Intermolecular Forces I Phases of Matter Liquids Solids














































- Slides: 98

Intermolecular Forces I Phases of Matter Liquids, Solids (Crystals) & Solutions Colligative Properties Dr. Ron Rusay

Intermolecular Forces: Phases of Matter & Colligative Properties • Changes of State – Phase transitions – Phase Diagrams • Liquid State – Pure substances and colligative properties of solutions, which depend upon the ratio of the number of solute particles to the number of solvent molecules in a solution. They are independent of the nature of the solute particles.

Intermolecular Forces: Phases of Matter Solid State Classification of Solids by Type of Attraction between Units Crystalline solids; crystal lattices and unit cells Structures of some crystalline solids Determining the Crystal Structure by X-ray Diffraction

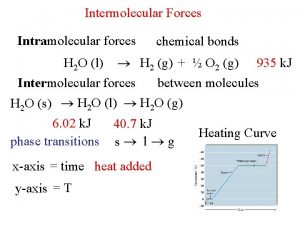
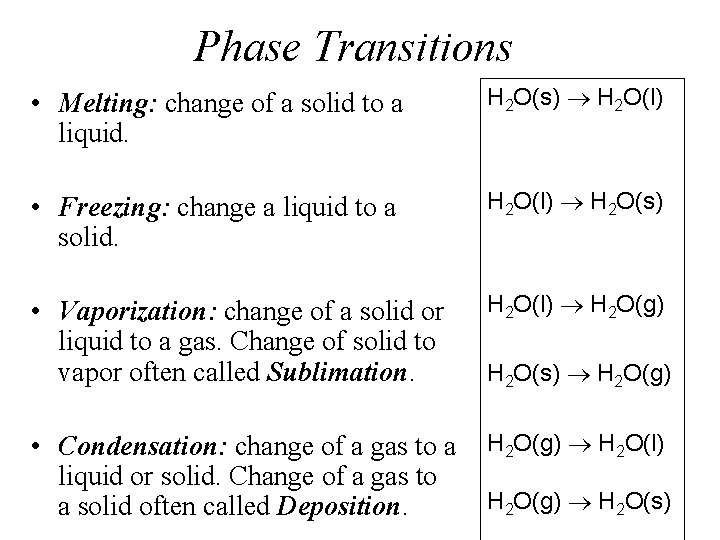
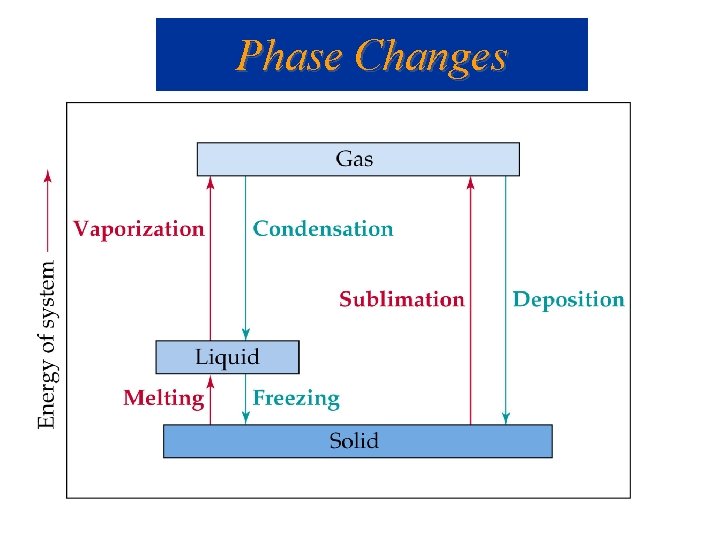
Phase Transitions • Melting: change of a solid to a liquid. H 2 O(s) H 2 O(l) • Freezing: change a liquid to a solid. H 2 O(l) H 2 O(s) • Vaporization: change of a solid or liquid to a gas. Change of solid to vapor often called Sublimation. H 2 O(l) H 2 O(g) H 2 O(s) H 2 O(g) • Condensation: change of a gas to a H 2 O(g) H 2 O(l) liquid or solid. Change of a gas to H 2 O(g) H 2 O(s) a solid often called Deposition.

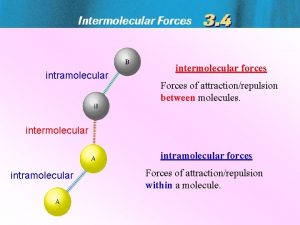
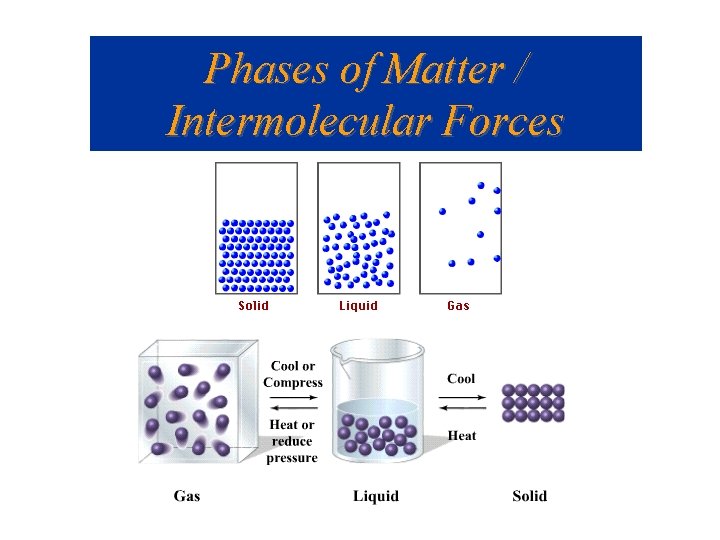
Phases of Matter / Intermolecular Forces

Phase Changes

QUESTION

ANSWER B) crystallization As a substance crystallizes, generally from a liquid, but a gaseous starting point is possible, the molecules, atoms, or ions lose kinetic energy to allow for bonding. This kinetic energy is emitted as heat.

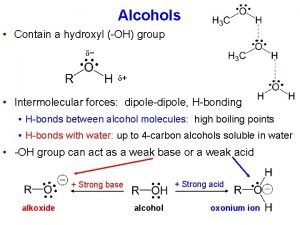
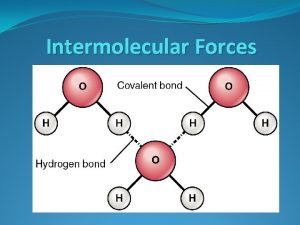
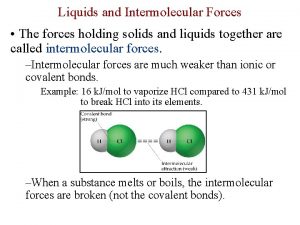
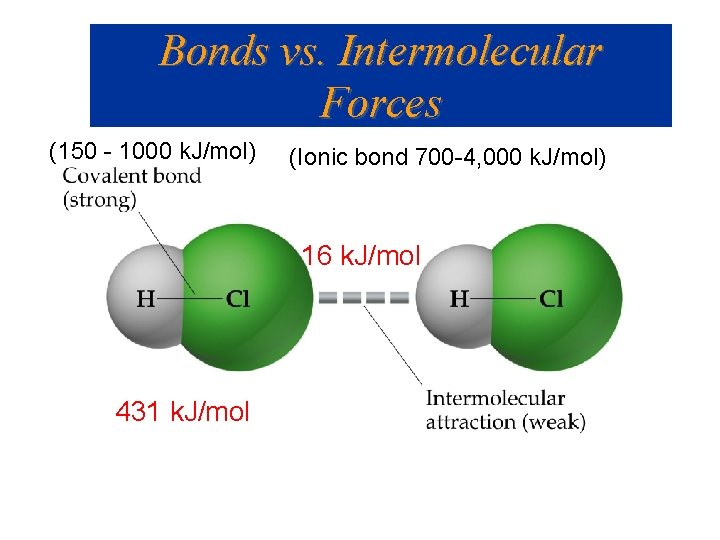
Bonds vs. Intermolecular Forces (150 - 1000 k. J/mol) (Ionic bond 700 -4, 000 k. J/mol) 16 k. J/mol 431 k. J/mol

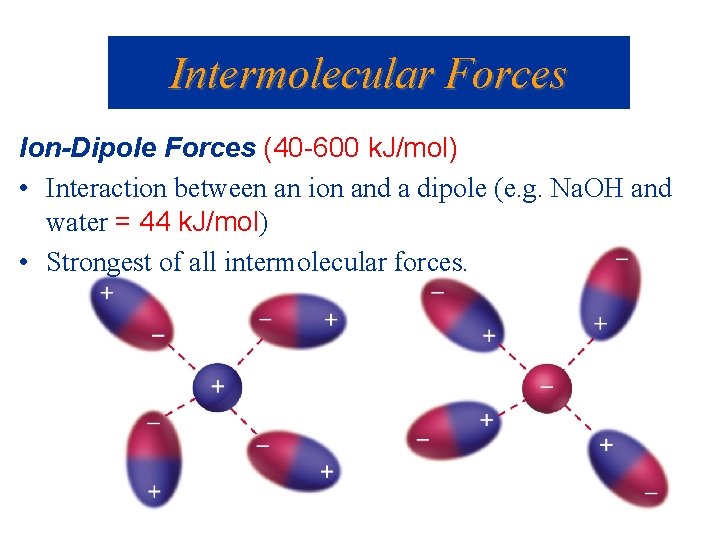
Intermolecular Forces Ion-Dipole Forces (40 -600 k. J/mol) • Interaction between an ion and a dipole (e. g. Na. OH and water = 44 k. J/mol) • Strongest of all intermolecular forces.

