elements Notice in what follows that all the 3 level orbitals are written together, even though the 3 d electrons are added to the atom after the 4 s. Sc: 1 s 22 p 63 s 23 p 63 d 14 s 2 Ti: 1 s 22 p 63 s 23 p 63 d 24 s 2 V : 1 s 22 p 63 s 23 p 63 d 34 s 2 Cr: 1 s 22 p 63 s 23 p 63 d 54 s 1 Whoops! Chromium breaks the sequence. In chromium, the electrons in the 3 d and 4 s orbitals rearrange so that there is one electron in each orbital. It would be convenient if the sequence was tidy - but it's not!

d-block elements Mn: 1 s 22 p 63 s 23 p 63 d 54 s 2 (back to being tidy again) Fe : 1 s 22 p 63 s 23 p 63 d 64 s 2 Co: 1 s 22 p 63 s 23 p 63 d 74 s 2 Ni : 1 s 22 p 63 s 23 p 63 d 84 s 2 Cu: 1 s 22 p 63 s 23 p 63 d 104 s 1 (another awkward one!) Zn: 1 s 22 p 63 s 23 p 63 d 104 s 2 (And at zinc the process of filling the d orbitals is complete. ) A transition element is defined as one which has partially filled d orbitals either in the element or any of its compounds. Zinc (at the right-hand end of the d-block) always has a completely full 3 d level (3 d 10) and so doesn't count as a transition element.

The metallic bond in molten metals In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. The metallic bond isn't fully broken until the metal boils. That means that boiling point is actually a better guide to the strength of the metallic bond than melting point is. On melting, the bond is loosened, not broken.

The physical properties of metals Melting points and boiling points Metals tend to have high melting and boiling points because of the strength of the metallic bond. The strength of the bond varies from metal to metal and depends on the number of electrons which each atom delocalises into the sea of electrons, and on the packing. Group 1 metals like sodium and potassium have relatively low melting and boiling points mainly because each atom only has one electron to contribute to the bond. They have relatively large atoms (meaning that the nuclei are some distance from the delocalised electrons) which also weakens the bond.


A metal that melts in hot water

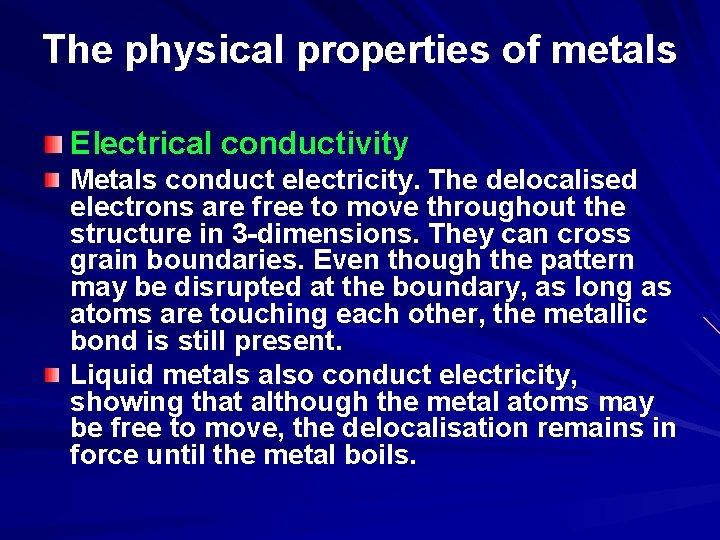
The physical properties of metals Electrical conductivity Metals conduct electricity. The delocalised electrons are free to move throughout the structure in 3 -dimensions. They can cross grain boundaries. Even though the pattern may be disrupted at the boundary, as long as atoms are touching each other, the metallic bond is still present. Liquid metals also conduct electricity, showing that although the metal atoms may be free to move, the delocalisation remains in force until the metal boils.

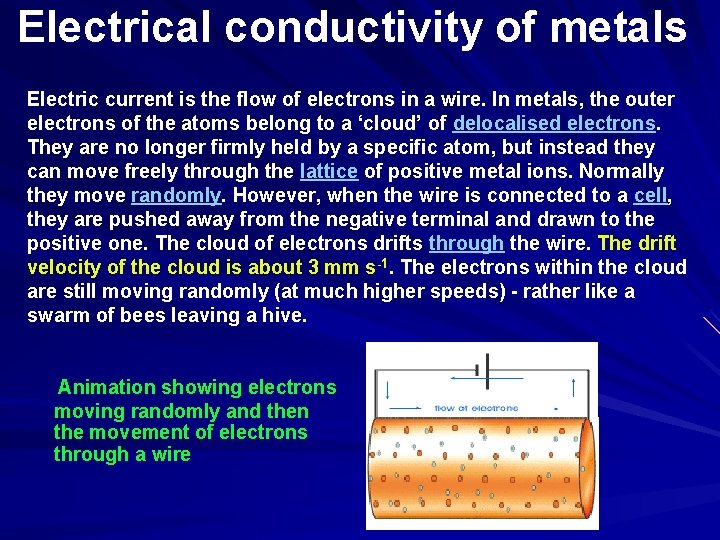
Electrical conductivity of metals Electric current is the flow of electrons in a wire. In metals, the outer electrons of the atoms belong to a ‘cloud’ of delocalised electrons. They are no longer firmly held by a specific atom, but instead they can move freely through the lattice of positive metal ions. Normally they move randomly. However, when the wire is connected to a cell, they are pushed away from the negative terminal and drawn to the positive one. The cloud of electrons drifts through the wire. The drift velocity of the cloud is about 3 mm s-1. The electrons within the cloud are still moving randomly (at much higher speeds) - rather like a swarm of bees leaving a hive. Animation showing electrons moving randomly and then the movement of electrons through a wire

Thermal conductivity Metals are good conductors of heat. Heat energy is picked up by the electrons as additional kinetic energy (it makes them move faster). The energy is transferred throughout the rest of the metal by the moving electrons.

Ionic vibrations The positive metal ions in a metal structure are packed closely together in a symmetrical geometric arrangement. They don’t move from their position in the lattice but they are constantly vibrating. If a metal is heated, the positive metal ions vibrate more vigorously. These ions collide with neighbouring ions and make them vibrate more vigorously too. In this way, the energy is passed, or conducted, through the metal. A cool lattice. If we heat the left hand end, then the energy will be carried along by conduction.

Free electrons However, metals are particularly good conductors of heat. In general, they are better than ionic compounds which also have strong bonds. So we need another mechanism to explain their especially good conductivity. It is their free electrons. Free electrons. The ions in the lattice are vibrating. The ions at the hot end of a piece of metal vibrate more. Let's look at just a few electrons. The electrons at the hot end will speed up – they gain kinetic energy from the vigorously vibrating ions. Some of them will move down to the cooler end and collide with ions that are vibrating less vigorously than those at the hot end. In these collisions, the electrons will lose kinetic energy and make the ions vibrate more vigorously. In effect, the electrons have carried the vibrational energy from the hot end to the cold end. And, because they are free to move through the lattice, they are able to do this more quickly than the bonds between the ions in the lattice

Thermal conductivity of metals Metals are good conductors of heat. There are two reasons for this: §the close packing of the metal ions in the lattice §the delocalised electrons can carry kinetic energy through the lattice How a metal conducts by the movement of free electrons.

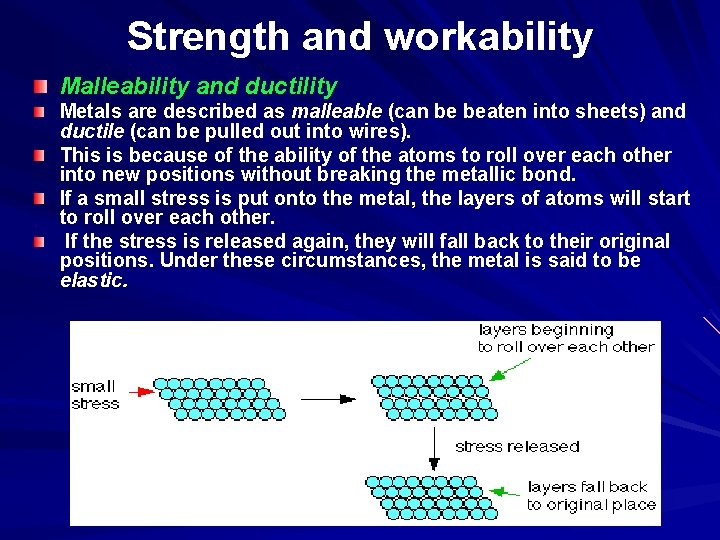
Strength and workability Malleability and ductility Metals are described as malleable (can be beaten into sheets) and ductile (can be pulled out into wires). This is because of the ability of the atoms to roll over each other into new positions without breaking the metallic bond. If a small stress is put onto the metal, the layers of atoms will start to roll over each other. If the stress is released again, they will fall back to their original positions. Under these circumstances, the metal is said to be elastic.

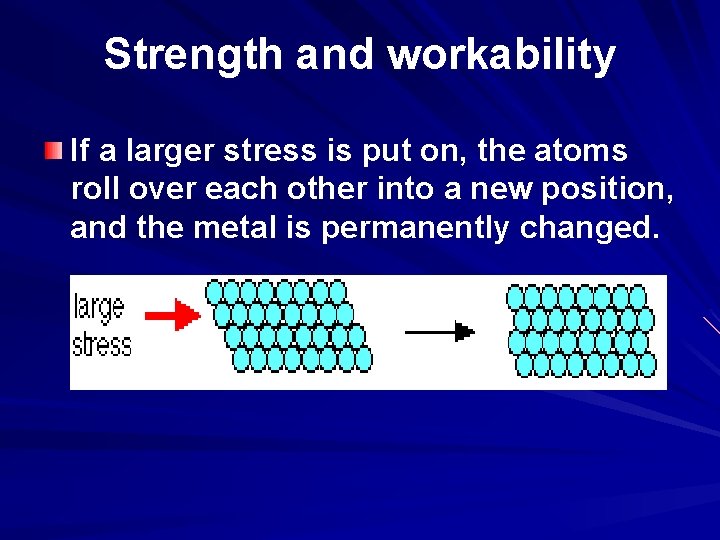
Strength and workability If a larger stress is put on, the atoms roll over each other into a new position, and the metal is permanently changed.

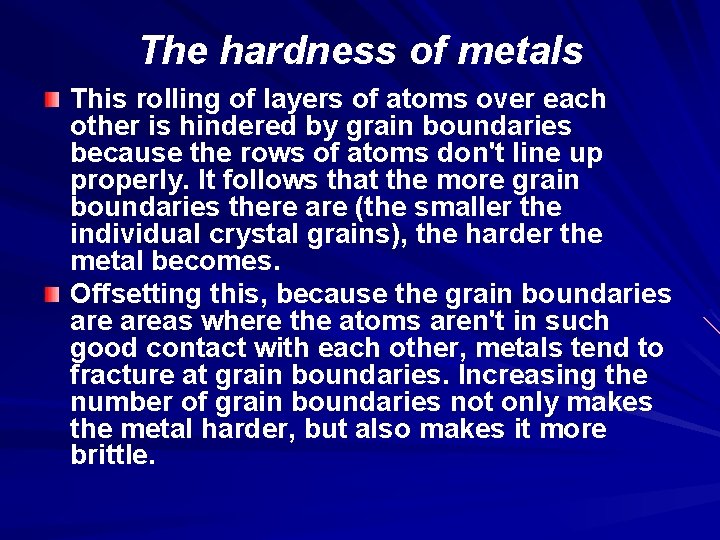
The hardness of metals This rolling of layers of atoms over each other is hindered by grain boundaries because the rows of atoms don't line up properly. It follows that the more grain boundaries there are (the smaller the individual crystal grains), the harder the metal becomes. Offsetting this, because the grain boundaries areas where the atoms aren't in such good contact with each other, metals tend to fracture at grain boundaries. Increasing the number of grain boundaries not only makes the metal harder, but also makes it more brittle.

Controlling the size of the crystal grains If you have a pure piece of metal, you can control the size of the grains by heat treatment or by working the metal. Heating a metal tends to shake the atoms into a more regular arrangement - decreasing the number of grain boundaries, and so making the metal softer. Banging the metal around when it is cold tends to produce lots of small grains. Cold working therefore makes a metal harder. To restore its workability, you would need to reheat it. You can also break up the regular arrangement of the atoms by inserting atoms of a slightly different size into the structure. Alloys such as brass (a mixture of copper and zinc) are harder than the original metals because the irregularity in the structure helps to stop rows of atoms from slipping over each other.

