Intermolecular Forces Intramolecular Forces The attractive forces between
Intermolecular Forces
Intramolecular Forces: – The attractive forces between atoms and ions within a molecule – e. g Ionic bond, covalent bonds (double and triple bonds) – Strong Intermolecular Forces: – The attractive forces between molecules – E. g. Van der Waals forces (London dispersion forces, dipole-dipole forces), hydrogen bonds – Weak (in comparison to intramolecular forces) – I. e. much less energy to melt H 2 O (inter) than for it to decompose into H 2 and O 2 (intra)
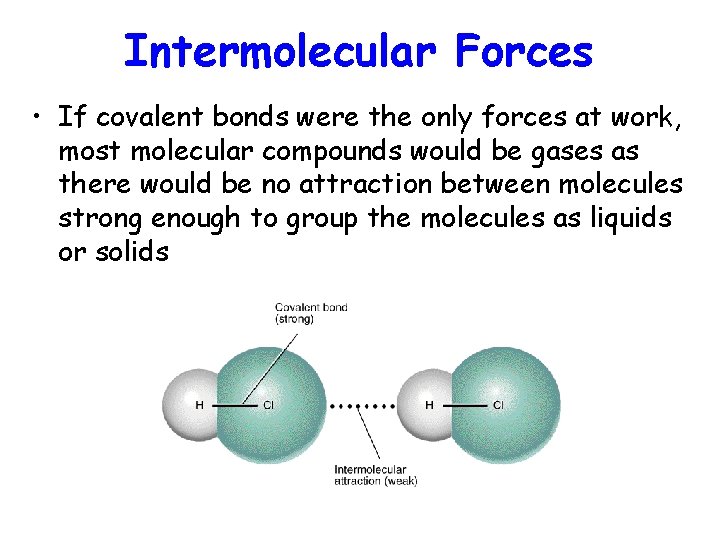
Intermolecular Forces • If covalent bonds were the only forces at work, most molecular compounds would be gases as there would be no attraction between molecules strong enough to group the molecules as liquids or solids

van der Waals Forces • Dipole-dipole • London Dispersion
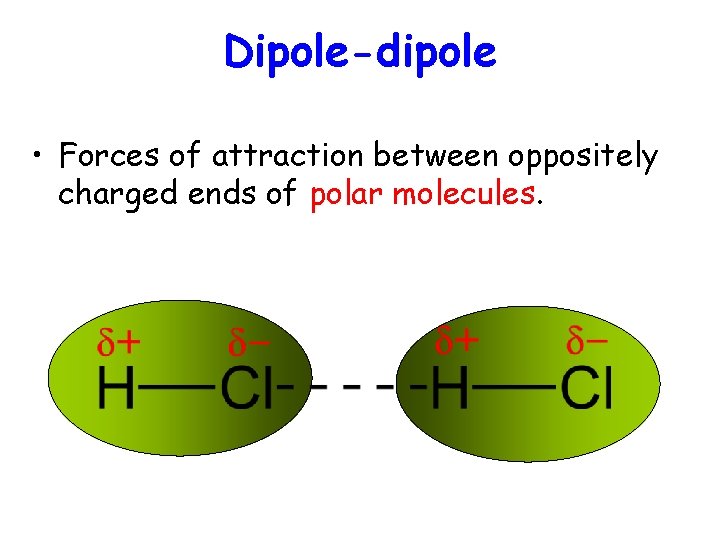
Dipole-dipole • Forces of attraction between oppositely charged ends of polar molecules.
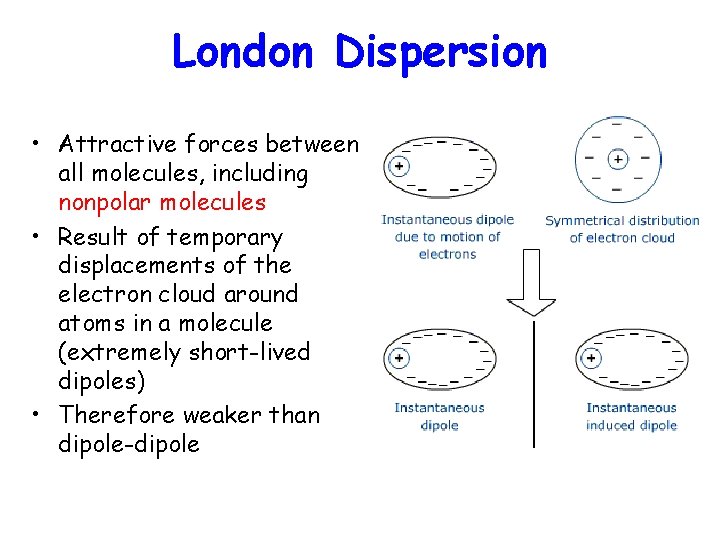
London Dispersion • Attractive forces between all molecules, including nonpolar molecules • Result of temporary displacements of the electron cloud around atoms in a molecule (extremely short-lived dipoles) • Therefore weaker than dipole-dipole
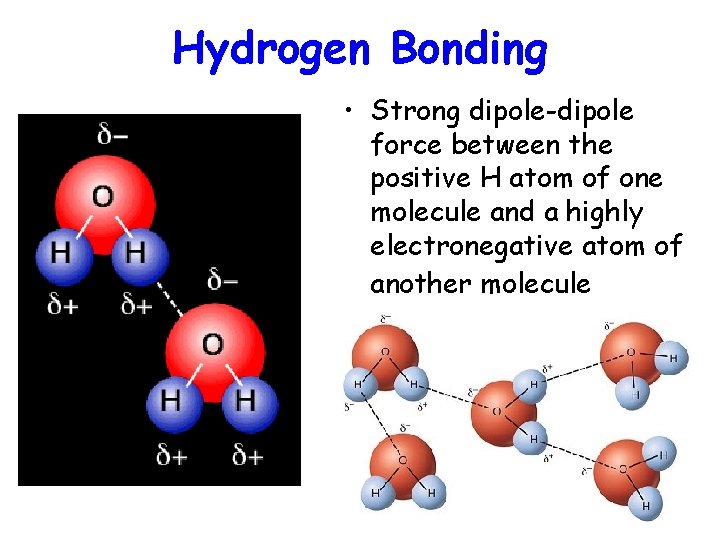
Hydrogen Bonding • Strong dipole-dipole force between the positive H atom of one molecule and a highly electronegative atom of another molecule

Practice Problems • p. 88 #7 -12
- Slides: 8