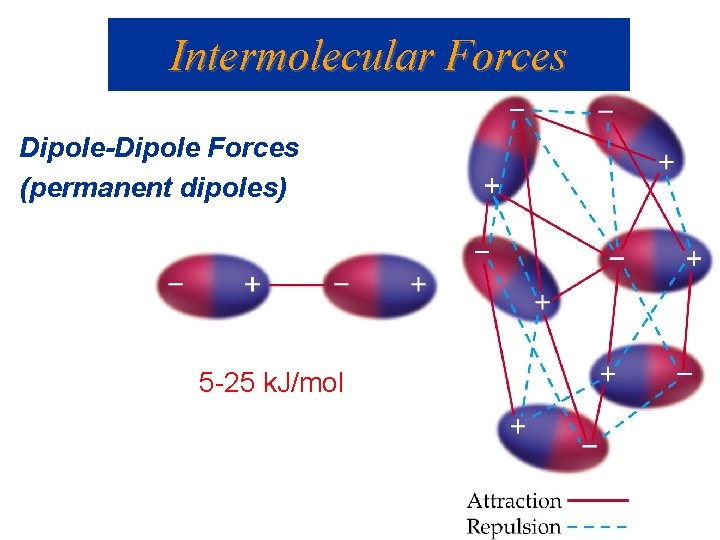
Intermolecular Forces Dipole-Dipole Forces (permanent dipoles) 5 -25 k. J/mol

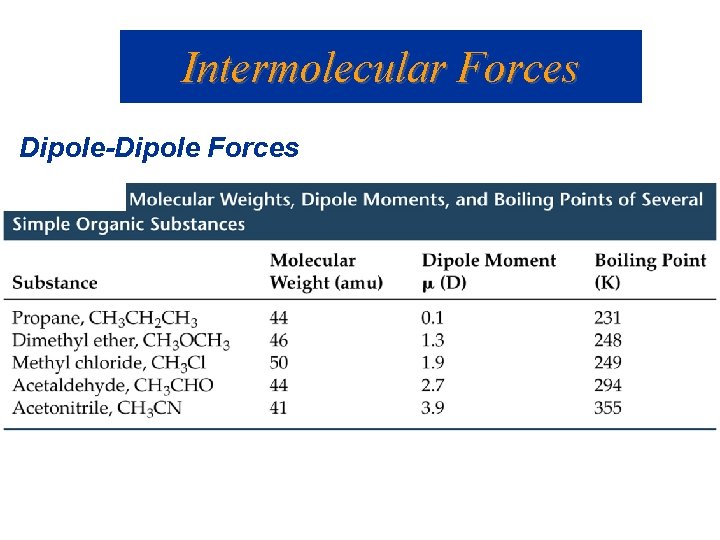
Intermolecular Forces Dipole-Dipole Forces

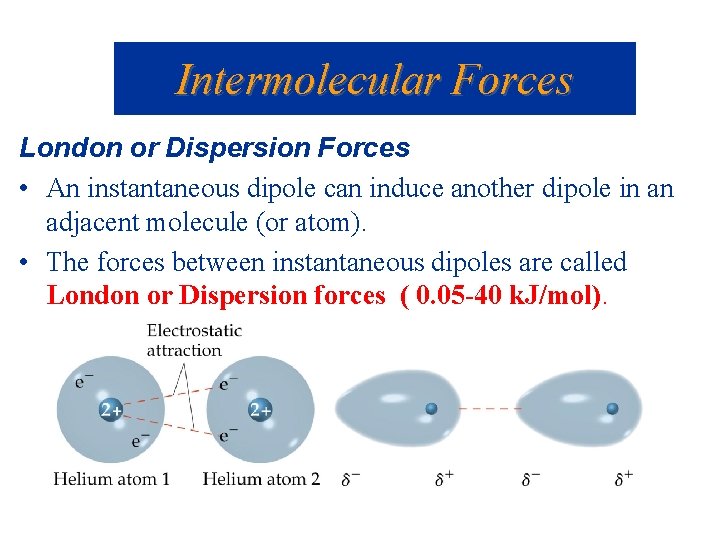
Intermolecular Forces London or Dispersion Forces • An instantaneous dipole can induce another dipole in an adjacent molecule (or atom). • The forces between instantaneous dipoles are called London or Dispersion forces ( 0. 05 -40 k. J/mol).

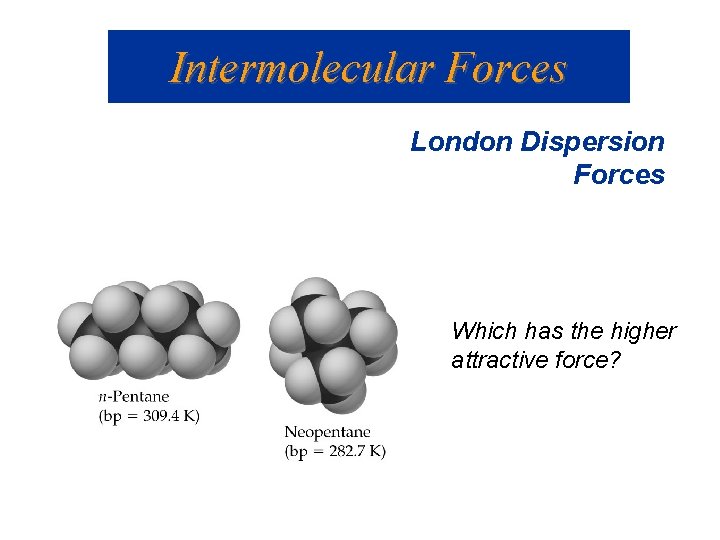
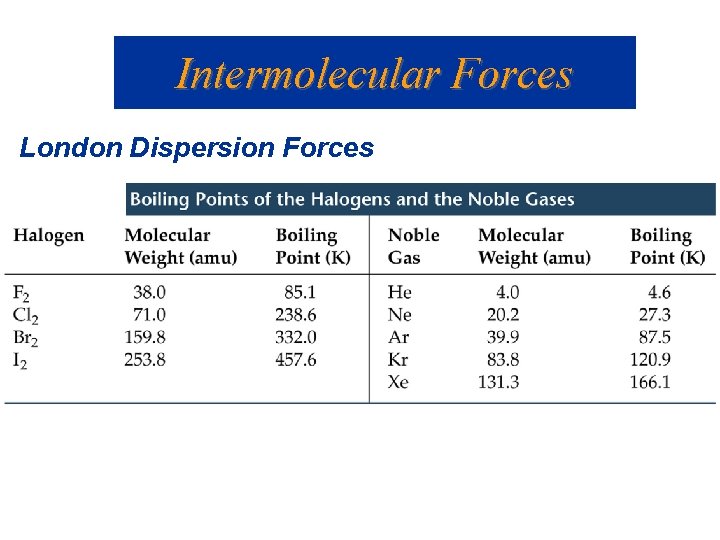
Intermolecular Forces London Dispersion Forces Which has the higher attractive force?

Intermolecular Forces London Dispersion Forces

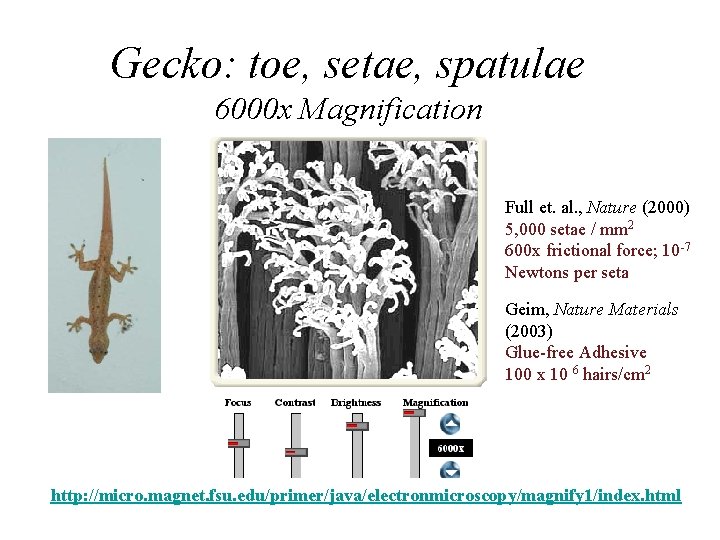
Gecko: toe, setae, spatulae 6000 x Magnification Full et. al. , Nature (2000) 5, 000 setae / mm 2 600 x frictional force; 10 -7 Newtons per seta Geim, Nature Materials (2003) Glue-free Adhesive 100 x 10 6 hairs/cm 2 http: //micro. magnet. fsu. edu/primer/java/electronmicroscopy/magnify 1/index. html

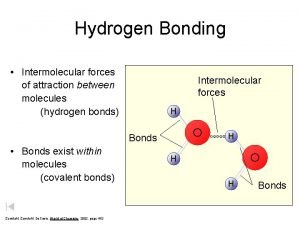
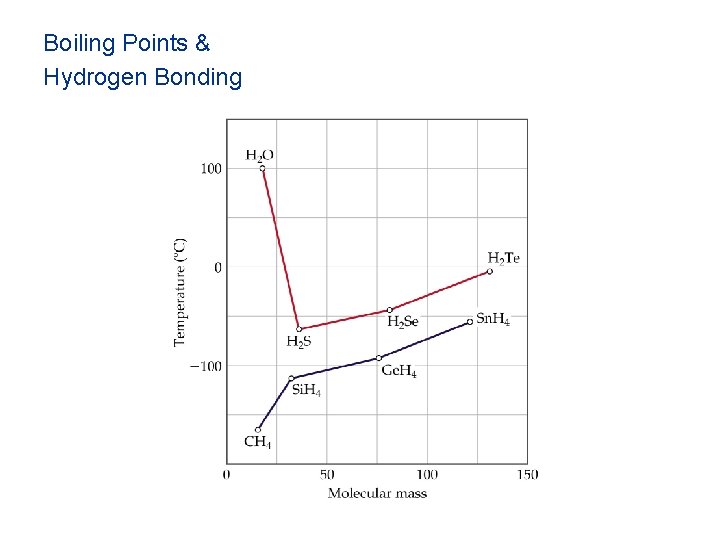
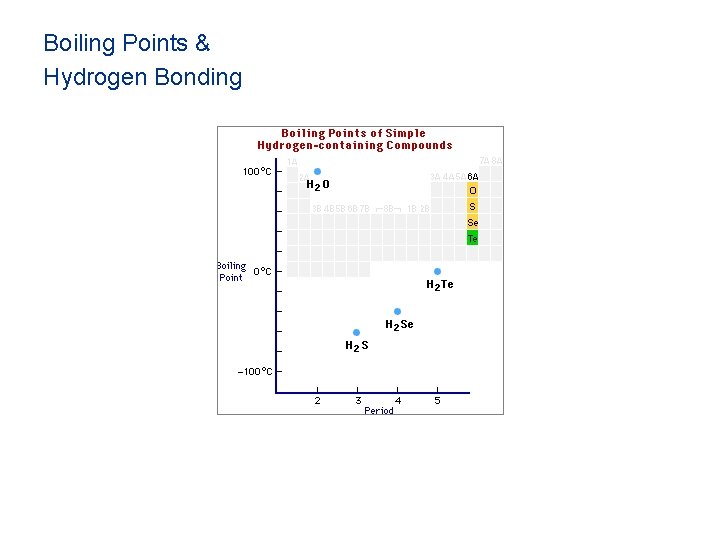
Boiling Points & Hydrogen Bonding