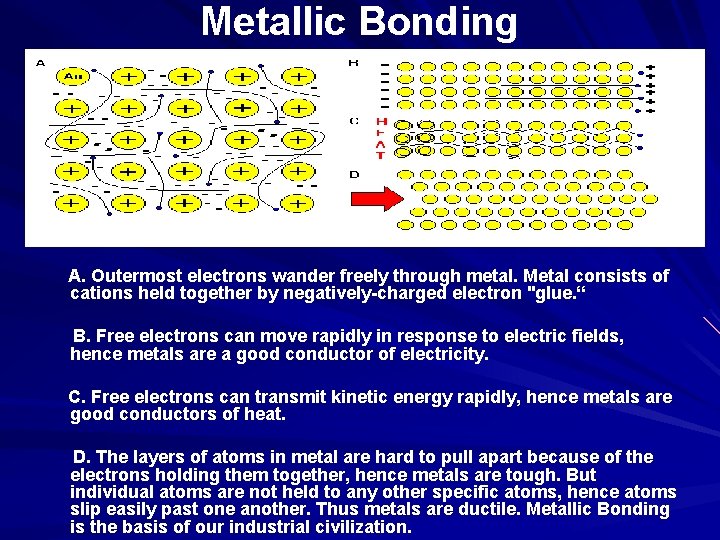
Metallic Bonding A. Outermost electrons wander freely through metal. Metal consists of cations held together by negatively-charged electron "glue. “ B. Free electrons can move rapidly in response to electric fields, hence metals are a good conductor of electricity. C. Free electrons can transmit kinetic energy rapidly, hence metals are good conductors of heat. D. The layers of atoms in metal are hard to pull apart because of the electrons holding them together, hence metals are tough. But individual atoms are not held to any other specific atoms, hence atoms slip easily past one another. Thus metals are ductile. Metallic Bonding is the basis of our industrial civilization.

2. DIPOLE FORCES Polar covalent molecules are sometimes described as "dipoles", meaning that the molecule has two "poles". One end (pole) of the molecule has a partial positive charge while the other end has a partial negative charge. The molecules will orientate themselves so that the opposite charges attract principle operates effectively.

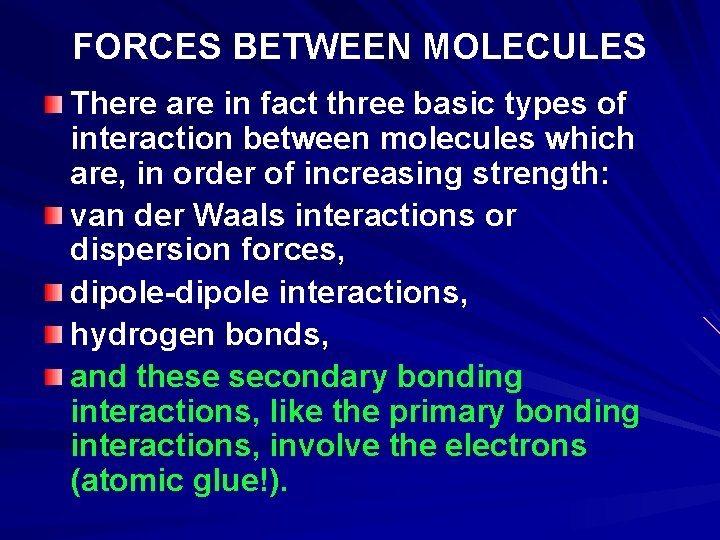
FORCES BETWEEN MOLECULES There are in fact three basic types of interaction between molecules which are, in order of increasing strength: van der Waals interactions or dispersion forces, dipole-dipole interactions, hydrogen bonds, and these secondary bonding interactions, like the primary bonding interactions, involve the electrons (atomic glue!).

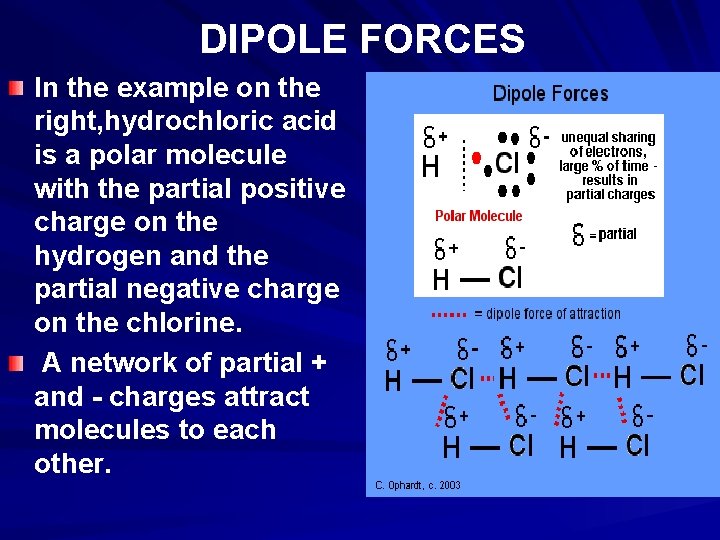
DIPOLE FORCES In the example on the right, hydrochloric acid is a polar molecule with the partial positive charge on the hydrogen and the partial negative charge on the chlorine. A network of partial + and - charges attract molecules to each other.

Polar Covalent Compounds Introduction to Covalent Bonding: Bonding between non-metals consists of two electrons shared between two atoms. In covalent bonding, the two electrons shared by the atoms are attracted to the nucleus of both atoms. Neither atom completely loses or gains electrons as in ionic bonding. There are two types of covalent bonding: 1. Non-polar bonding with an equal sharing of electrons. 2. Polar bonding with an unequal sharing of electrons. The number of shared electrons depends on the number of electrons needed to complete the octet.

Polar Covalent Compounds POLAR BONDING results when two different non-metals unequally share electrons between them. One well known exception to the identical atom rule is the combination of carbon and hydrogen in all organic compounds. The non-metal closer to fluorine in the Periodic Table has a greater tendency to keep its own electron and also draw away the other atom's electron. It is NOT completely successful. As a result only partial charges are established. One atom becomes partially positive since it has lost control of its electron some of the time. The other atom becomes partially negative since it gains electron some of the time.

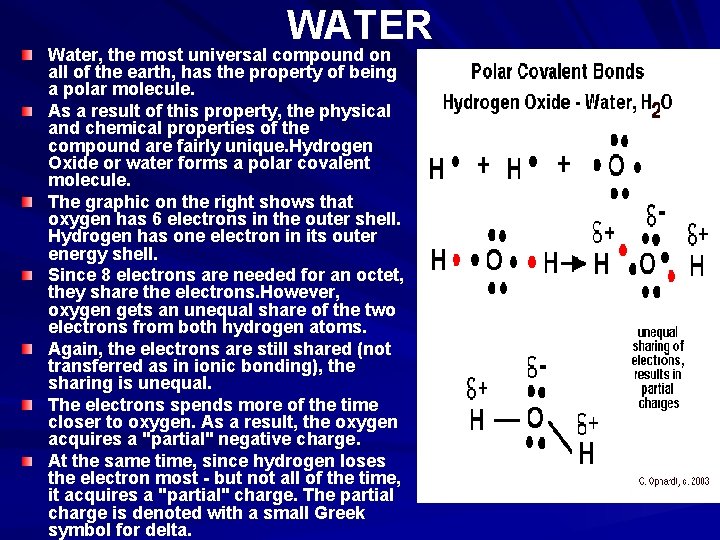
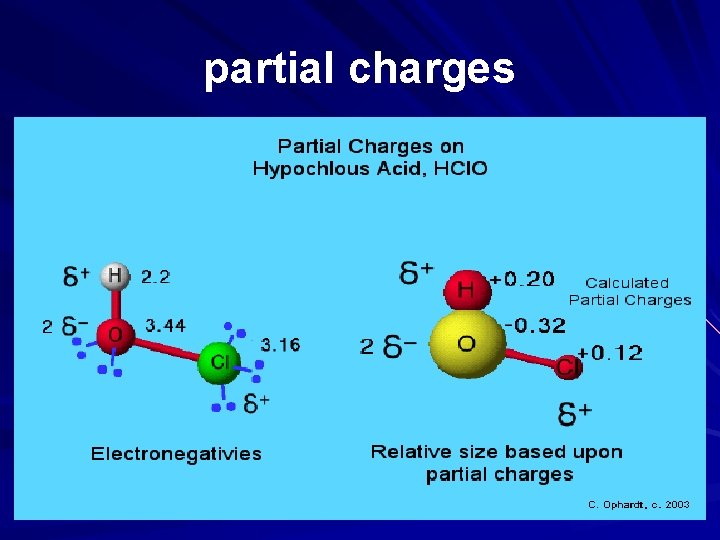
WATER Water, the most universal compound on all of the earth, has the property of being a polar molecule. As a result of this property, the physical and chemical properties of the compound are fairly unique. Hydrogen Oxide or water forms a polar covalent molecule. The graphic on the right shows that oxygen has 6 electrons in the outer shell. Hydrogen has one electron in its outer energy shell. Since 8 electrons are needed for an octet, they share the electrons. However, oxygen gets an unequal share of the two electrons from both hydrogen atoms. Again, the electrons are still shared (not transferred as in ionic bonding), the sharing is unequal. The electrons spends more of the time closer to oxygen. As a result, the oxygen acquires a "partial" negative charge. At the same time, since hydrogen loses the electron most - but not all of the time, it acquires a "partial" charge. The partial charge is denoted with a small Greek symbol for delta.

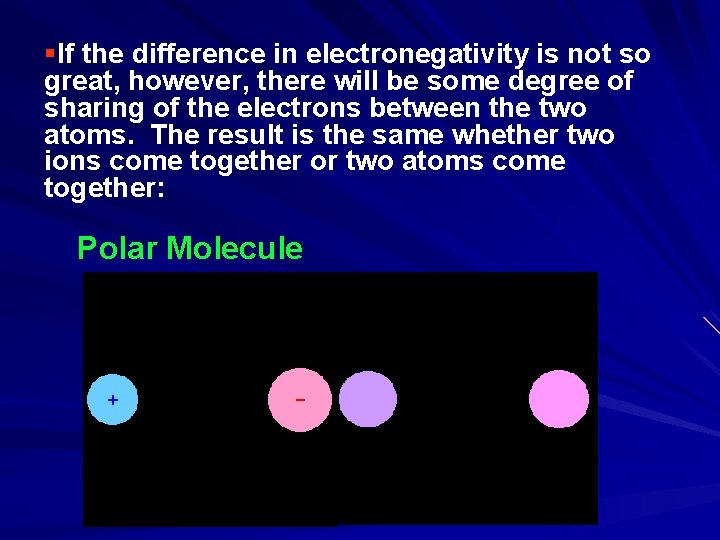
§If the difference in electronegativity is not so great, however, there will be some degree of sharing of the electrons between the two atoms. The result is the same whether two ions come together or two atoms come together: Polar Molecule

Polar molecules can interact with ions: Ion - Dipole Interactions

or with other polar molecules: Dipole - Dipole Interactions

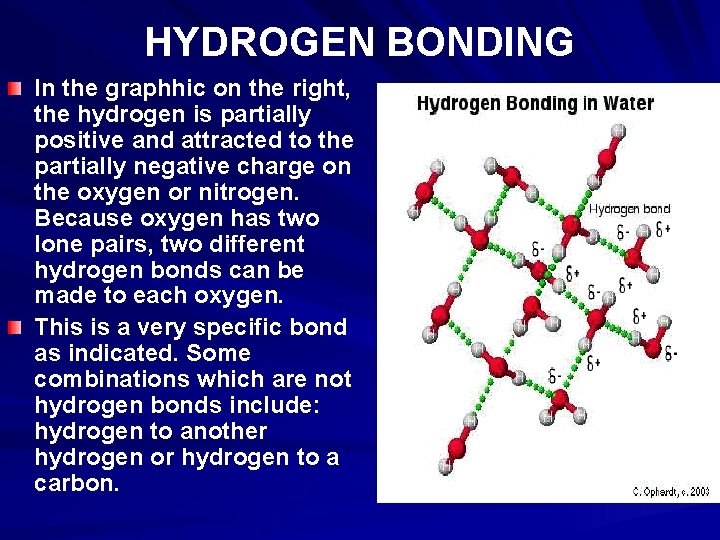
HYDROGEN BONDING To recognize the possibility of hydrogen bonding, examine the Lewis structure of the molecule. The electronegative atom must have one or more unshared electron pairs as in the case of oxygen and nitrogen, and has a negative partial charge. The hydrogen, which has a partial positive charge tries to find another atom of oxygen or nitrogen with excess electrons to share and is attracted to the partial negative charge. This forms the basis for the hydrogen bond.

3. HYDROGEN BONDING The hydrogen bond is really a special case of dipole forces. A hydrogen bond is the attractive force between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule. Usually the electronegative atom is oxygen, nitrogen, or fluorine. In other words - The hydrogen on one molecule attached to O or N that is attracted to an O or N of a different molecule.

HYDROGEN BONDING In the graphhic on the right, the hydrogen is partially positive and attracted to the partially negative charge on the oxygen or nitrogen. Because oxygen has two lone pairs, two different hydrogen bonds can be made to each oxygen. This is a very specific bond as indicated. Some combinations which are not hydrogen bonds include: hydrogen to another hydrogen or hydrogen to a carbon.


HYDROGEN BONDING Hydrogen bonding is usually stronger than normal dipole forces between molecules. Of course hydrogen bonding is not nearly as strong as normal covalent bonds within a molecule - it is only about 1/10 as strong. This is still strong enough to have many important ramifications on the properties of water.