Boiling Points & Hydrogen Bonding

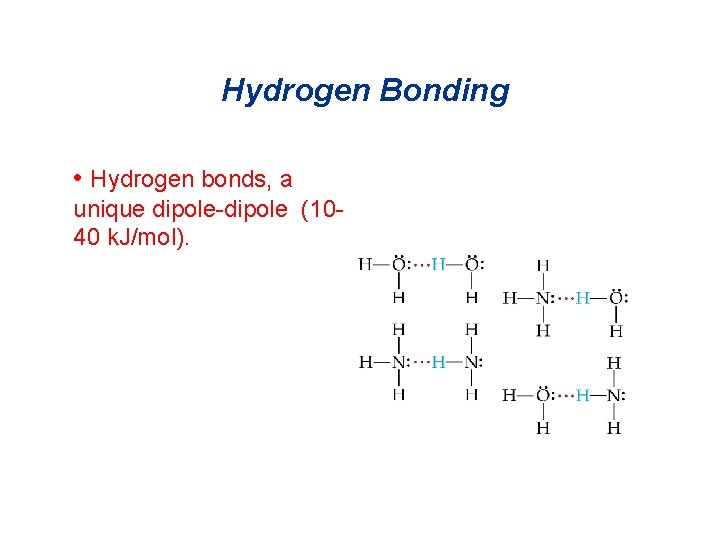
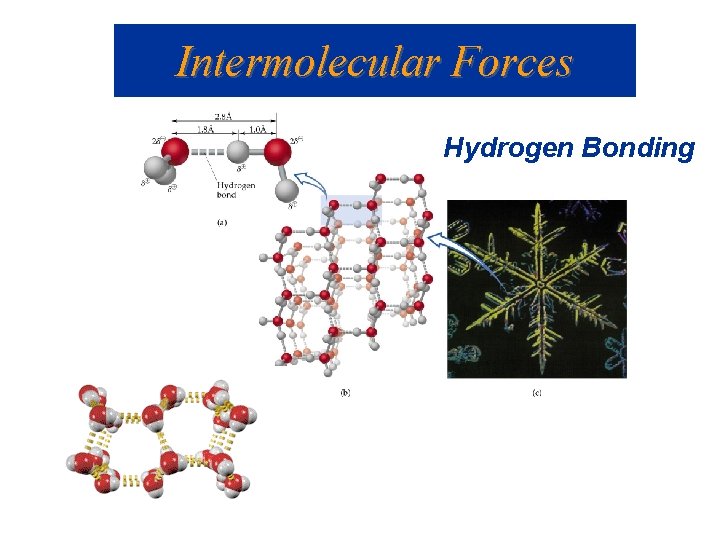
Hydrogen Bonding • Hydrogen bonds, a unique dipole-dipole (1040 k. J/mol).


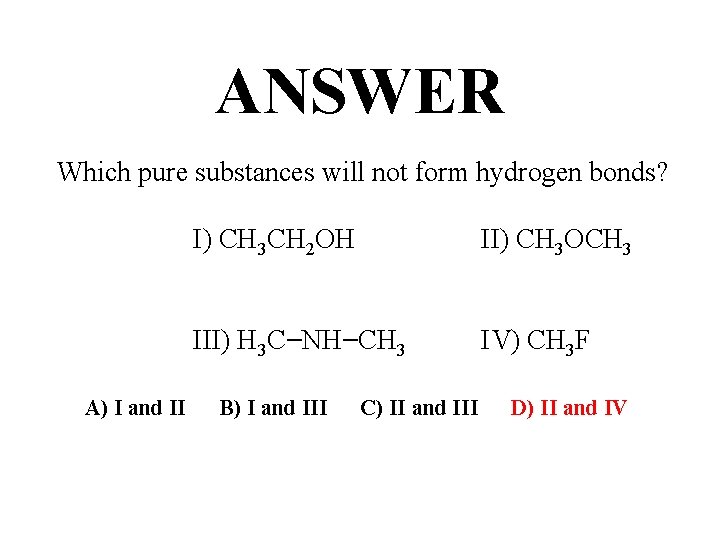
QUESTION Which pure substances will not form hydrogen bonds? I) CH 3 CH 2 OH II) CH 3 OCH 3 III) H 3 C−NH−CH 3 IV) CH 3 F A) I and II B) I and III C) II and III D) II and IV

ANSWER Which pure substances will not form hydrogen bonds? I) CH 3 CH 2 OH II) CH 3 OCH 3 III) H 3 C−NH−CH 3 IV) CH 3 F A) I and II B) I and III C) II and III D) II and IV

Intermolecular Forces Hydrogen Bonding

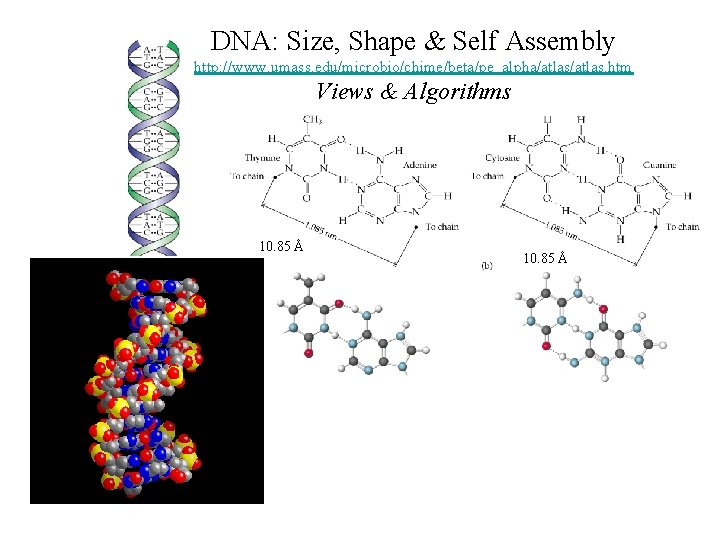
DNA: Size, Shape & Self Assembly http: //www. umass. edu/microbio/chime/beta/pe_alpha/atlas. htm Views & Algorithms 10. 85 Å

Intermolecular Forces

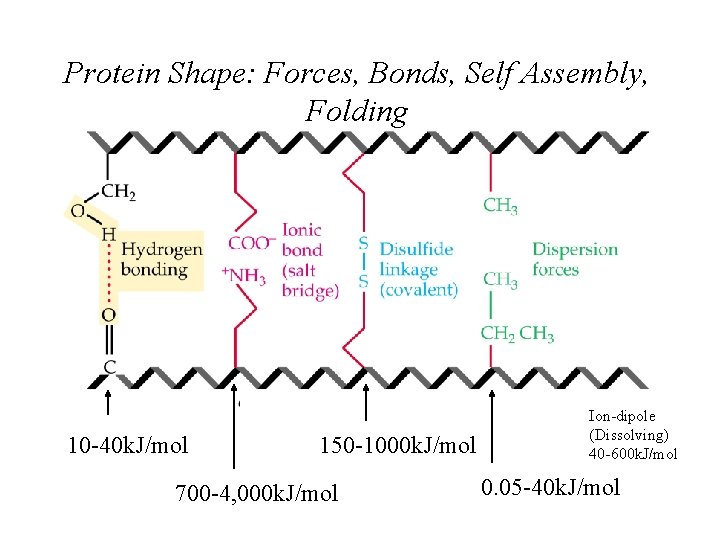
Protein Shape: Forces, Bonds, Self Assembly, Folding 10 -40 k. J/mol 150 -1000 k. J/mol 700 -4, 000 k. J/mol Ion-dipole (Dissolving) 40 -600 k. J/mol 0. 05 -40 k. J/mol

QUESTION Predict which liquid will have the strongest intermolecular forces of attraction (neglect the small differences in molar masses). A) CH 3 COCH 2 CH 3 (molar mass = 86 g/mol) B) CH 3 CH 2 CH 2 OH (molar mass = 88 g/mol) C) CH 3 CH 2 CH 2 CH 3 (molar mass = 86 g/mol) D) HOH 2 C−CH=CH−CH 2 OH (molar mass = 88 g/mol)

ANSWER Predict which liquid will have the strongest intermolecular forces of attraction (neglect the small differences in molar masses). A) CH 3 COCH 2 CH 3 (molar mass = 86 g/mol) B) CH 3 CH 2 CH 2 OH (molar mass = 88 g/mol) C) CH 3 CH 2 CH 2 CH 3 (molar mass = 86 g/mol) D) HOH 2 C−CH=CH−CH 2 OH (molar mass = 88 g/mol)

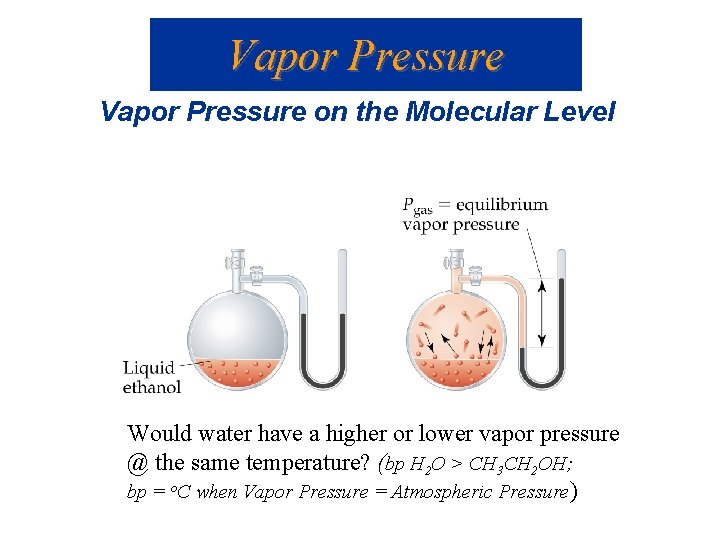
Vapor Pressure on the Molecular Level Would water have a higher or lower vapor pressure @ the same temperature? (bp H 2 O > CH 3 CH 2 OH; bp = o. C when Vapor Pressure = Atmospheric Pressure)

Vapor Pressure Explaining Vapor Pressure on a Molecular Level

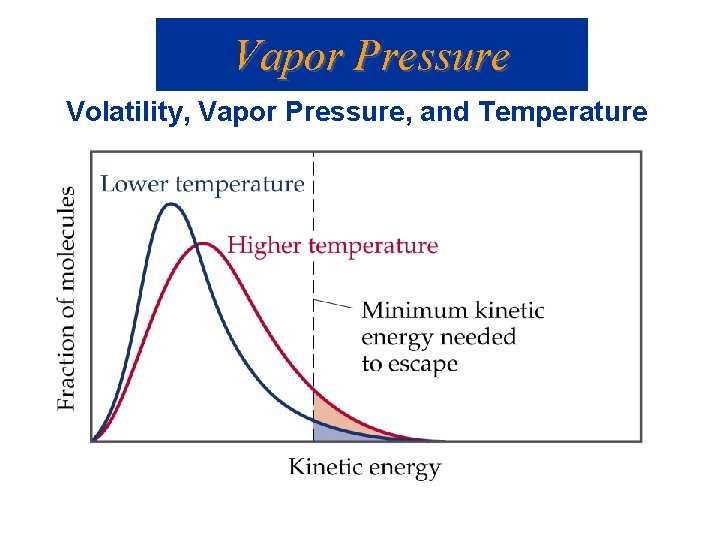
Vapor Pressure Volatility, Vapor Pressure, and Temperature

Vapor Pressure Solid Liquid Gas

QUESTION

ANSWER E) The vapor pressure of a liquid. Molecules at the surface of a liquid will be held tighter by stronger intermolecular forces, making it more difficult for them to escape into the vapor phase.

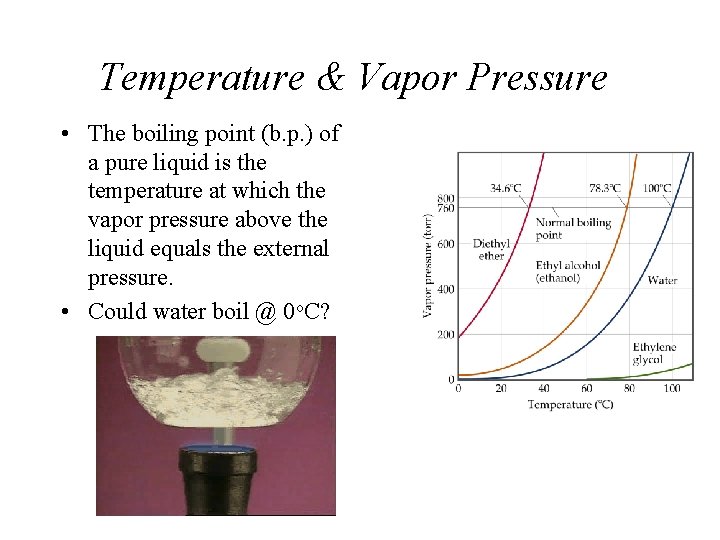
Temperature & Vapor Pressure • The boiling point (b. p. ) of a pure liquid is the temperature at which the vapor pressure above the liquid equals the external pressure. • Could water boil @ 0 o. C?

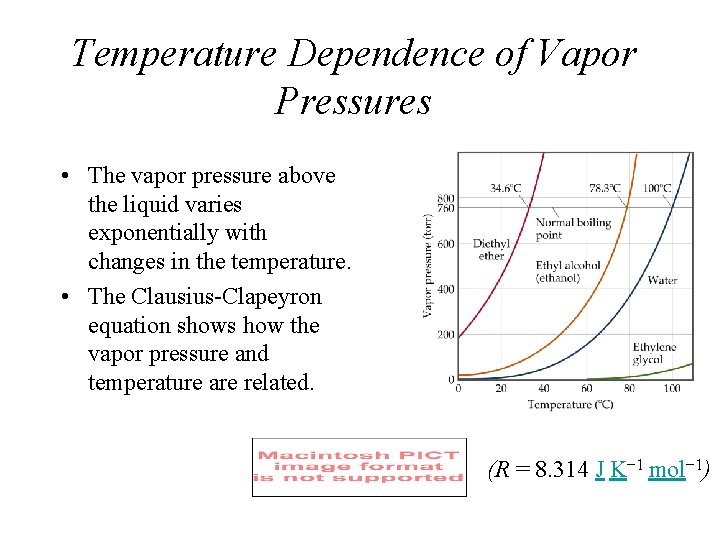
Temperature Dependence of Vapor Pressures • The vapor pressure above the liquid varies exponentially with changes in the temperature. • The Clausius-Clapeyron equation shows how the vapor pressure and temperature are related. (R = 8. 314 J K− 1 mol− 1)

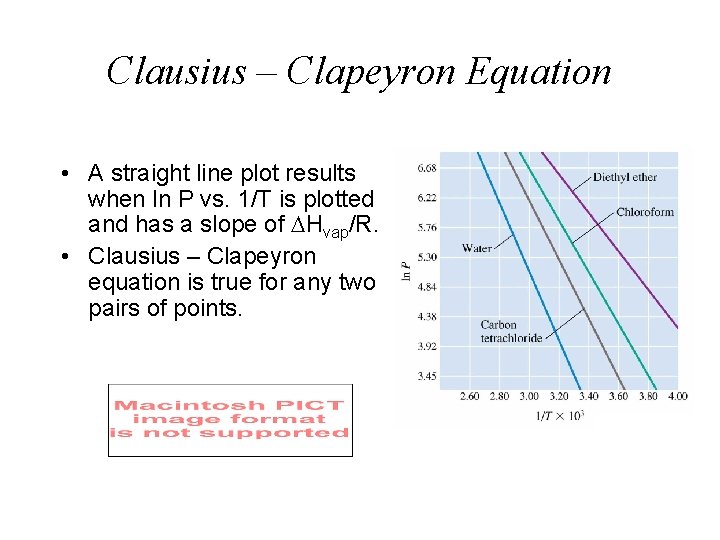
Clausius – Clapeyron Equation • A straight line plot results when ln P vs. 1/T is plotted and has a slope of Hvap/R. • Clausius – Clapeyron equation is true for any two pairs of points.

QUESTION

ANSWER D) diethyl ether, CH 3 CH 2–O–CH 2 CH 3 Diethyl ether boils first as the temperature increases, leading one to believe that its vapor pressure at any temperature is also the highest of the given group of compounds.

Heating Curve http: //chemconnections. org/general/movies/Heating. Curves. swf

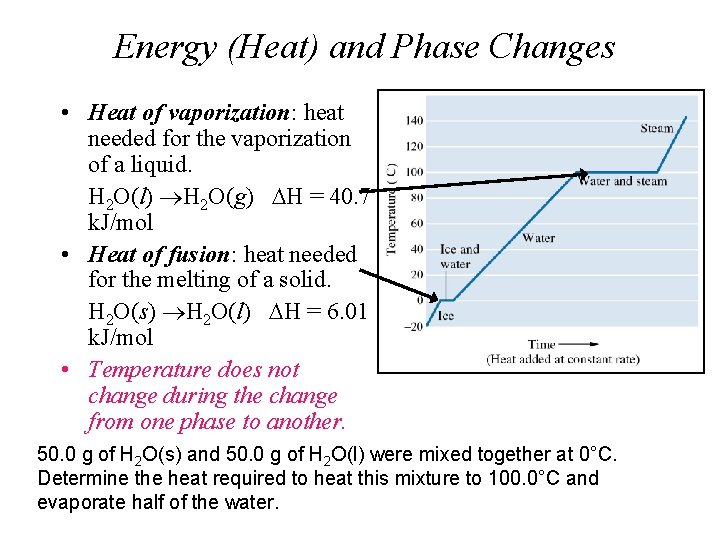
Energy (Heat) and Phase Changes • Heat of vaporization: heat needed for the vaporization of a liquid. H 2 O(l) H 2 O(g) H = 40. 7 k. J/mol • Heat of fusion: heat needed for the melting of a solid. H 2 O(s) H 2 O(l) H = 6. 01 k. J/mol • Temperature does not change during the change from one phase to another. 50. 0 g of H 2 O(s) and 50. 0 g of H 2 O(l) were mixed together at 0°C. Determine the heat required to heat this mixture to 100. 0°C and evaporate half of the water.

Intermolecular Forces II Phases of Matter / Phases Diagrams Solids (Crystals) & Solutions Colligative Properties Dr. Ron Rusay

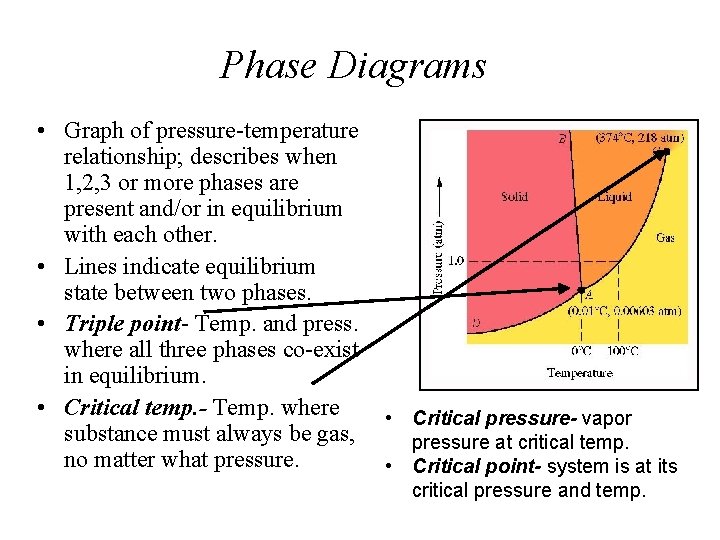
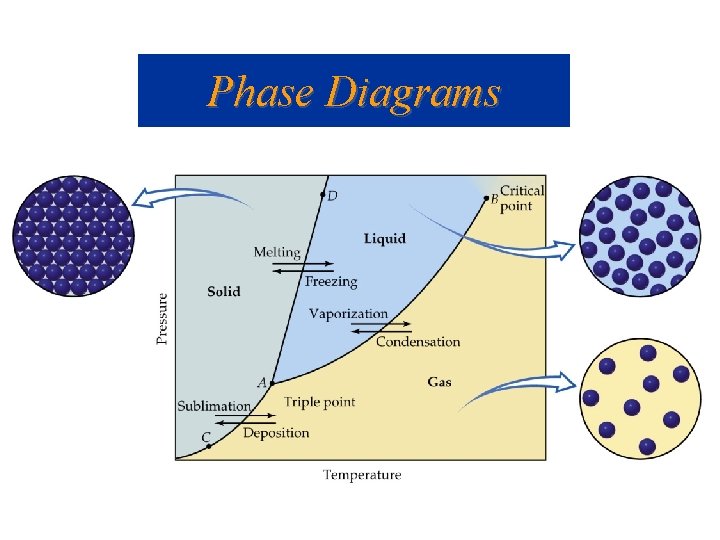
Phase Diagrams • Graph of pressure-temperature relationship; describes when 1, 2, 3 or more phases are present and/or in equilibrium with each other. • Lines indicate equilibrium state between two phases. • Triple point- Temp. and press. where all three phases co-exist in equilibrium. • Critical temp. - Temp. where • Critical pressure- vapor substance must always be gas, pressure at critical temp. no matter what pressure. • Critical point- system is at its critical pressure and temp.