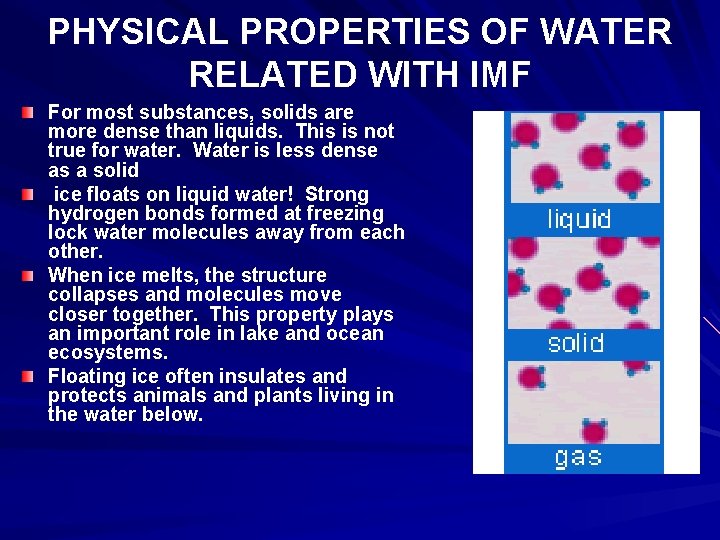
PHYSICAL PROPERTIES OF WATER RELATED WITH IMF For most substances, solids are more dense than liquids. This is not true for water. Water is less dense as a solid ice floats on liquid water! Strong hydrogen bonds formed at freezing lock water molecules away from each other. When ice melts, the structure collapses and molecules move closer together. This property plays an important role in lake and ocean ecosystems. Floating ice often insulates and protects animals and plants living in the water below.

Hydrogen Bonds in ice and liquid water In liquid water each molecule is hydrogen bonded to approximately 3. 4 other water molecules. In ice each molecule is hydrogen bonded to 4 other molecules. Compare the two structures below. Notice the empty spaces within the ice structure

Hydrogen Bonds in liquid water Hydrogen bonds are much weaker than covalent bonds. However, when a large number of hydrogen bonds act in unison they will make a strong contributory effect. This is the case in water.

Hydogen Bonds in liquid water Liquid water has a partially ordered structure in which hydrogen bonds are constantly being formed and breaking up.

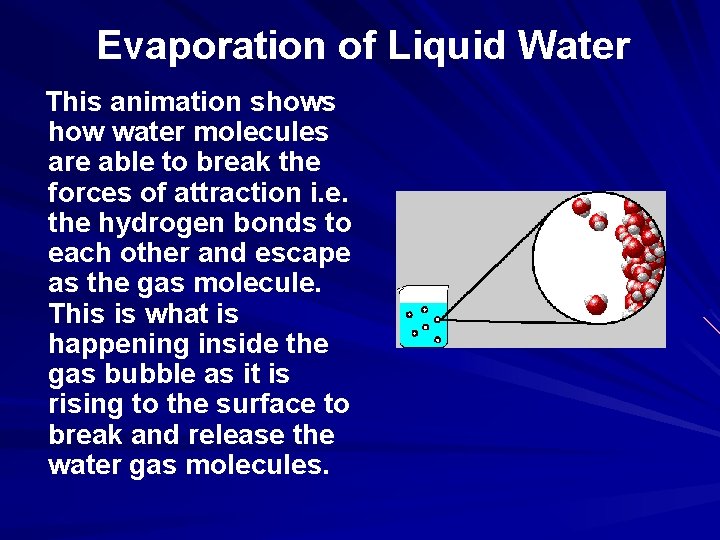
Evaporation of Liquid Water This animation shows how water molecules are able to break the forces of attraction i. e. the hydrogen bonds to each other and escape as the gas molecule. This is what is happening inside the gas bubble as it is rising to the surface to break and release the water gas molecules.

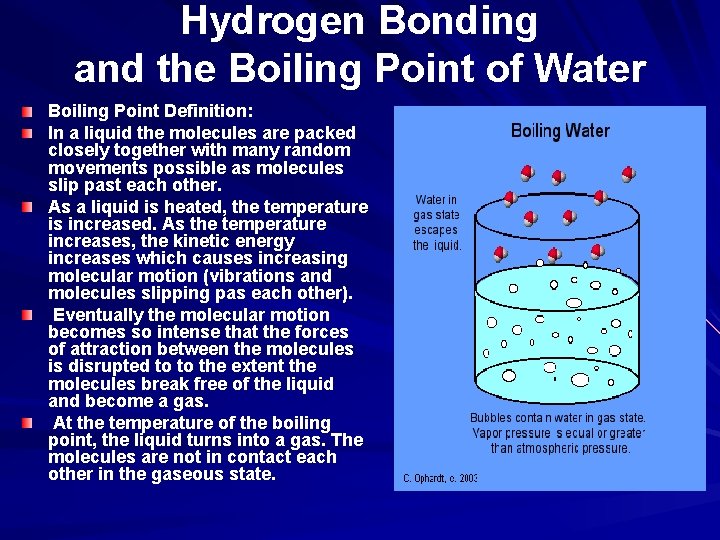
Hydrogen Bonding and the Boiling Point of Water Boiling Point Definition: In a liquid the molecules are packed closely together with many random movements possible as molecules slip past each other. As a liquid is heated, the temperature is increased. As the temperature increases, the kinetic energy increases which causes increasing molecular motion (vibrations and molecules slipping pas each other). Eventually the molecular motion becomes so intense that the forces of attraction between the molecules is disrupted to to the extent the molecules break free of the liquid and become a gas. At the temperature of the boiling point, the liquid turns into a gas. The molecules are not in contact each other in the gaseous state.

Evaporation

Polarity and Boiling Point: The polarity of the molecules determines the forces of attraction between the molecules in the liquid state. Polar molecules are attracted by the opposite charge effect (the positive end of one molecule is attracted to the negative end of another molecule. Molecules have different degrees of polarity as determined by the functional group present. The greater the forces of attraction the higher the boiling point or the greater the polarity the higher the boiling point.

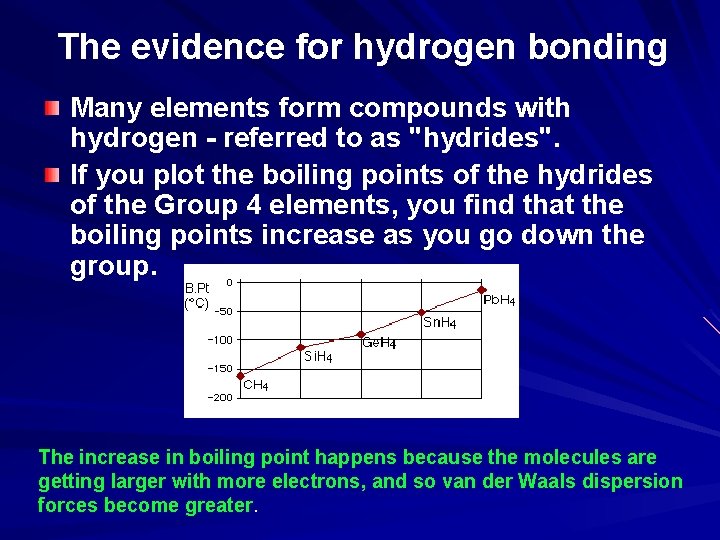
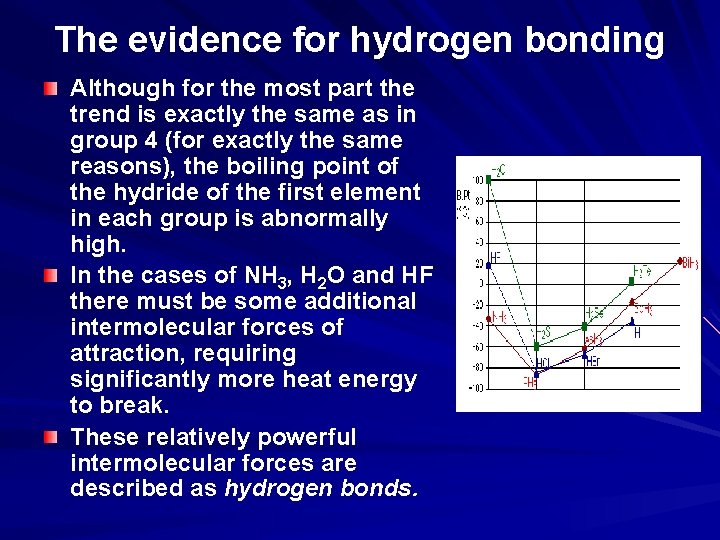
The evidence for hydrogen bonding Many elements form compounds with hydrogen - referred to as "hydrides". If you plot the boiling points of the hydrides of the Group 4 elements, you find that the boiling points increase as you go down the group. The increase in boiling point happens because the molecules are getting larger with more electrons, and so van der Waals dispersion forces become greater.

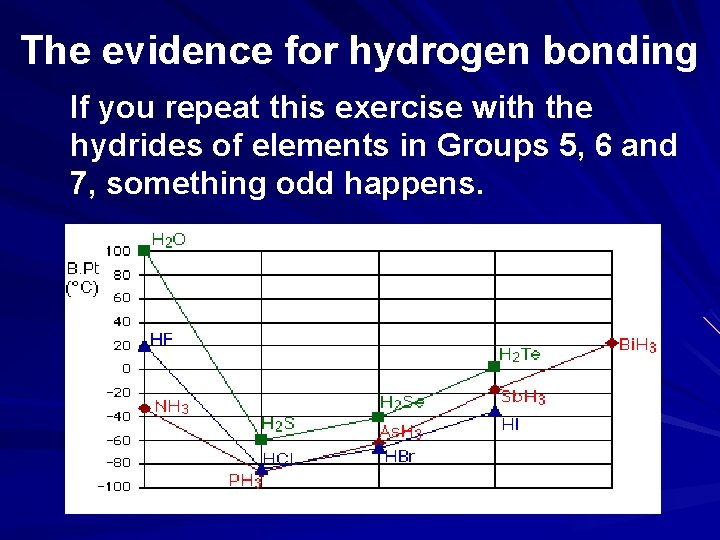
The evidence for hydrogen bonding If you repeat this exercise with the hydrides of elements in Groups 5, 6 and 7, something odd happens.

The evidence for hydrogen bonding Although for the most part the trend is exactly the same as in group 4 (for exactly the same reasons), the boiling point of the hydride of the first element in each group is abnormally high. In the cases of NH 3, H 2 O and HF there must be some additional intermolecular forces of attraction, requiring significantly more heat energy to break. These relatively powerful intermolecular forces are described as hydrogen bonds.

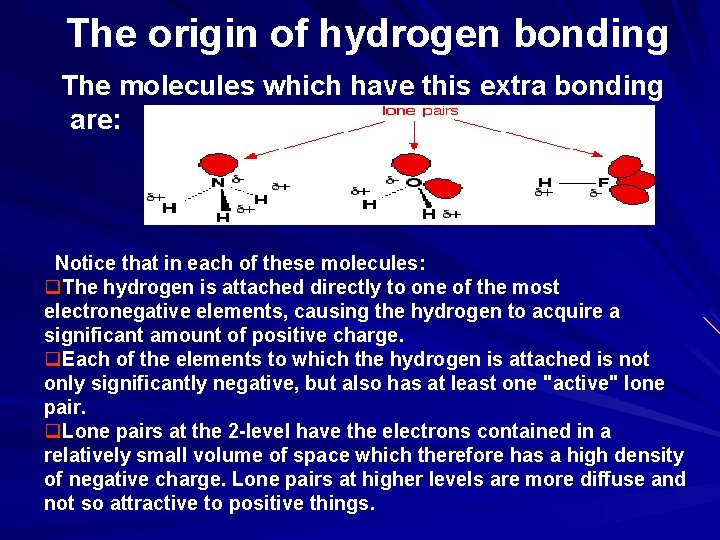
The origin of hydrogen bonding The molecules which have this extra bonding are: Notice that in each of these molecules: q. The hydrogen is attached directly to one of the most electronegative elements, causing the hydrogen to acquire a significant amount of positive charge. q. Each of the elements to which the hydrogen is attached is not only significantly negative, but also has at least one "active" lone pair. q. Lone pairs at the 2 -level have the electrons contained in a relatively small volume of space which therefore has a high density of negative charge. Lone pairs at higher levels are more diffuse and not so attractive to positive things.

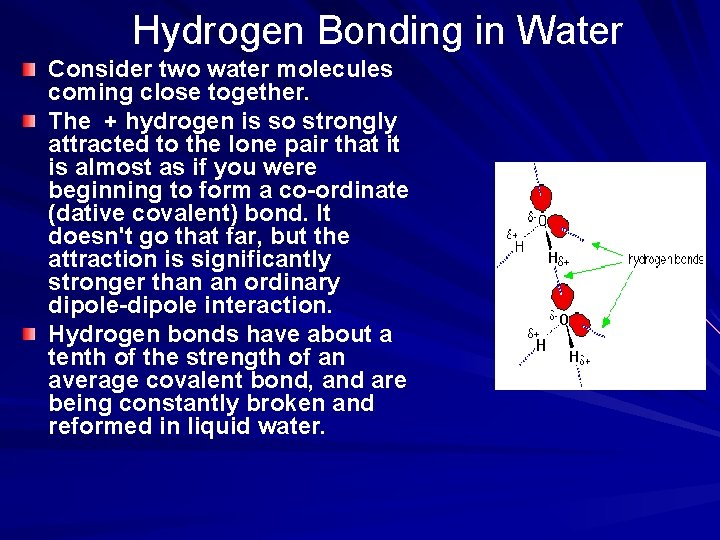
Hydrogen Bonding in Water Consider two water molecules coming close together. The + hydrogen is so strongly attracted to the lone pair that it is almost as if you were beginning to form a co-ordinate (dative covalent) bond. It doesn't go that far, but the attraction is significantly stronger than an ordinary dipole-dipole interaction. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are being constantly broken and reformed in liquid water.