Phase Diagrams

Phase Diagrams

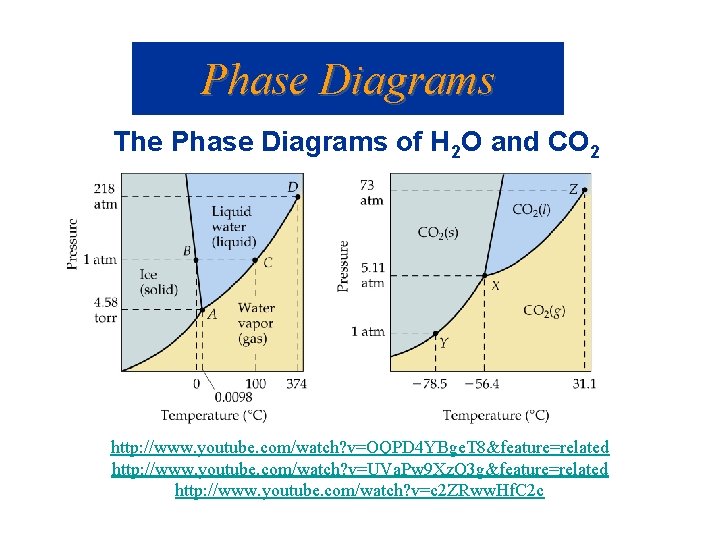
Phase Diagrams The Phase Diagrams of H 2 O and CO 2 http: //www. youtube. com/watch? v=OQPD 4 YBge. T 8&feature=related http: //www. youtube. com/watch? v=UVa. Pw 9 Xz. Q 3 g&feature=related http: //www. youtube. com/watch? v=c 2 ZRww. Hf. C 2 c

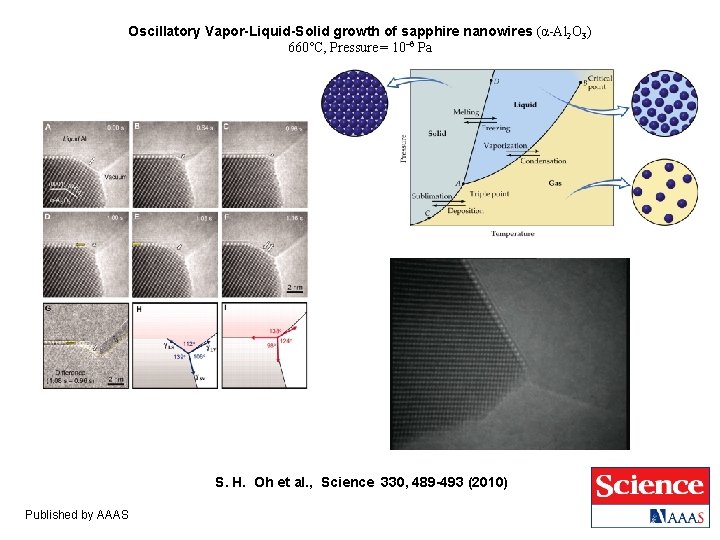
Oscillatory Vapor-Liquid-Solid growth of sapphire nanowires (α-Al 2 O 3) 660°C, Pressure = 10– 6 Pa S. H. Oh et al. , Science 330, 489 -493 (2010) Published by AAAS

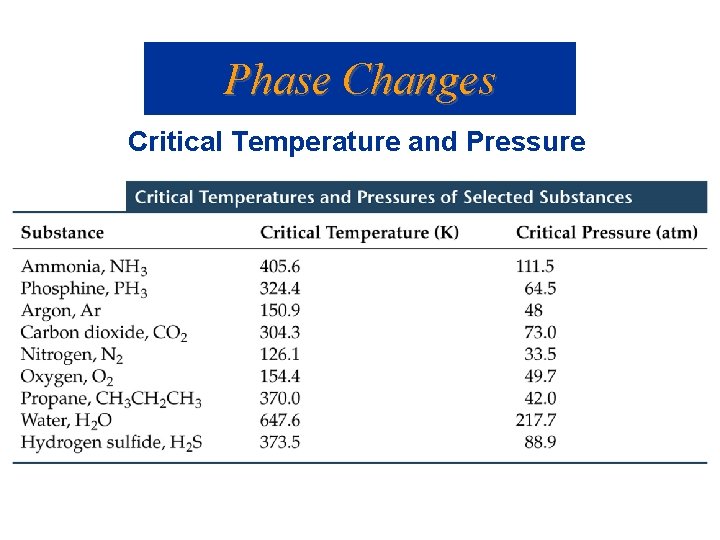
Phase Changes Critical Temperature and Pressure

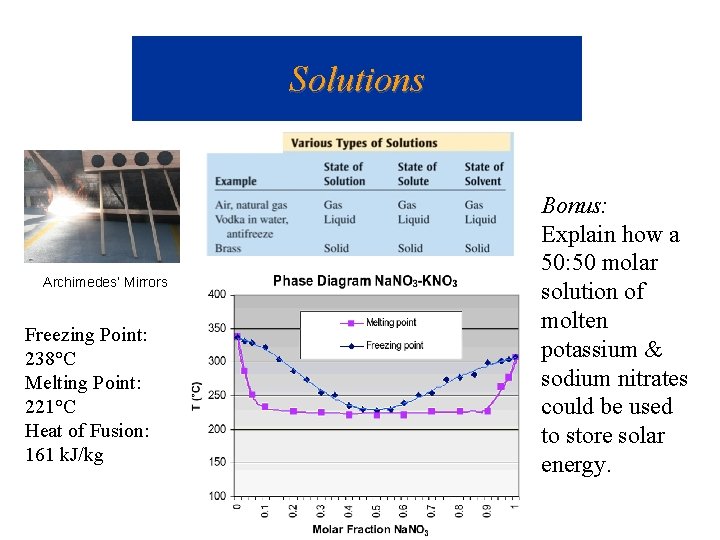
Solutions Archimedes’ Mirrors Freezing Point: 238°C Melting Point: 221°C Heat of Fusion: 161 k. J/kg Bonus: Explain how a 50: 50 molar solution of molten potassium & sodium nitrates could be used to store solar energy.

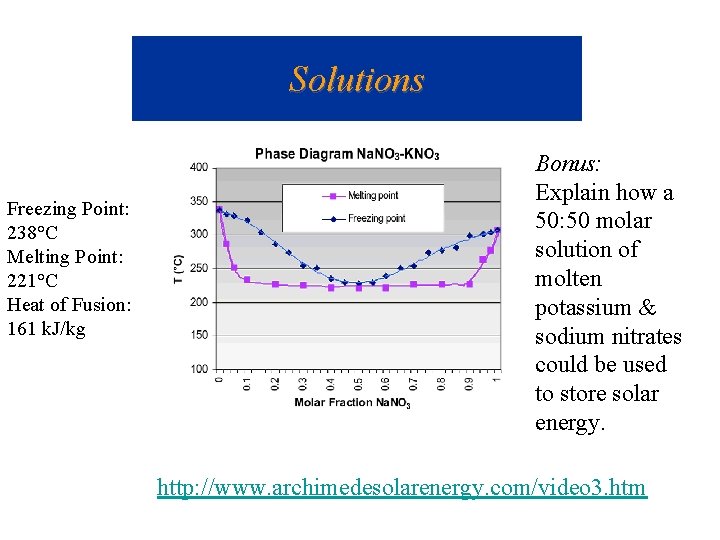
Solutions Freezing Point: 238°C Melting Point: 221°C Heat of Fusion: 161 k. J/kg Bonus: Explain how a 50: 50 molar solution of molten potassium & sodium nitrates could be used to store solar energy. http: //www. archimedesolarenergy. com/video 3. htm

Solana $ 2 billion 3 years 2, 200, 000 m 2 2, 700 mirrors 280 Mega. Watts 70, 000 homes - 475, 000 MT CO 2 Equivalent Bonus: The heat from a solution of molten potassium & sodium nitrates is used to store the energy, which turns a turbine in 6 hrs of darkness/ day. http: //www. youtube. com/watch/? v=G 1 hdo. Wk 17 w. U

Solutions

QUESTION

ANSWER B) decreases over time. The concentration of the solution increases as the water evaporates. The higher the concentration of salt, the lower the vapor pressure.

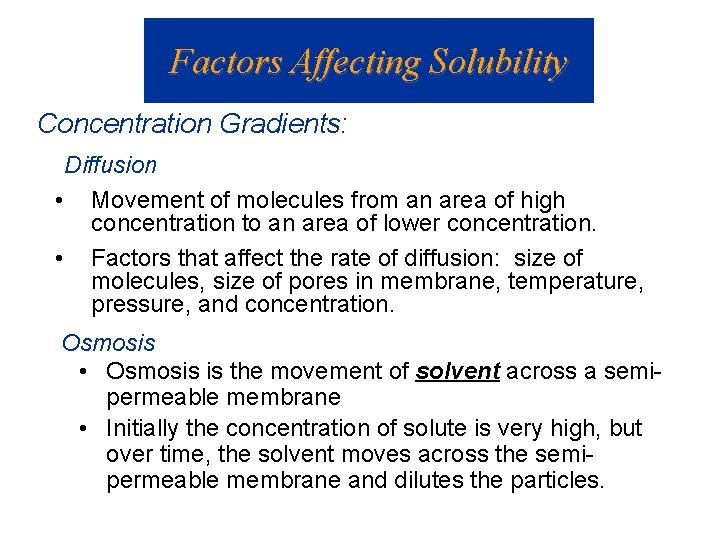
Factors Affecting Solubility Concentration Gradients: Diffusion • Movement of molecules from an area of high concentration to an area of lower concentration. • Factors that affect the rate of diffusion: size of molecules, size of pores in membrane, temperature, pressure, and concentration. Osmosis • Osmosis is the movement of solvent across a semipermeable membrane • Initially the concentration of solute is very high, but over time, the solvent moves across the semipermeable membrane and dilutes the particles.


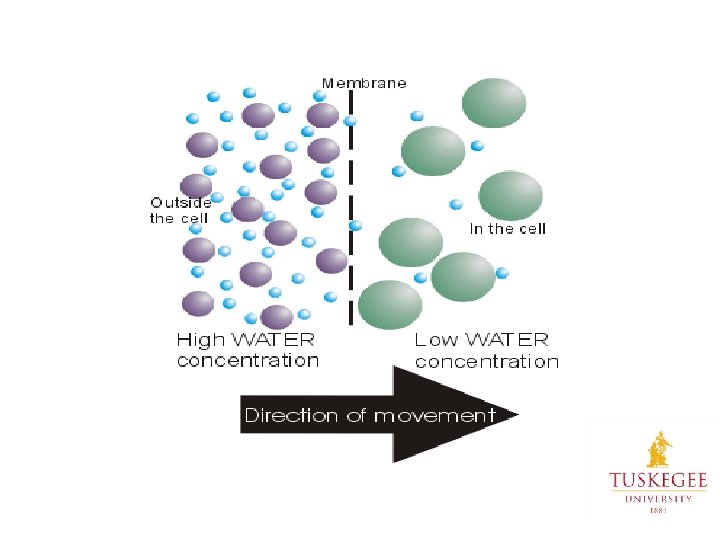
Osmosis – A Special kind of Diffusion of water across a selectively permeable membrane (a barrier that allows some substances to pass but not others). The cell membrane is such a barrier. Small molecules pass through – eg. water Large molecules can’t pass through – eg. proteins and complex carbohydrates https: //www. youtube. com/watch? v=sdi. Jt. DRJQEc

Over time molecules move across the membrane until the concentration of solute is equal on both sides. This type of solution is called ISOTONIC.

Factors Affecting Solubility Pressure Effects If Sg is the solubility of a gas, k is a constant, and Pg is the partial pressure of a gas, then Henry’s Law gives:

QUESTION A minimum of 1. 3 10– 4 M O 2 must be maintained in freshwater supplies to sustain aquatic life. In the mountains of Montana, the partial pressure of O 2 may drop to 0. 15 atm. What is the water concentration of O 2 there? Henry’s constant for O 2 = 1. 3 10– 3 mol/L-atm. At the lower elevations at the base of those mountains, would more or less O 2 be dissolved in water? • M = 2. 0 10– 4; more dissolved • M = 8. 7 10– 4; more dissolved • M = 2. 0 10– 4; less dissolved • M = 8. 7 10– 4; less dissolved

ANSWER A) provides the correct M and the correct change in concentration. Henry’s Law relates pressure of a gas over a solution to the concentration of the gas in the solution: C = k P. At lower altitudes, the partial pressure of O 2 would be higher, thus more O 2 would dissolve. The huge fishing population of Montana is very appreciative of Henry’s Law.

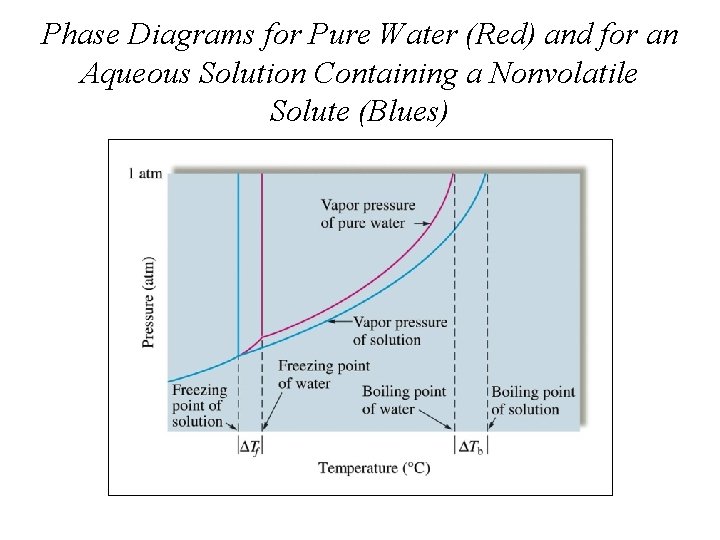
Phase Diagrams for Pure Water (Red) and for an Aqueous Solution Containing a Nonvolatile Solute (Blues)

Sugar Dissolved in Water to Make Candy Causes the Boiling Point to be Elevated

Spreading Salt on a Highway The Addition of Antifreeze Lowers the Freezing Point of Water in a Car's Radiator

Colligative Properties • Colligative properties depend on quantity and type of solute/solvent molecules. (E. g. freezing point depression and melting point elevation. ) Lowering Vapor Pressure • Non-volatile solvents reduce the ability of the surface solvent molecules to escape the liquid. • Therefore, vapor pressure is lowered. • The amount of vapor pressure lowering depends on the amount of solute.

Colligative Properties Lowering Vapor Pressure • Raoult’s Law: PA is the vapor pressure with solute, PA is the vapor pressure without solvent, and A is the mole fraction of A, then • Recall Dalton’s Law:

QUESTION

ANSWER C) 0. 800 Remember to convert grams to moles before attempting to find the mole fraction.

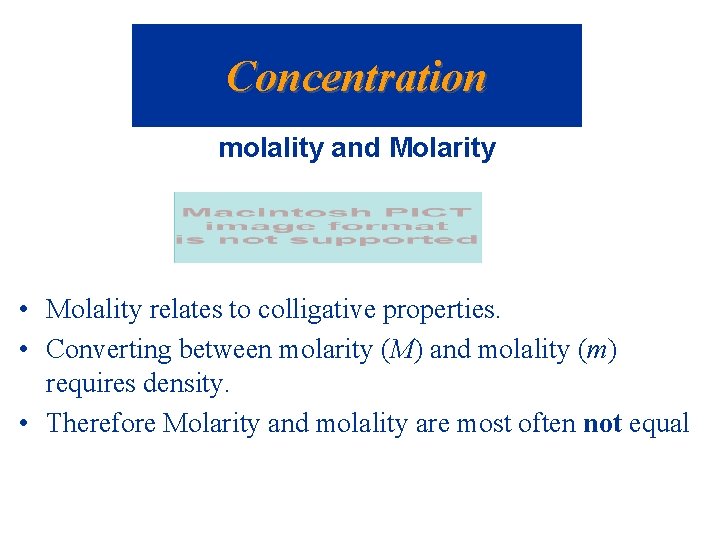
Concentration molality and Molarity • Molality relates to colligative properties. • Converting between molarity (M) and molality (m) requires density. • Therefore Molarity and molality are most often not equal

QUESTION

ANSWER A) 5. 47 m Using the density, the mass of the solution is found. Don’t forget that molality has units of kg of solvent and the mass of the solute must be subtracted from the calculated mass of solution.

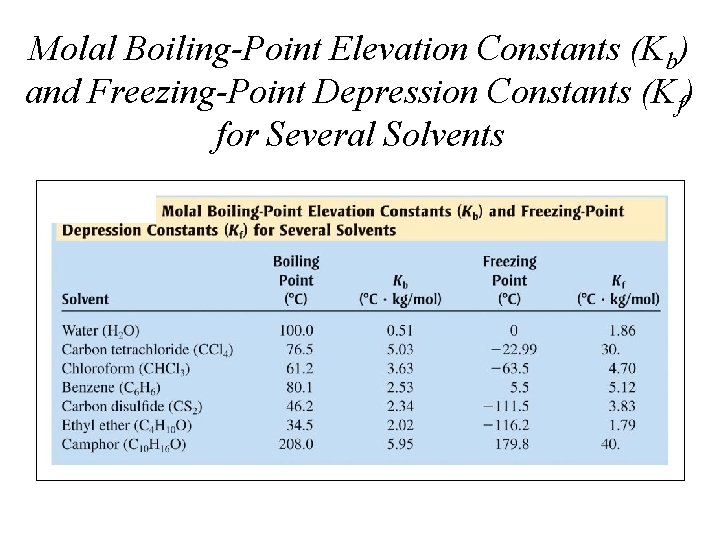
Molal Boiling-Point Elevation Constants (Kb) and Freezing-Point Depression Constants (Kf) for Several Solvents


QUESTION Household bleach is an aqueous solution of sodium hypochlorite. If 5. 25 g of Na. OCl (molar mass = 74. 5 g/mol) were placed in 94. 75 g of water, what would you calculate as the molality? The density of the solution is slightly greater than water. Would the molarity of the solution be greater, less or the same as the molality? A. 0. 0705 m; M would be greater B. 0. 705 m; M would be the same C. 0. 744 m; M would be greater D. 0. 744 m; M would be less

ANSWER D) provides the correct answer to both parts of the question. The molality involves moles/kg of water, so the given mass of solute can be converted to moles of solute and then divided by 0. 09475 kg to obtain molality. The molarity of the solution will be greater than the molality because the density of the solution shows that one milliliter of solution has a mass greater than one. So one liter of solution will contain more mass of solute than would be found mixed with one kilogram of water.

QUESTION Suppose you want to keep the water in your car cooling system from freezing during a cold Alaska winter night. If you added 5. 00 kg of ethylene glycol (C 2 H 4(OH)2 MM = 62. 0 g/mol) to 5. 50 kg of water, what would be the freezing temperature of the coolant/water mixture in your automobile? k f. p. H 2 O = – 1. 86°C kg/ mol A. – 0. 0367°C B. – 7. 90°C C. – 14. 7°C D. – 27. 3°C

ANSWER D) -17. 14 o. F provides the correct, although very cold, answer for that Alaska night. The molality of ethylene glycol must be calculated from the mass (in grams) and molar mass. This is then multiplied by the freezing point depression constant for the solvent to obtain the drop in freezing point. Since water normally freezes at zero degrees Celsius, the change is the actual new freezing point.

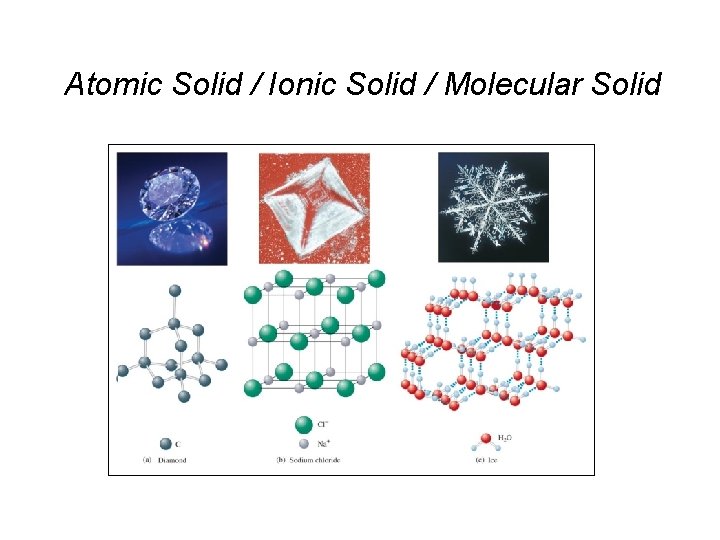
Atomic Solid / Ionic Solid / Molecular Solid

Structure of Solids Types of solids: – Crystalline – a well defined arrangement of atoms; this arrangement is often seen on a macroscopic level. • Ionic solids – ionic bonds hold the solids in a regular three dimensional arrangement. Eg. “Galena”, lead sulfide ore http: //en. wikipedia. org/wiki/Crystal_radio http: //www. crystalradio. net/ • Molecular solid – solids like ice that are held together by intermolecular forces. • Covalent network – a solid consists of atoms held together in large networks or chains by covalent networks. Eg. Diamond & graphite • Metallic – similar to covalent network except with metals. Provides high conductivity. – Amorphous – atoms are randomly arranged. No order exists in the solid. Eg. Glass, gels, thin films

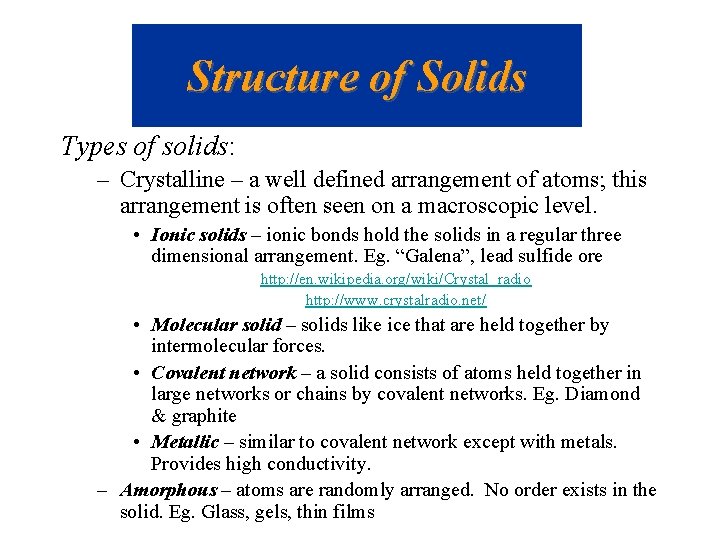
Closest Packing Arrangement of Uniform Spheres Arrays of atoms act as if they are spheres. Two or more layers produce a 3 -D structure.