Water as a "perfect" example of hydrogen bonding Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. There are exactly the right numbers of + hydrogens and lone pairs so that every one of them can be involved in hydrogen bonding. This is why the boiling point of water is higher than that of ammonia or hydrogen fluoride. In the case of ammonia, the amount of hydrogen bonding is limited by the fact that each nitrogen only has one lone pair. In a group of ammonia molecules, there aren't enough lone pairs to go around to satisfy all the hydrogens. In hydrogen fluoride, the problem is a shortage of hydrogens. In water, there are exactly the right number of each. Water could be considered as the "perfect" hydrogen bonded system.

Harmony molecule Distilled water Tap water

Ice sphere

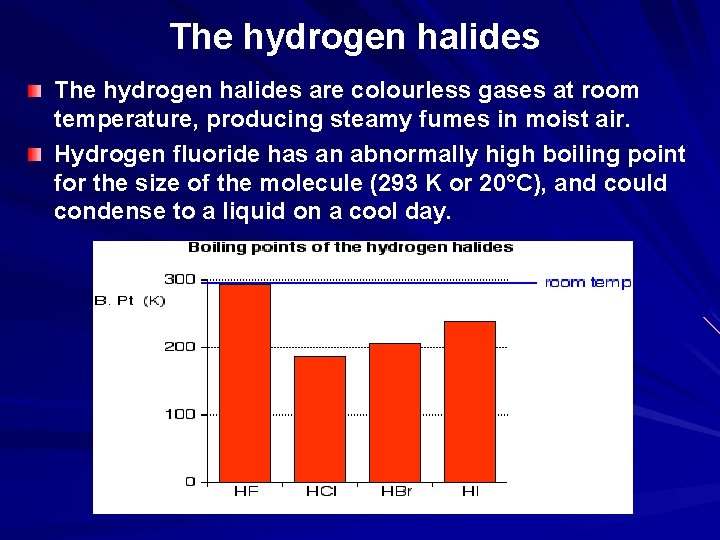
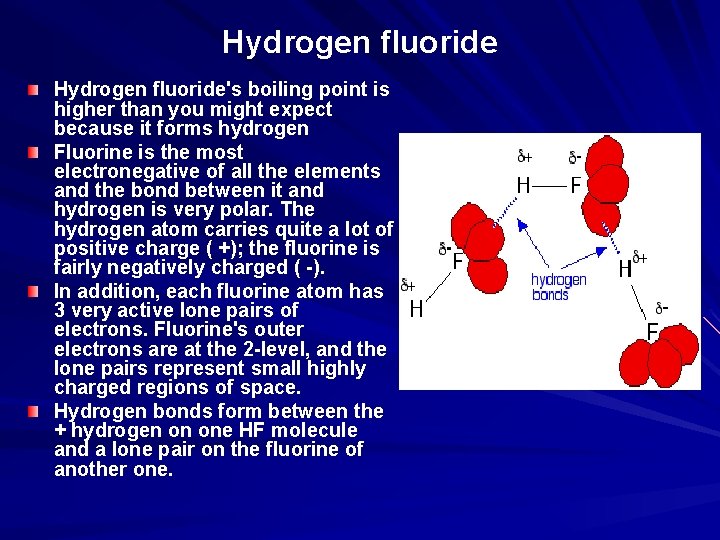
The hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist air. Hydrogen fluoride has an abnormally high boiling point for the size of the molecule (293 K or 20°C), and could condense to a liquid on a cool day.

Hydrogen fluoride's boiling point is higher than you might expect because it forms hydrogen Fluorine is the most electronegative of all the elements and the bond between it and hydrogen is very polar. The hydrogen atom carries quite a lot of positive charge ( +); the fluorine is fairly negatively charged ( -). In addition, each fluorine atom has 3 very active lone pairs of electrons. Fluorine's outer electrons are at the 2 -level, and the lone pairs represent small highly charged regions of space. Hydrogen bonds form between the + hydrogen on one HF molecule and a lone pair on the fluorine of another one.

The other hydrogen halides don't form hydrogen bonds. The other halogens aren't as electronegative as fluorine, and so the bonds in HX are less polar. As well as that, their lone pairs are at higher energy levels. That makes the lone pairs bigger, and so they don't carry such an intensely concentrated negative charge for the hydrogens to be attracted to.

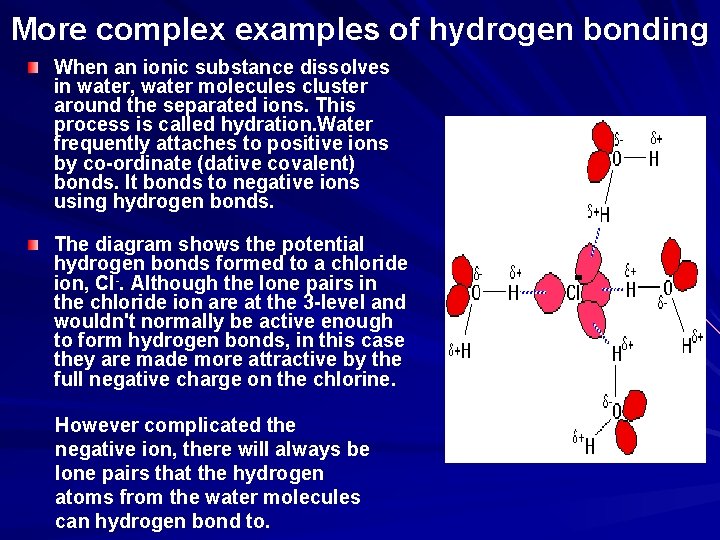
More complex examples of hydrogen bonding When an ionic substance dissolves in water, water molecules cluster around the separated ions. This process is called hydration. Water frequently attaches to positive ions by co-ordinate (dative covalent) bonds. It bonds to negative ions using hydrogen bonds. The diagram shows the potential hydrogen bonds formed to a chloride ion, Cl-. Although the lone pairs in the chloride ion are at the 3 -level and wouldn't normally be active enough to form hydrogen bonds, in this case they are made more attractive by the full negative charge on the chlorine. However complicated the negative ion, there will always be lone pairs that the hydrogen atoms from the water molecules can hydrogen bond to.

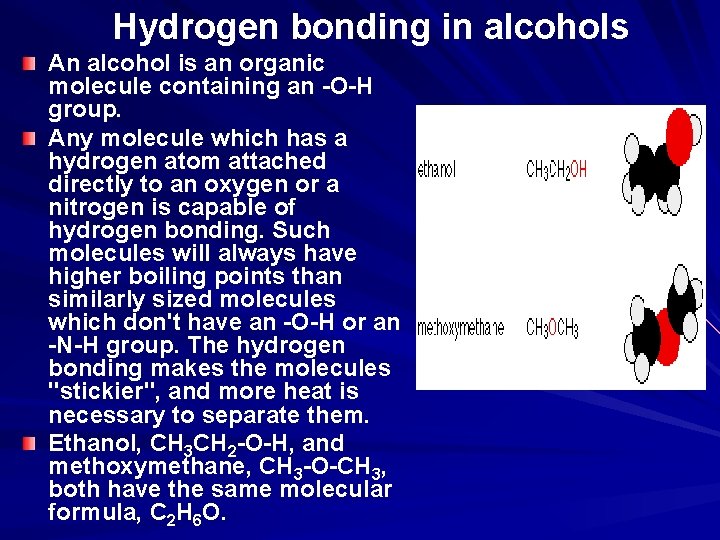
Hydrogen bonding in alcohols An alcohol is an organic molecule containing an -O-H group. Any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Such molecules will always have higher boiling points than similarly sized molecules which don't have an -O-H or an -N-H group. The hydrogen bonding makes the molecules "stickier", and more heat is necessary to separate them. Ethanol, CH 3 CH 2 -O-H, and methoxymethane, CH 3 -O-CH 3, both have the same molecular formula, C 2 H 6 O.

Hydrogen bonding in alcohols Ethanol and methoxymethane have the same number of electrons, and a similar length to the molecule. The van der Waals attractions (both dispersion forces and dipole-dipole attractions) in each will be much the same. However, ethanol has a hydrogen atom attached directly to an oxygen - and that oxygen still has exactly the same two lone pairs as in a water molecule. Hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. The hydrogen bonding is limited by the fact that there is only one hydrogen in each ethanol molecule with sufficient + charge. In methoxymethane, the lone pairs on the oxygen are still there, but the hydrogens aren't sufficiently + for hydrogen bonds to form. Except in some rather unusual cases, the hydrogen atom has to be attached directly to the very electronegative element for hydrogen bonding to occur. The boiling points of ethanol and methoxymethane show the dramatic effect that the hydrogen bonding has on the stickiness of the ethanol molecules: ethanol (with hydrogen bonding) 78. 5°C methoxymethane (without hydrogen bonding) -24. 8°C The hydrogen bonding in the ethanol has lifted its boiling point about 100°C.

Hydrogen bonding in alcohols It is important to realise that hydrogen bonding exists in addition to van der Waals attractions. For example, all the following molecules contain the same number of electrons, and the first two are much the same length. The higher boiling point of the butan-1 -ol is due to the additional hydrogen bonding. Comparing the two alcohols (containing -OH groups), both boiling points are high because of the additional hydrogen bonding due to the hydrogen attached directly to the oxygen - but they aren't the same. The boiling point of the 2 -methylpropan-1 -ol isn't as high as the butan-1 -ol because the branching in the molecule makes the van der Waals attractions less effective than in the longer butan -1 -ol.

Hydrogen bonding in organic molecules containing nitrogen Hydrogen bonding also occurs in organic molecules containing N-H groups - in the same sort of way that it occurs in ammonia. Examples range from simple molecules like CH 3 NH 2 (methylamine) to large molecules like proteins and DNA. The two strands of the famous alpha-helix in DNA are held together by hydrogen bonds involving N-H groups.

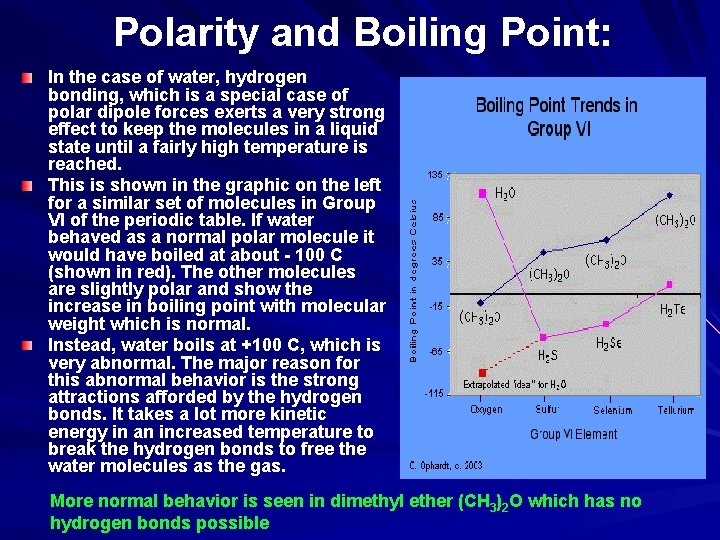
Polarity and Boiling Point: In the case of water, hydrogen bonding, which is a special case of polar dipole forces exerts a very strong effect to keep the molecules in a liquid state until a fairly high temperature is reached. This is shown in the graphic on the left for a similar set of molecules in Group VI of the periodic table. If water behaved as a normal polar molecule it would have boiled at about - 100 C (shown in red). The other molecules are slightly polar and show the increase in boiling point with molecular weight which is normal. Instead, water boils at +100 C, which is very abnormal. The major reason for this abnormal behavior is the strong attractions afforded by the hydrogen bonds. It takes a lot more kinetic energy in an increased temperature to break the hydrogen bonds to free the water molecules as the gas. More normal behavior is seen in dimethyl ether (CH 3)2 O which has no hydrogen bonds possible

Surface tension of water Water molecules at the surface (next to the air) hold closely together, forming an invisible film. We call this water’s surface tension. Water’s surface tension can hold weight that would normally sink. You can carefully float a sewing needle or paper clip on top of water in a glass. Surface tension allows many aquatic insects, like water spiders and pod skaters, to “walk” across rivers and streams. Next to mercury water has the highest surface tension of all commonly occurring liquids Surface tension is essential for the transfer of energy from wind to water to create waves. Waves are necessary for rapid oxygen diffusion in lakes and seas

Surface tension of water The water strider is an insect of characteristic length 1 cm and weight 10 dynes that resides on the surface of ponds, rivers, lakes and the open ocean. Its weight is supported by the surface tension force generated by curvature of the free surface. Its body and legs are covered by thousands of hairs that render its legs effectively non-wetting (Andersen 1982). The water strider propels itself by driving its central pair of legs in a sculling motion. In order for it to move, it must transfer momentum to the underlying fluid. It was previously assumed that this transfer occurs exclusively through capillary waves excited by the leg stroke (Denny 1993). Our experiments reveal that, conversely, the strider transfers momentum to the fluid principally through dipolar vortices shed by its driving legs. The strider thus generates thrust by rowing, using its legs as oars, and the meniscii beneath its driving legs as blades.