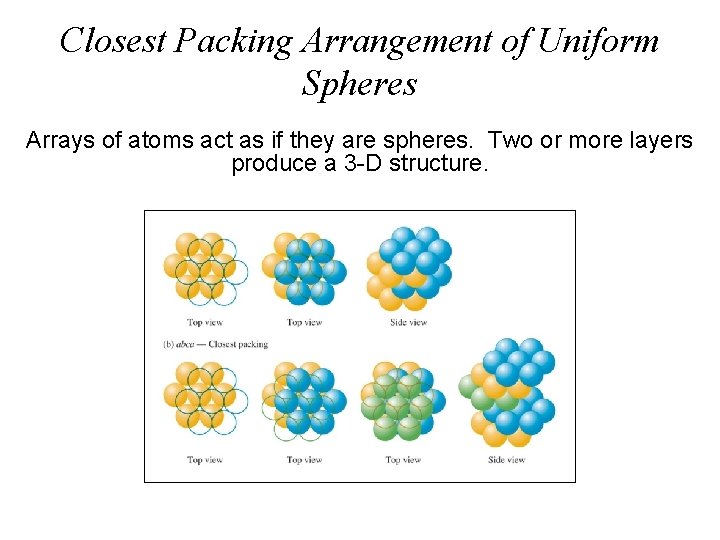
Close Packed: The Red Sphere has 12 Nearest Neighbors

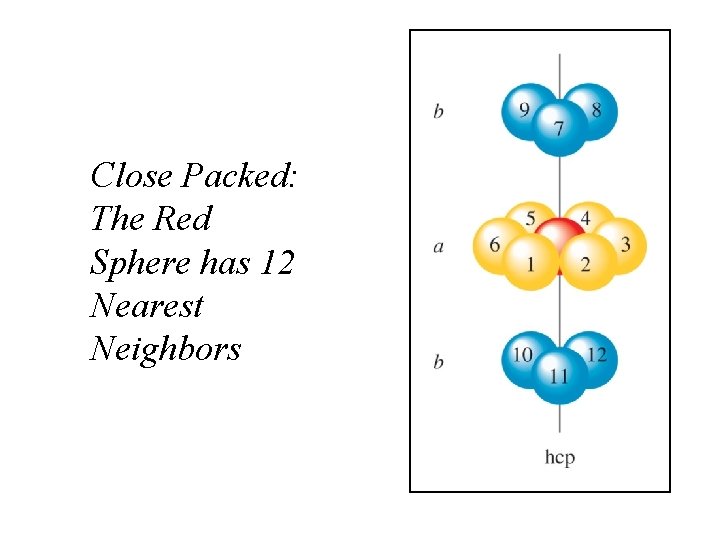
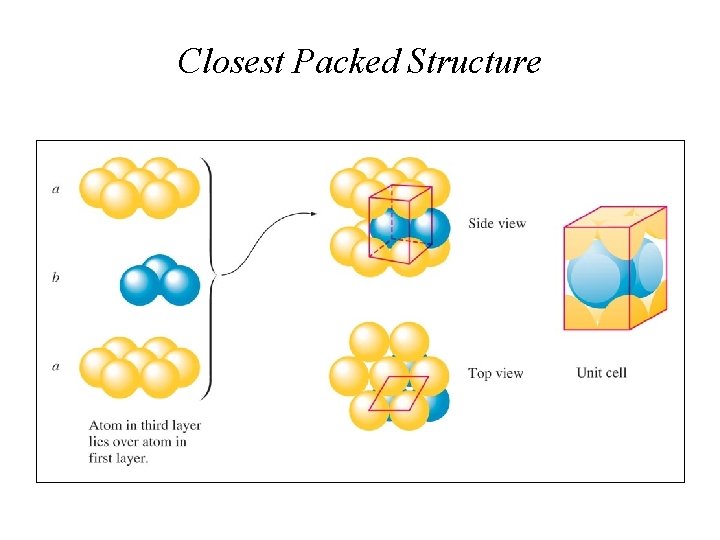
Closest Packed Structure

Unit Cells • Crystals are made up regular arrays – the smallest repeating array of atoms is called the unit cell. • There are 14 different unit cells that are observed which vary in terms of the angles between atoms some are 90°, but others are not.

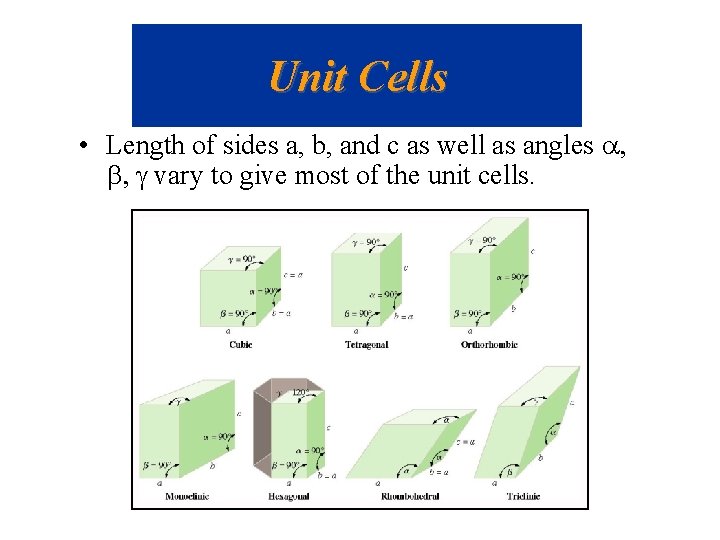
Unit Cells • Length of sides a, b, and c as well as angles a, b, g vary to give most of the unit cells.

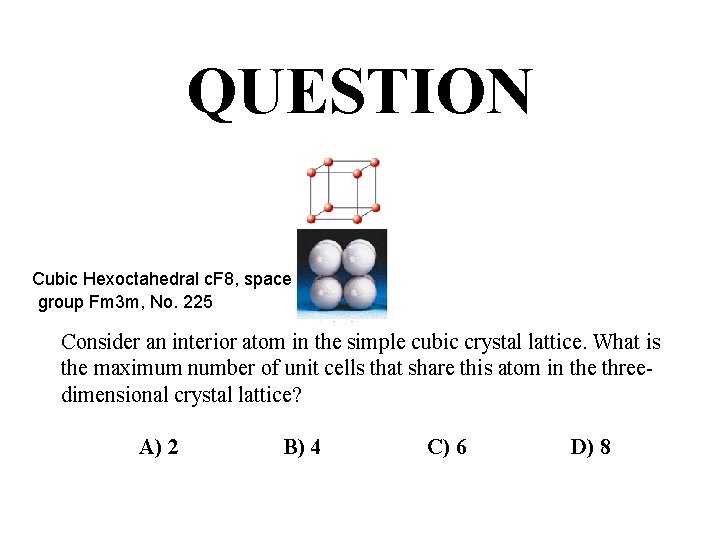
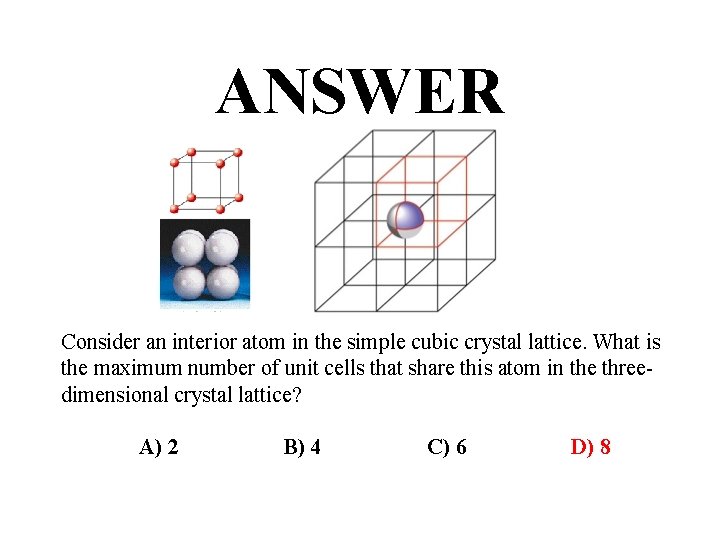
QUESTION Cubic Hexoctahedral c. F 8, space group Fm 3 m, No. 225 Consider an interior atom in the simple cubic crystal lattice. What is the maximum number of unit cells that share this atom in the threedimensional crystal lattice? A) 2 B) 4 C) 6 D) 8

ANSWER Consider an interior atom in the simple cubic crystal lattice. What is the maximum number of unit cells that share this atom in the threedimensional crystal lattice? A) 2 B) 4 C) 6 D) 8

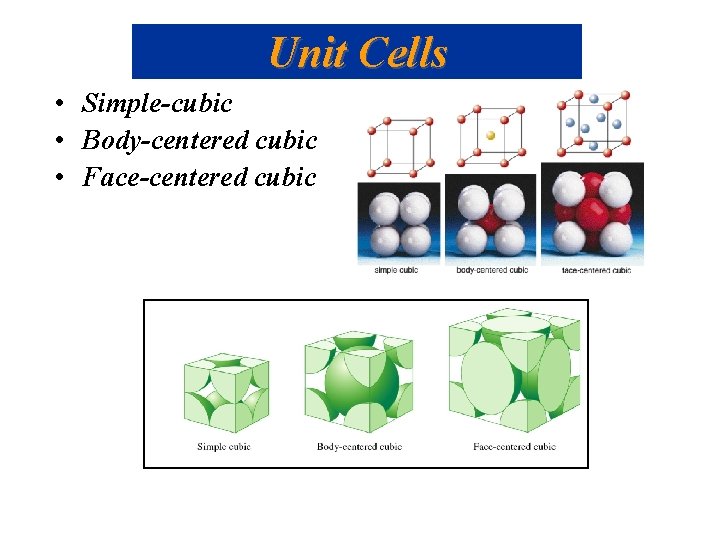
Unit Cells • Simple-cubic • Body-centered cubic • Face-centered cubic

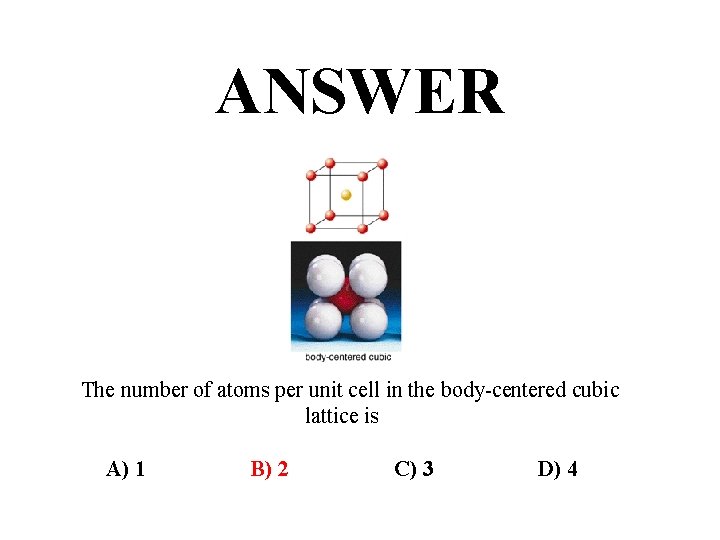
QUESTION The number of atoms per unit cell in the body-centered cubic lattice is A) 1 B) 2 C) 3 D) 4