Cohesion and Adhesion

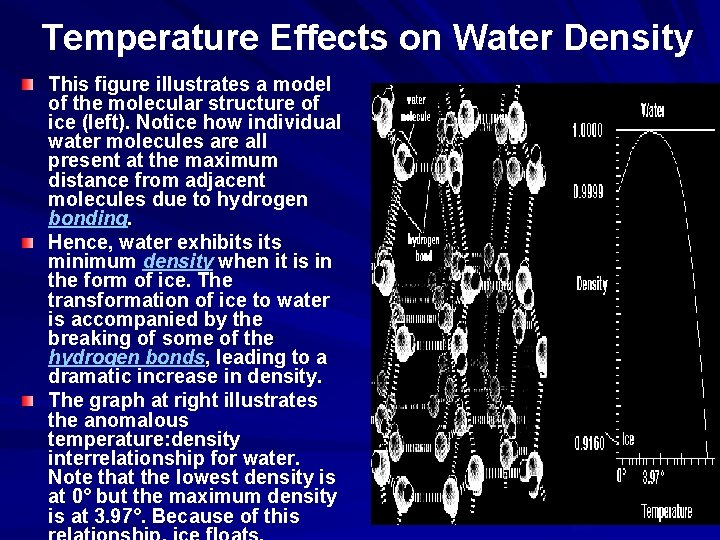
Temperature Effects on Water Density This figure illustrates a model of the molecular structure of ice (left). Notice how individual water molecules are all present at the maximum distance from adjacent molecules due to hydrogen bonding. Hence, water exhibits minimum density when it is in the form of ice. The transformation of ice to water is accompanied by the breaking of some of the hydrogen bonds, leading to a dramatic increase in density. The graph at right illustrates the anomalous temperature: density interrelationship for water. Note that the lowest density is at 0° but the maximum density is at 3. 97°. Because of this

Six dancers

The abnormal behaviour of water: At 4 ºC, water has its highest density. It actually contracts as the temperature rises from 0 ºC to 4ºC. This means that in regions with severe winters, lakes cool at the surface, and when the surface water reaches 4 ºC, that water, being more dense, sinks. A temperature gradient is set up, and when the water freezes, it does so at the surface. Eventually, the ice, which floats on water, acts as an insulator, protecting the water below it from further cooling. This results in lakes freezing from the top down, and not from the bottom up. This means that fishes can survive below the ice even if the air temperature is far below 0 ºC for prolonged periods.

ice floats on water

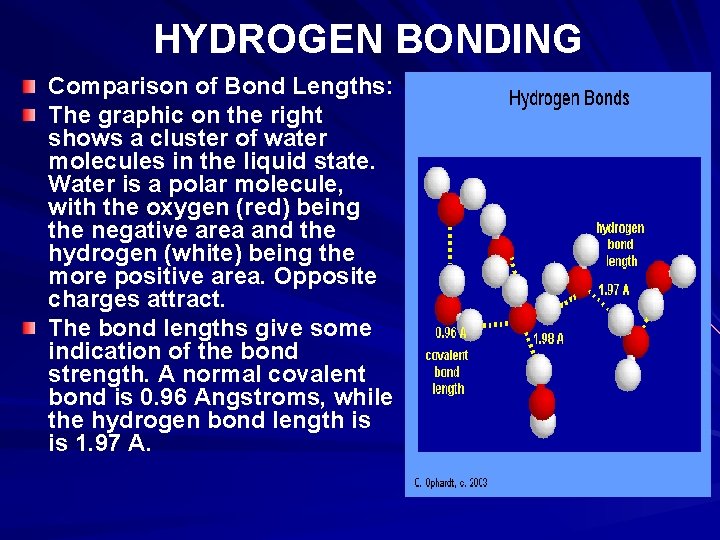
HYDROGEN BONDING Comparison of Bond Lengths: The graphic on the right shows a cluster of water molecules in the liquid state. Water is a polar molecule, with the oxygen (red) being the negative area and the hydrogen (white) being the more positive area. Opposite charges attract. The bond lengths give some indication of the bond strength. A normal covalent bond is 0. 96 Angstroms, while the hydrogen bond length is is 1. 97 A.

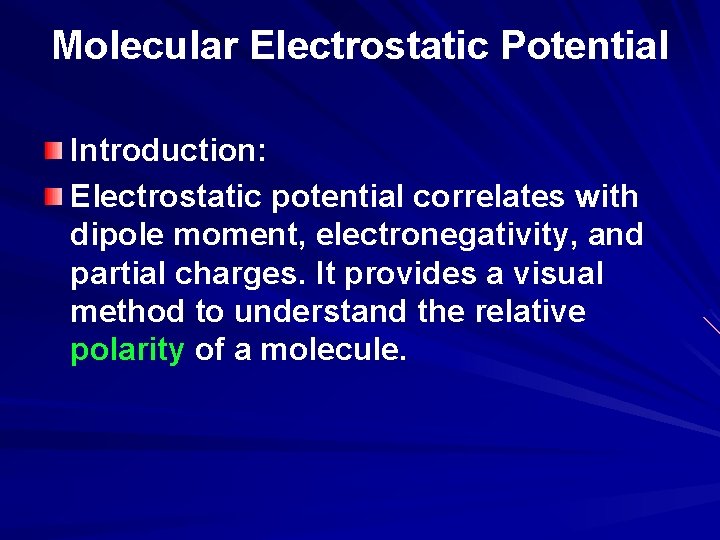
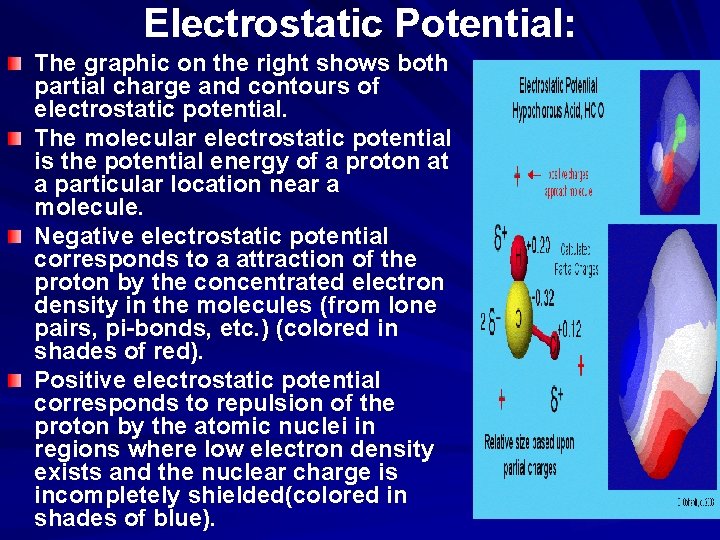
Electrostatic Potential as an Indication of Polarity The molecular electrostatic potential is the potential energy of a proton at a particular location near a molecule. Negative electrostatic potential corresponds to a attraction of the proton by the concentrated electron density in the molecules (from lone pairs, pi-bonds, etc. ) (colored in shades of red). Positive electrostatic potential corresponds to repulsion of the proton by the atomic nuclei in regions where low electron density exists and the nuclear charge is incompletely shielded(colored in shades of blue). The polarity of the water molecule with the attraction of the positive and negative partial charges is the basis for the hydrogen bonding.

Molecular Electrostatic Potential Introduction: Electrostatic potential correlates with dipole moment, electronegativity, and partial charges. It provides a visual method to understand the relative polarity of a molecule.

Molecular Electrostatic Potential Electronegativity: Linus Pauling first defined electronegativity as: "The power of an atom in a molecule to attract electrons to itself. " A numerical scale based upon a physical measurements allows a comparison between atoms. A rough approximation for comparison of atoms is to say, the closer an atom is to Fluorine in the periodic table, the greater the electronegativity compared to an atom further away. The greater the electronegativity difference between atoms in a bond, the more polar the bond.

Molecular Electrostatic Potential Partial Charges: Electronegativity of atoms in molecules indicates where partial charges are likely to be found - the most electronegative atoms are most negative, the others are less negative or more positive. Quantum mechanical calculations generate values for partial charges for the atoms in a molecule. These are related to electron densities around various atoms resulting from bonding and lone pairs of electrons. The calculated partial charges represented as spheres (yellow is negative, red is positive) show the molecule would interact with an approaching proton. The greater the difference in partial charges, the more polar the molecule.

partial charges

Electrostatic Potential: The graphic on the right shows both partial charge and contours of electrostatic potential. The molecular electrostatic potential is the potential energy of a proton at a particular location near a molecule. Negative electrostatic potential corresponds to a attraction of the proton by the concentrated electron density in the molecules (from lone pairs, pi-bonds, etc. ) (colored in shades of red). Positive electrostatic potential corresponds to repulsion of the proton by the atomic nuclei in regions where low electron density exists and the nuclear charge is incompletely shielded(colored in shades of blue).

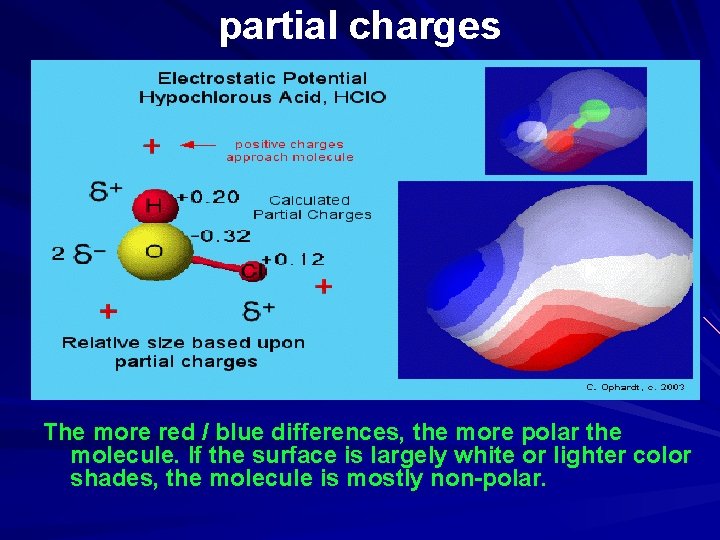
partial charges The calculated partial charges represented as spheres (yellow is negative, red is positive) show the molecule would interact with approaching protons or positive charges. When a unit of positive charge (proton) approaches a positive region of the molecule, the repulsive interaction results in an increasing positive potential energy (colored in shades of blue). As a proton approaches a negative region an attractive interaction results in negative potential energy (colored in shades of red). The electron density isosurface is a surface on which the molecule's electron density has a particular value and that encloses a specified fraction of the molecule's electron probability density. The electrostatic potential at different points on the electron density isosurface is shown by coloring the isosurface with contours.

partial charges The more red / blue differences, the more polar the molecule. If the surface is largely white or lighter color shades, the molecule is mostly non-polar.

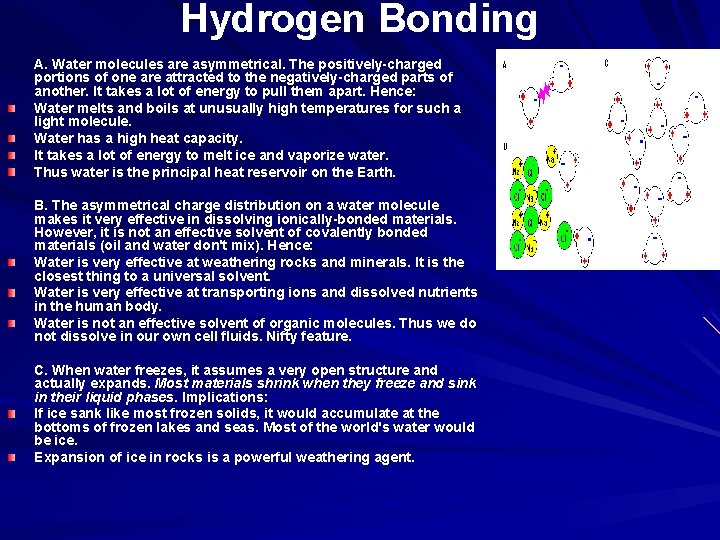
Hydrogen Bonding A. Water molecules are asymmetrical. The positively-charged portions of one are attracted to the negatively-charged parts of another. It takes a lot of energy to pull them apart. Hence: Water melts and boils at unusually high temperatures for such a light molecule. Water has a high heat capacity. It takes a lot of energy to melt ice and vaporize water. Thus water is the principal heat reservoir on the Earth. B. The asymmetrical charge distribution on a water molecule makes it very effective in dissolving ionically-bonded materials. However, it is not an effective solvent of covalently bonded materials (oil and water don't mix). Hence: Water is very effective at weathering rocks and minerals. It is the closest thing to a universal solvent. Water is very effective at transporting ions and dissolved nutrients in the human body. Water is not an effective solvent of organic molecules. Thus we do not dissolve in our own cell fluids. Nifty feature. C. When water freezes, it assumes a very open structure and actually expands. Most materials shrink when they freeze and sink in their liquid phases. Implications: If ice sank like most frozen solids, it would accumulate at the bottoms of frozen lakes and seas. Most of the world's water would be ice. Expansion of ice in rocks is a powerful weathering agent.


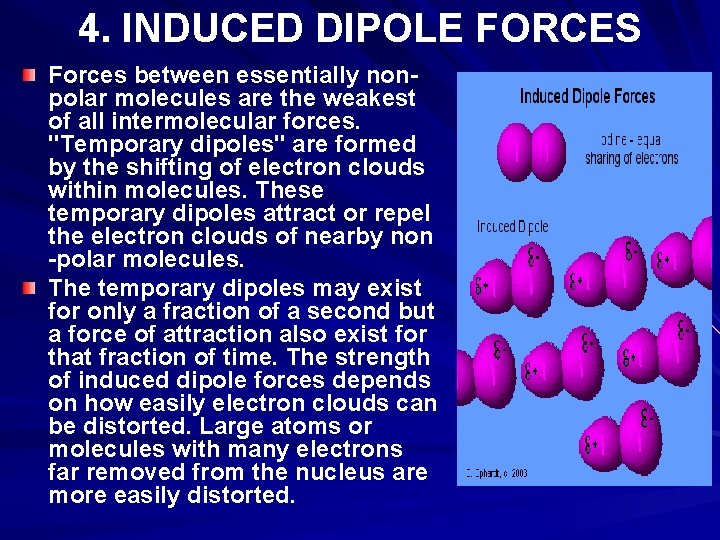
4. INDUCED DIPOLE FORCES Forces between essentially nonpolar molecules are the weakest of all intermolecular forces. "Temporary dipoles" are formed by the shifting of electron clouds within molecules. These temporary dipoles attract or repel the electron clouds of nearby non -polar molecules. The temporary dipoles may exist for only a fraction of a second but a force of attraction also exist for that fraction of time. The strength of induced dipole forces depends on how easily electron clouds can be distorted. Large atoms or molecules with many electrons far removed from the nucleus are more easily distorted.

Nonpolar Covalent Compounds Introduction to Covalent Bonding: Bonding between non-metals consists of two electrons shared between two atoms. In covalent bonding, the two electrons shared by the atoms are attracted to the nucleus of both atoms. Neither atom completely loses or gains electrons as in ionic bonding. There are two types of covalent bonding: 1. Non-polar bonding with an equal sharing of electrons. 2. Polar bonding with an unequal sharing of electrons. The number of shared electrons depends on the number of electrons needed to complete the octet

Nonpolar Covalent Compounds NON-POLAR BONDING results when two identical non-metals equally share electrons between them. One well known exception to the identical atom rule is the combination of carbon and hydrogen in all organic compounds.

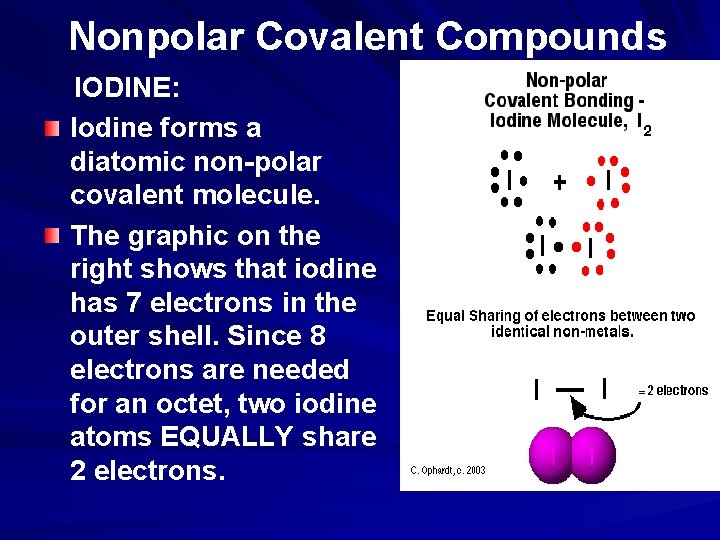
Nonpolar Covalent Compounds IODINE: Iodine forms a diatomic non-polar covalent molecule. The graphic on the right shows that iodine has 7 electrons in the outer shell. Since 8 electrons are needed for an octet, two iodine atoms EQUALLY share 2 electrons.

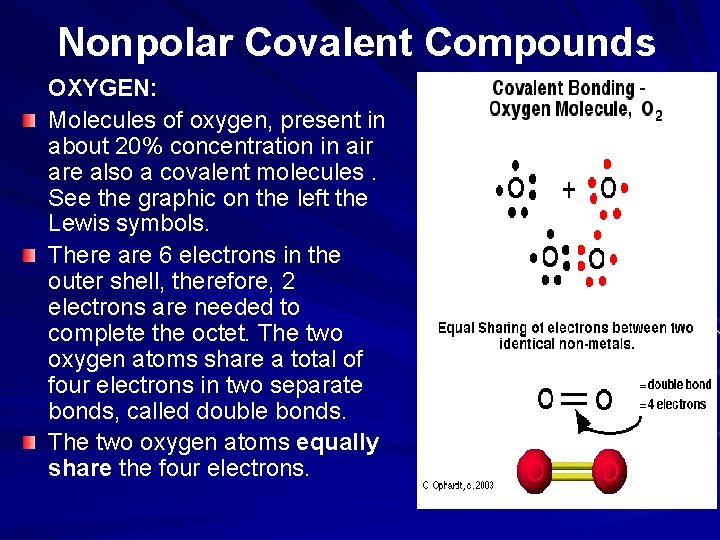
Nonpolar Covalent Compounds OXYGEN: Molecules of oxygen, present in about 20% concentration in air are also a covalent molecules. See the graphic on the left the Lewis symbols. There are 6 electrons in the outer shell, therefore, 2 electrons are needed to complete the octet. The two oxygen atoms share a total of four electrons in two separate bonds, called double bonds. The two oxygen atoms equally share the four electrons.

Non-polar Molecule

Fluctuating Dipole in a Non-polar Molecule These instantaneous dipoles may be induced and stabilized as an ion or a polar molecule approaches the non-polar molecule.

Ion - Induced Dipole Interaction

Dipole - Induced Dipole Interaction

Dispersion Forces §Interactions between ions, dipoles, and induced dipoles account for many properties of molecules - deviations from ideal gas behavior in the vapor state, and the condensation of gases to the liquid or solid states. §In general, stronger interactions allow the solid and liquid states to persist to higher temperatures. §However, non-polar molecules show similar behavior, indicating that there are some types of intermolecular interactions that cannot be attributed to simple electrical attractions. These interactions are generally called dispersion forces.

Dispersion Forces Electrical forces operate when the molecules are several molecular diameters apart, and become stronger as the molecules or ions approach each other. Dispersion forces are very weak until the molecules or ions are almost touching each other, as in the liquid state. These forces appear to increase with the number of "contact points" with other molecules, so that long non-polar molecules such as n-octane (C 8 H 18) may have stronger intermolecular interactions than very polar molecules such as water (H 2 O), and the boiling point of n-octane is actually higher than that of water.

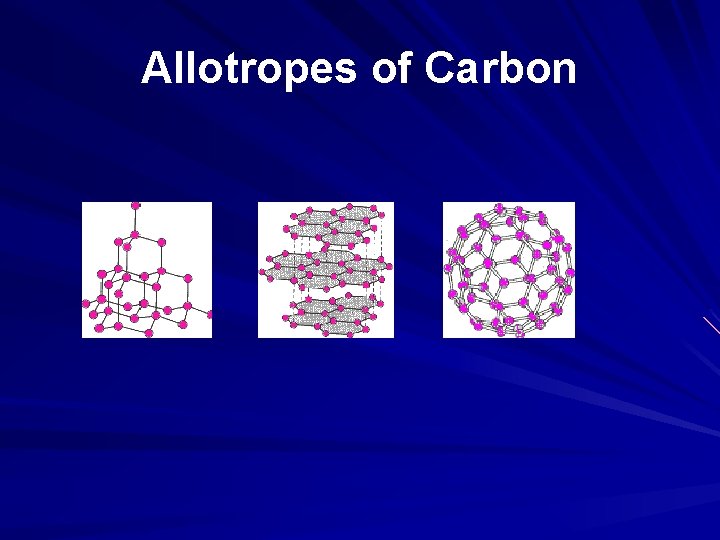
GIANT COVALENT STRUCTURES Giant covalent substances like diamond, graphite and silicon dioxide (silicon(IV) oxide), and relates those structures to the physical properties of the substances.

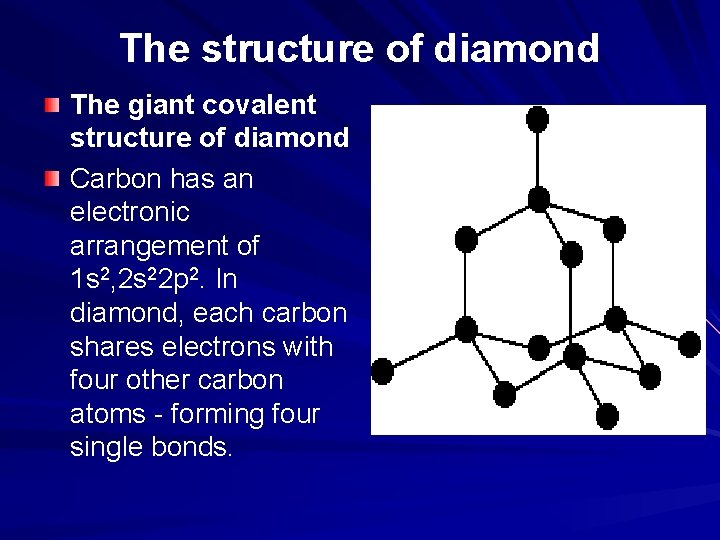
The structure of diamond The giant covalent structure of diamond Carbon has an electronic arrangement of 1 s 2, 2 s 22 p 2. In diamond, each carbon shares electrons with four other carbon atoms - forming four single bonds.

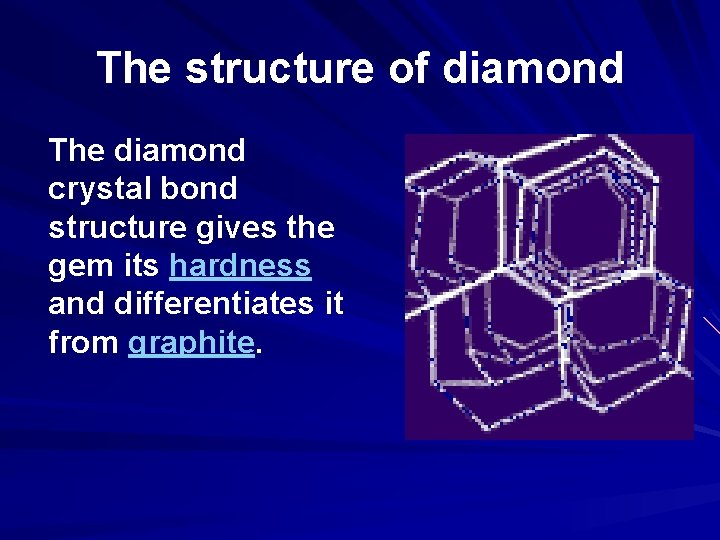
The structure of diamond The diamond crystal bond structure gives the gem its hardness and differentiates it from graphite.

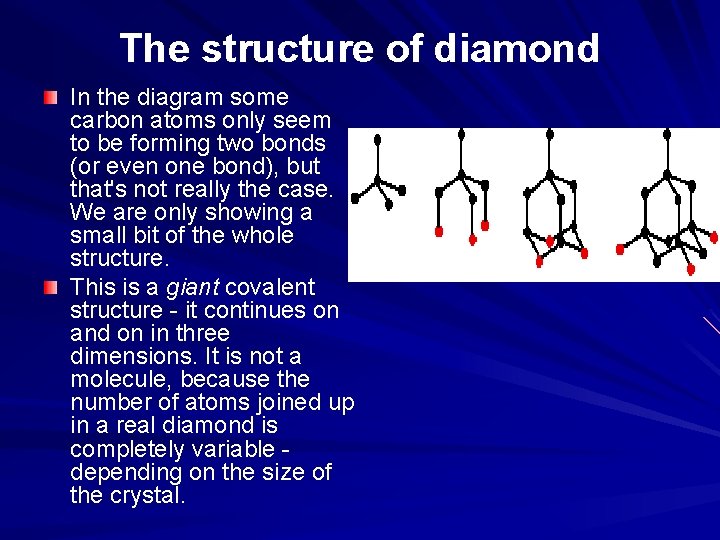
The structure of diamond In the diagram some carbon atoms only seem to be forming two bonds (or even one bond), but that's not really the case. We are only showing a small bit of the whole structure. This is a giant covalent structure - it continues on and on in three dimensions. It is not a molecule, because the number of atoms joined up in a real diamond is completely variable - depending on the size of the crystal.

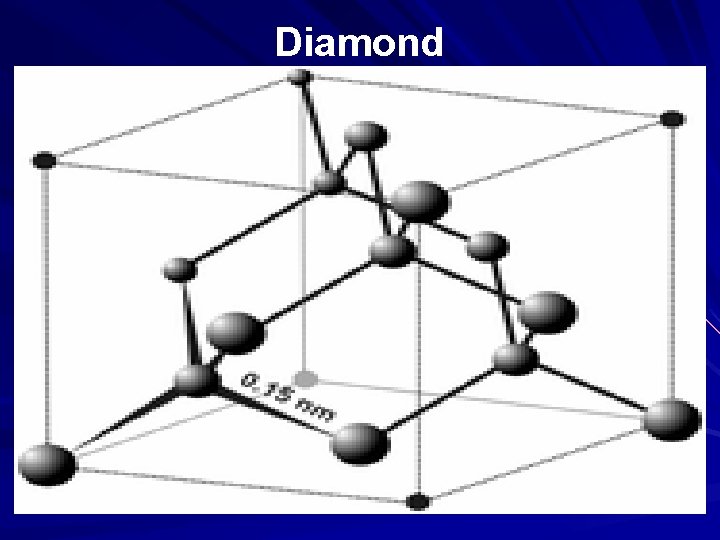
Diamond

The physical properties of diamond Diamond has a very high melting point (almost 4000°C). Very strong carbon-carbon covalent bonds have to be broken throughout the structure before melting occurs. is very hard. This is again due to the need to break very strong covalent bonds operating in 3 -dimensions. doesn't conduct electricity. All the electrons are held tightly between the atoms, and aren't free to move. is insoluble in water and organic solvents. There are no possible attractions which could occur between solvent molecules and carbon atoms which could outweigh the attractions between the covalently bound carbon atoms.

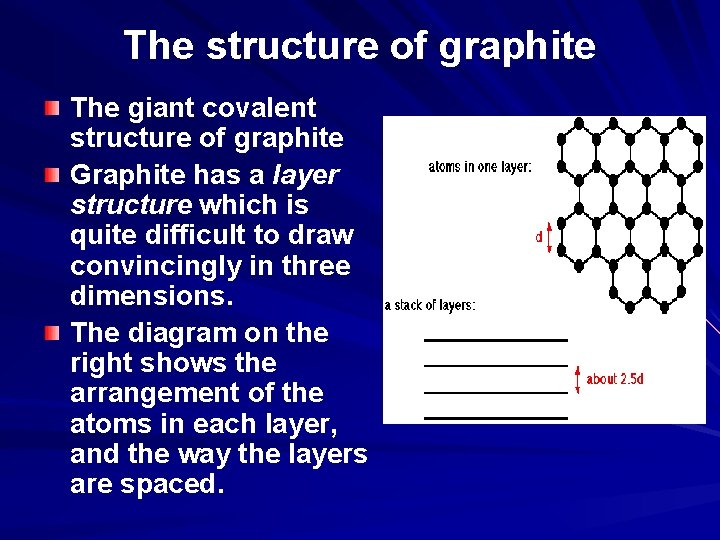
The structure of graphite The giant covalent structure of graphite Graphite has a layer structure which is quite difficult to draw convincingly in three dimensions. The diagram on the right shows the arrangement of the atoms in each layer, and the way the layers are spaced.

The structure of graphite Notice that you can't really draw the side view of the layers to the same scale as the atoms in the layer without one or other part of the diagram being either very spread out or very squashed. In that case, it is important to give some idea of the distances involved. The distance between the layers is about 2. 5 times the distance between the atoms within each layer. The layers, of course, extend over huge numbers of atoms - not just the few shown above. You might argue that carbon has to form 4 bonds because of its 4 unpaired electrons, whereas in this diagram it only seems to be forming 3 bonds to the neighbouring carbons. This diagram is something of a simplification, and shows the arrangement of atoms rather than the bonding.

The bonding in graphite Each carbon atom uses three of its electrons to form simple bonds to its three close neighbours. That leaves a fourth electron in the bonding level. These "spare" electrons in each carbon atom become delocalised over the whole of the sheet of atoms in one layer. They are no longer associated directly with any particular atom or pair of atoms, but are free to wander throughout the whole sheet.

The bonding in graphite is like a vastly extended version of the bonding in benzene. Each carbon atom undergoes sp 2 hybridisation, and then the unhybridised p orbitals on each carbon atom overlap sideways to give a massive pi system above and below the plane of the sheet of atoms.

Allotropes of Carbon

Graphite to diamond animation This animation shows how graphite becomes diamond under extreme heat and pressure

An orbital model for the benzene (C 6 H 6) structure Building the orbital model Benzene is built from hydrogen atoms (1 s 1) and carbon atoms (1 s 22 px 12 py 1). Each carbon atom has to join to three other atoms (one hydrogen and two carbons) and doesn't have enough unpaired electrons to form the required number of bonds, so it needs to promote one of the 2 s 2 pair into the empty 2 pz orbital. This is exactly the same as happens whenever carbon forms bonds - whatever else it ends up joined to.

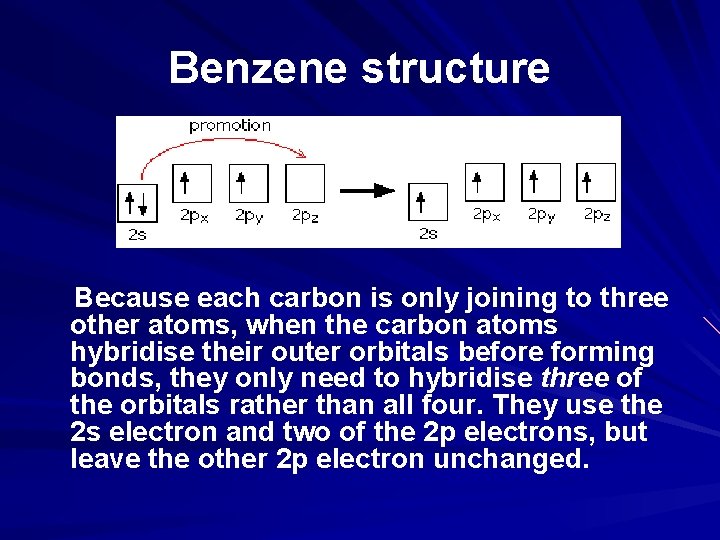
Benzene structure Because each carbon is only joining to three other atoms, when the carbon atoms hybridise their outer orbitals before forming bonds, they only need to hybridise three of the orbitals rather than all four. They use the 2 s electron and two of the 2 p electrons, but leave the other 2 p electron unchanged.

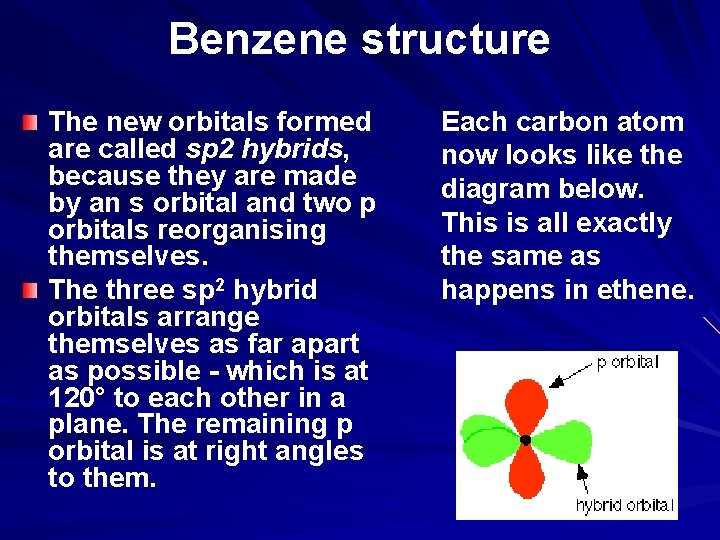
Benzene structure The new orbitals formed are called sp 2 hybrids, because they are made by an s orbital and two p orbitals reorganising themselves. The three sp 2 hybrid orbitals arrange themselves as far apart as possible - which is at 120° to each other in a plane. The remaining p orbital is at right angles to them. Each carbon atom now looks like the diagram below. This is all exactly the same as happens in ethene.

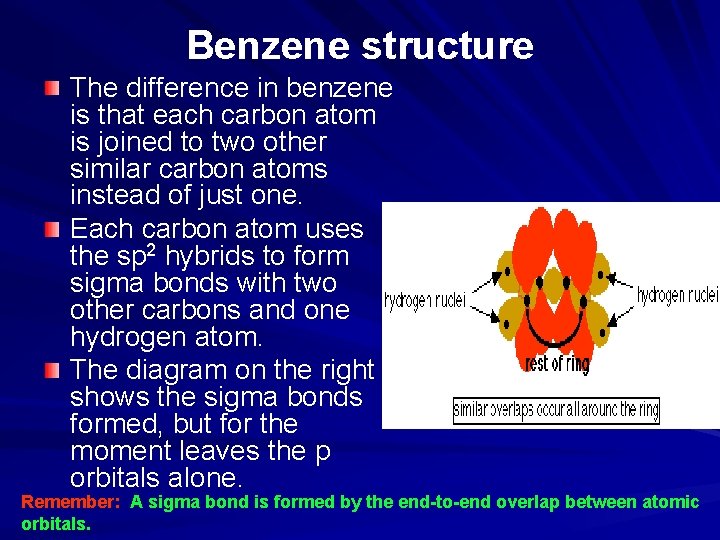
Benzene structure The difference in benzene is that each carbon atom is joined to two other similar carbon atoms instead of just one. Each carbon atom uses the sp 2 hybrids to form sigma bonds with two other carbons and one hydrogen atom. The diagram on the right shows the sigma bonds formed, but for the moment leaves the p orbitals alone. Remember: A sigma bond is formed by the end-to-end overlap between atomic orbitals.

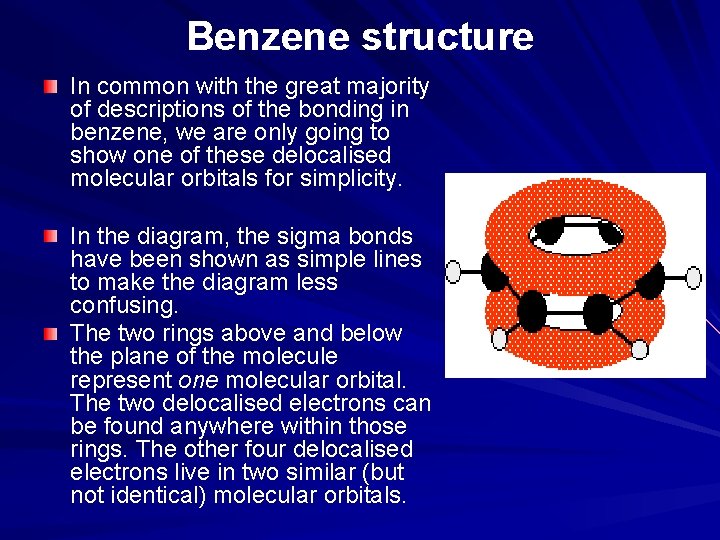
Benzene structure In common with the great majority of descriptions of the bonding in benzene, we are only going to show one of these delocalised molecular orbitals for simplicity. In the diagram, the sigma bonds have been shown as simple lines to make the diagram less confusing. The two rings above and below the plane of the molecule represent one molecular orbital. The two delocalised electrons can be found anywhere within those rings. The other four delocalised electrons live in two similar (but not identical) molecular orbitals.

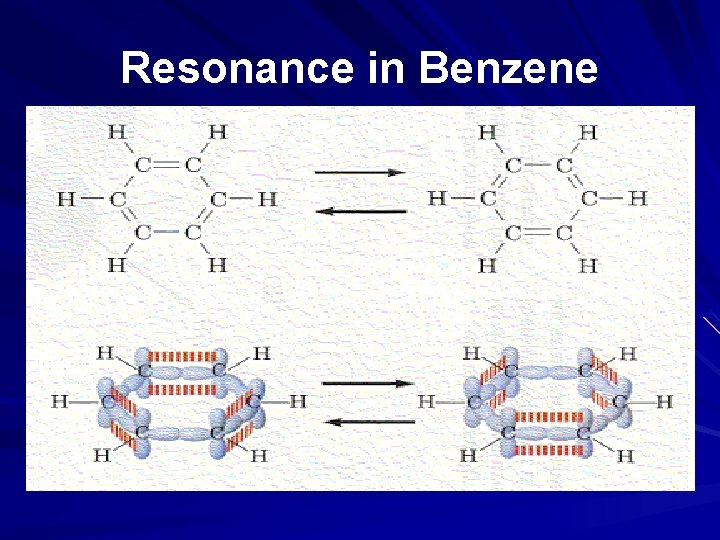
Resonance in Benzene

Warning! Be very careful how you phrase this in exams. You must never talk about the p orbitals on the carbons overlapping sideways to produce a delocalised pi bond. This upsets examiners because a pi bond can only hold 2 electrons - whereas in benzene there are 6 delocalised electrons. Talk instead about a "pi system" - or just about the delocalised electrons

Relating the orbital model to the properties of benzene The shape of benzene This is easily explained. Benzene is a regular hexagon because all the bonds are identical. The delocalisation of the electrons means that there aren't alternating double and single bonds. The energetic stability of benzene This is accounted for by the delocalisation. As a general principle, the more you can spread electrons around - in other words, the more they are delocalised - the more stable the molecule becomes. The extra stability of benzene is often referred to as "delocalisation energy".

Relating the orbital model to the properties of benzene The reluctance of benzene to undergo addition reactions With the delocalised electrons in place, benzene is about 150 k. J mol-1 more stable than it would otherwise be. If you added other atoms to a benzene ring you would have to use some of the delocalised electrons to join the new atoms to the ring. That would disrupt the delocalisation and the system would become less stable. Since about 150 k. J per mole of benzene would have to be supplied to break up the delocalisation, this isn't going to be an easy thing to do.

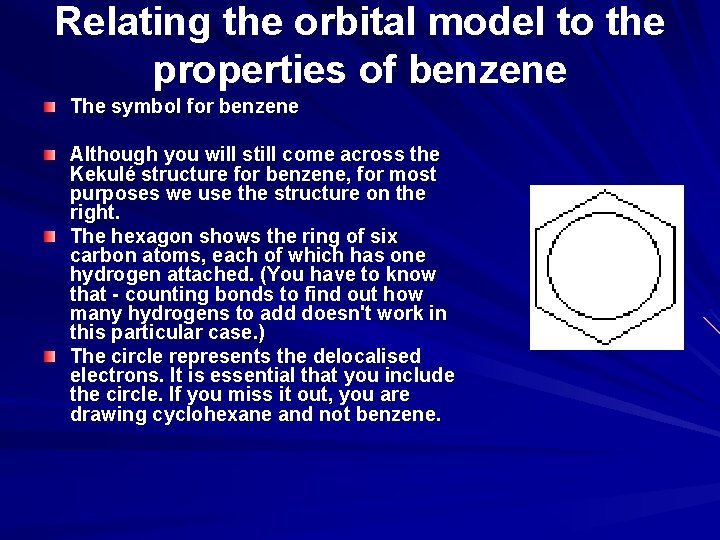
Relating the orbital model to the properties of benzene The symbol for benzene Although you will still come across the Kekulé structure for benzene, for most purposes we use the structure on the right. The hexagon shows the ring of six carbon atoms, each of which has one hydrogen attached. (You have to know that - counting bonds to find out how many hydrogens to add doesn't work in this particular case. ) The circle represents the delocalised electrons. It is essential that you include the circle. If you miss it out, you are drawing cyclohexane and not benzene.

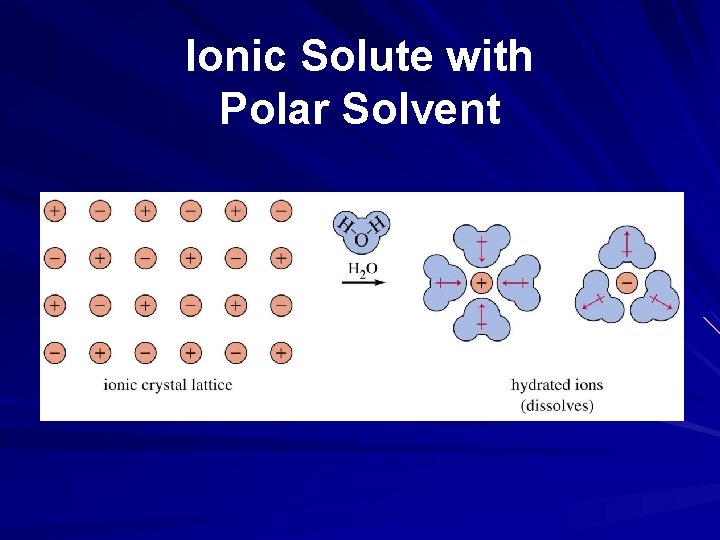
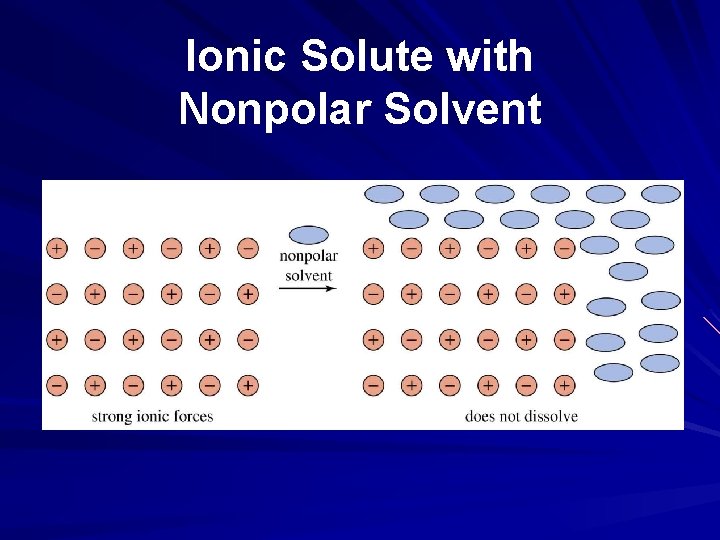
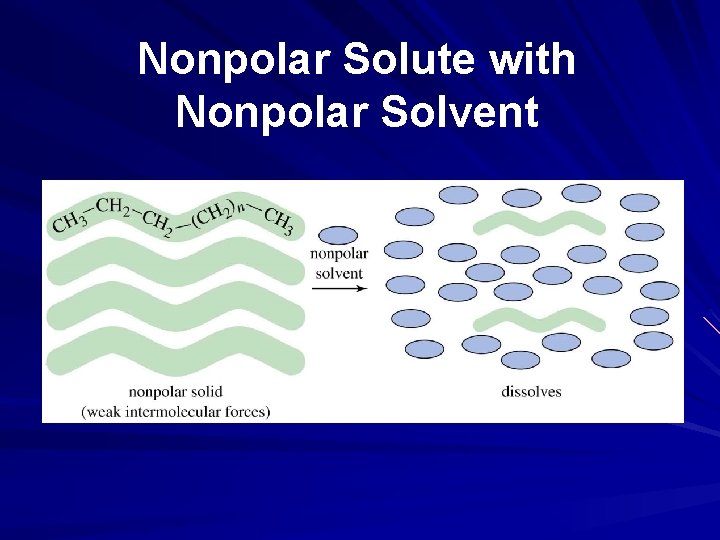
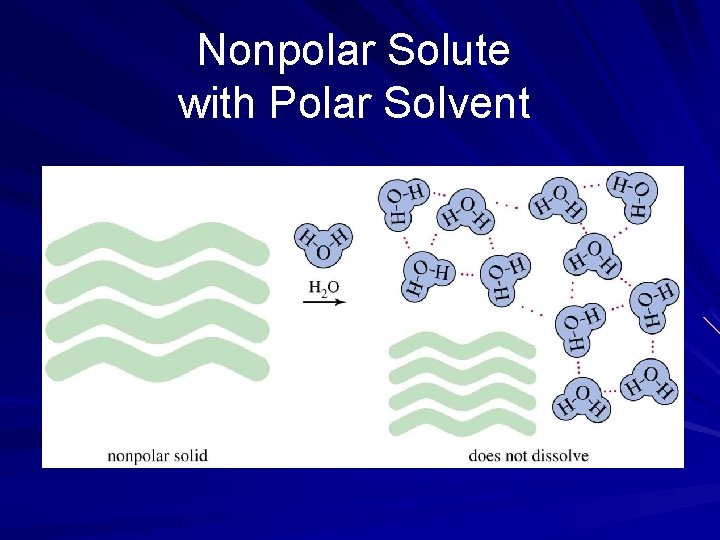
Solubility and Intermolecular Forces Like dissolves like – Polar solutes dissolve in polar solvents – Nonpolar solutes dissolve in nonpolar solvents Molecules with similar intermolecular forces will mix freely

Ionic Solute with Polar Solvent

Ionic Solute with Nonpolar Solvent

Nonpolar Solute with Nonpolar Solvent

Nonpolar Solute with Polar Solvent



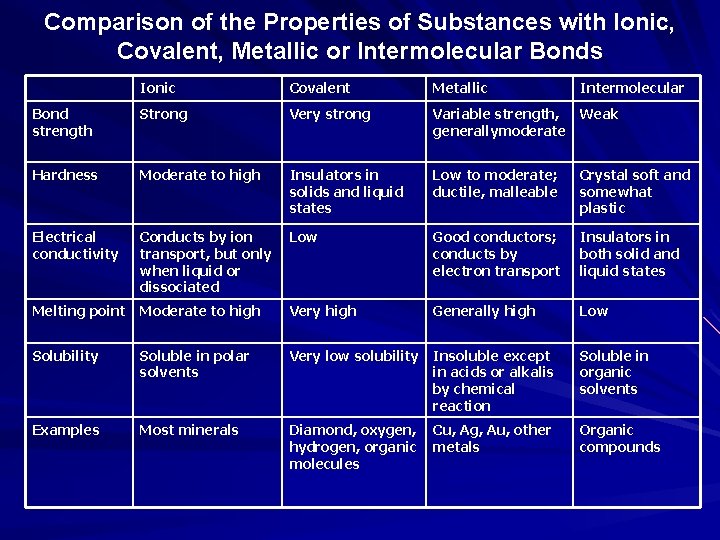
Comparison of the Properties of Substances with Ionic, Covalent, Metallic or Intermolecular Bonds Ionic Covalent Metallic Bond strength Strong Very strong Variable strength, Weak generallymoderate Hardness Moderate to high Insulators in solids and liquid states Low to moderate; ductile, malleable Crystal soft and somewhat plastic Electrical conductivity Conducts by ion transport, but only when liquid or dissociated Low Good conductors; conducts by electron transport Insulators in both solid and liquid states Melting point Moderate to high Very high Generally high Low Solubility Soluble in polar solvents Very low solubility Insoluble except in acids or alkalis by chemical reaction Soluble in organic solvents Examples Most minerals Diamond, oxygen, hydrogen, organic molecules Organic compounds Cu, Ag, Au, other metals Intermolecular

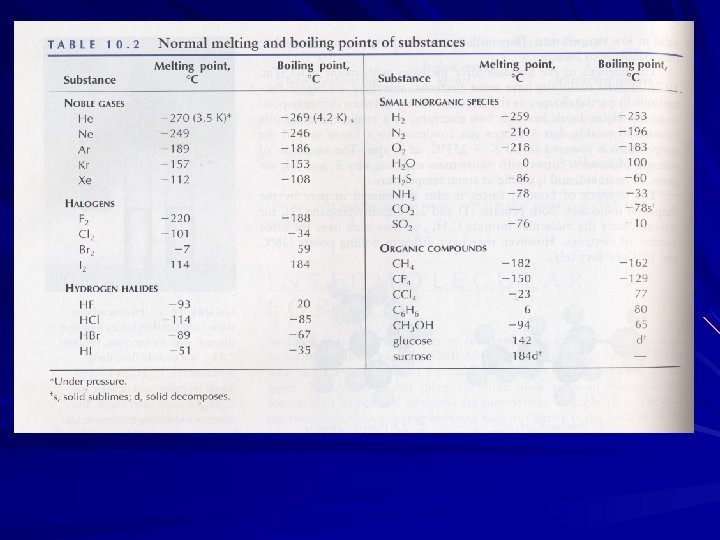
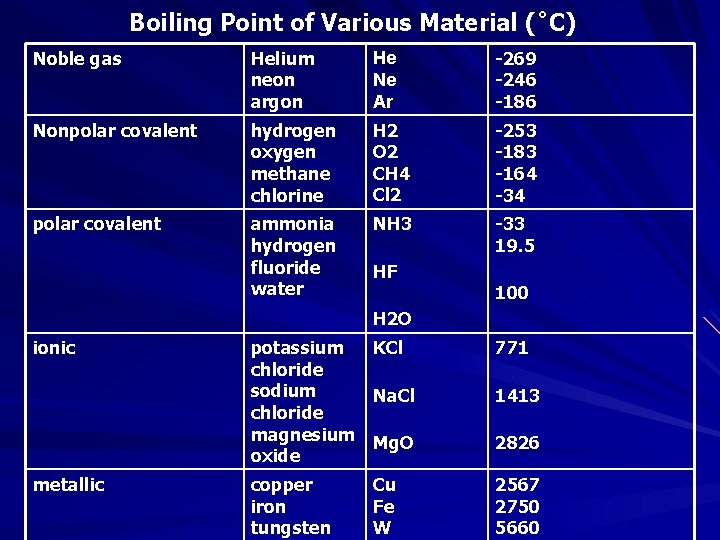
Boiling Point of Various Material (˚C) Noble gas Helium neon argon He Ne Ar -269 -246 -186 Nonpolar covalent hydrogen oxygen methane chlorine H 2 O 2 CH 4 Cl 2 -253 -183 -164 -34 polar covalent ammonia hydrogen fluoride water NH 3 -33 19. 5 HF 100 H 2 O ionic metallic potassium KCl chloride sodium Na. Cl chloride magnesium Mg. O oxide 771 copper iron tungsten 2567 2750 5660 Cu Fe W 1413 2826



REFERENCES q VIRTUAL CHEMBOOK http: //www. elmhurst. edu/%7 Echm/vchembook/index. html q Gary L. Bertrand Department of Chemistry University of Missouri-Rolla q chemguide. Helping you to understand Chemistry Jim Clark 2005 http: //www. chemguide. co. uk/ q Minerals http: //www. uwgb. edu/dutchs/Earth. SC 202 Notes/minerals. htm