ANSWER The number of atoms per unit cell in the body-centered cubic lattice is A) 1 B) 2 C) 3 D) 4

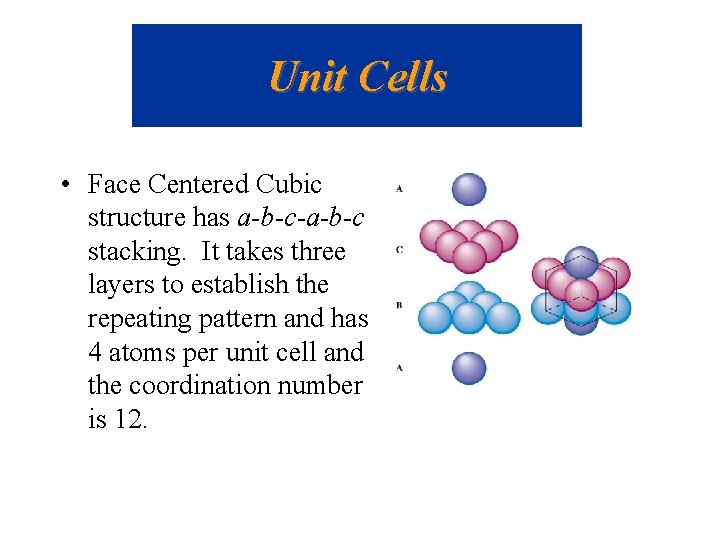
Unit Cells • Face Centered Cubic structure has a-b-c-a-b-c stacking. It takes three layers to establish the repeating pattern and has 4 atoms per unit cell and the coordination number is 12.

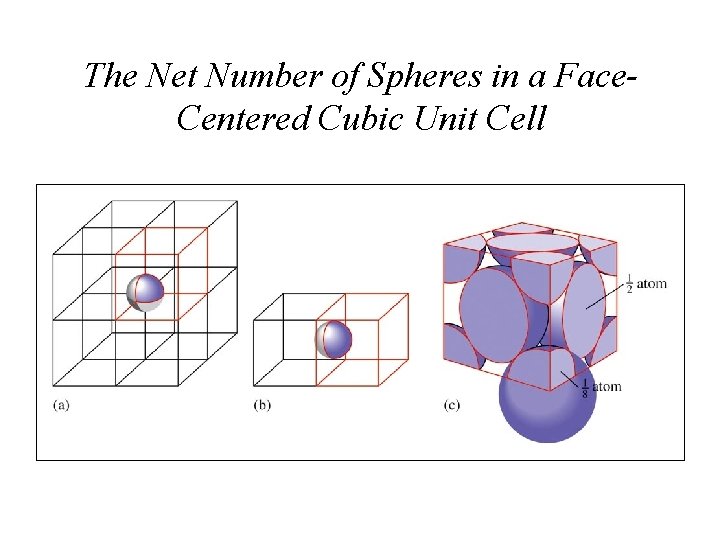
The Net Number of Spheres in a Face. Centered Cubic Unit Cell

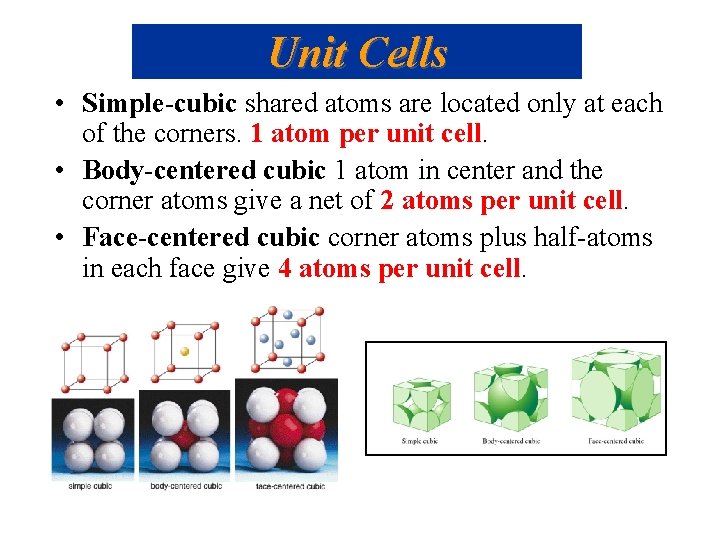
Unit Cells • Simple-cubic shared atoms are located only at each of the corners. 1 atom per unit cell. • Body-centered cubic 1 atom in center and the corner atoms give a net of 2 atoms per unit cell. • Face-centered cubic corner atoms plus half-atoms in each face give 4 atoms per unit cell.

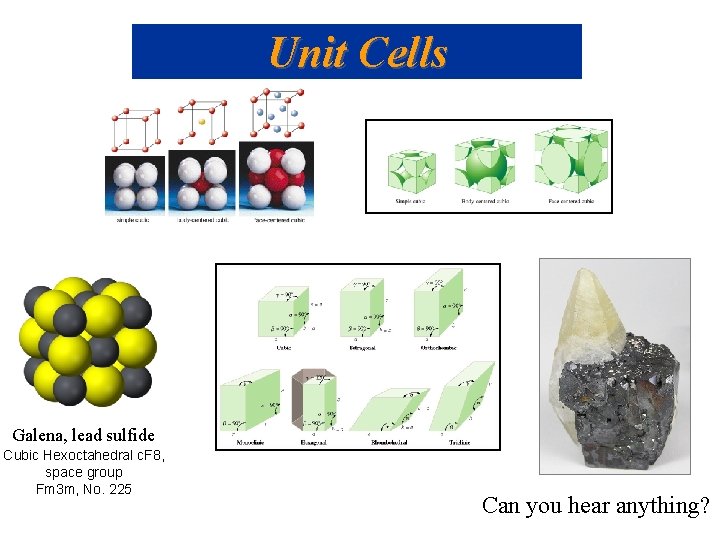
Unit Cells Galena, lead sulfide Cubic Hexoctahedral c. F 8, space group Fm 3 m, No. 225 Can you hear anything?

Crystals for the Classroom Bridging the realms of the macro and atomic/nano scale http: //crystals. llnl. gov Connecting: Science, Technology, Engineering and Mathematics (STEM)

Crystals for the Classroom Bridging the realms of the macro and atomic/nano scale http: //crystals. llnl. gov The story of NIF ( The National Ignition Facility) http: //crystals. llnl. gov/nif-kdp-frameset. html

Crystals for the Classroom Bridging the realms of the macro and atomic/nano scale http: //crystals. llnl. gov Time lapsed KDP Growth

Crystals for the Classroom Bridging the realms of the macro and atomic/nano scale http: //crystals. llnl. gov Simulation: Fusion

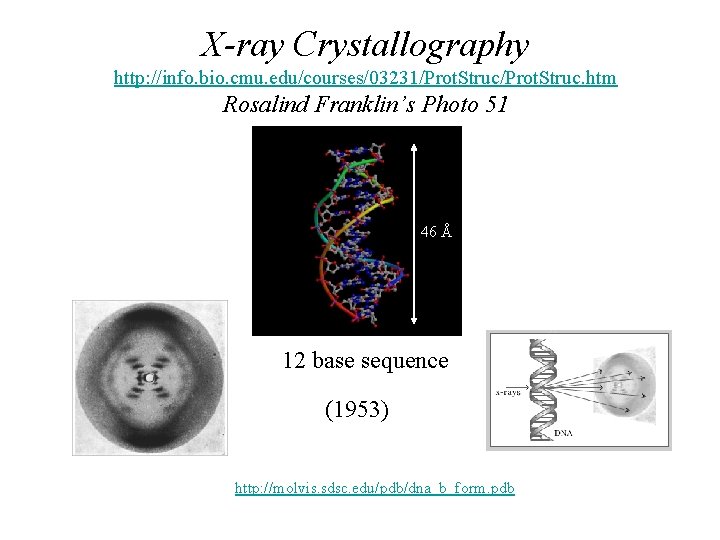
X-ray Crystallography http: //info. bio. cmu. edu/courses/03231/Prot. Struc. htm Rosalind Franklin’s Photo 51 46 Å 12 base sequence (1953) http: //molvis. sdsc. edu/pdb/dna_b_form. pdb
 Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Interu
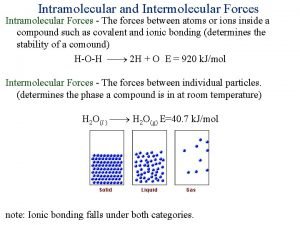
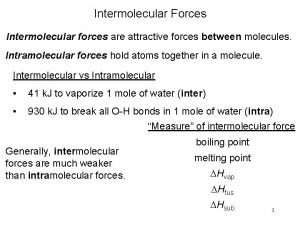
Interu Intramolecular forces vs intermolecular forces
Intramolecular forces vs intermolecular forces Intramolecular forces
Intramolecular forces Intermolecular forces in matter
Intermolecular forces in matter Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Diagram states of matter
Diagram states of matter Liquid information
Liquid information Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Buoyancyability
Buoyancyability Kesler science.com
Kesler science.com 20 examples of liquids
20 examples of liquids Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Liquids and solids menu
Liquids and solids menu Filtering solids from liquids
Filtering solids from liquids Kinetic molecular theory of solids
Kinetic molecular theory of solids Particle movement in solids liquids and gases
Particle movement in solids liquids and gases Why is gas easier to compress than liquid and solid
Why is gas easier to compress than liquid and solid Lesson outline lesson 1 solids liquids and gases answer key
Lesson outline lesson 1 solids liquids and gases answer key Solid liquid gas information
Solid liquid gas information How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Combined gas law def
Combined gas law def Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Interatomic and intermolecular forces
Interatomic and intermolecular forces Intermolecular forces capillary action
Intermolecular forces capillary action Pbr3 trigonal pyramidal
Pbr3 trigonal pyramidal Van der waals gecko
Van der waals gecko Factors affecting solvation worksheet answers
Factors affecting solvation worksheet answers Intermolecular forces in formaldehyde
Intermolecular forces in formaldehyde Intermolecular forces
Intermolecular forces Sucrose intermolecular forces
Sucrose intermolecular forces Ch5n polar or nonpolar
Ch5n polar or nonpolar Intramolecular forces list
Intramolecular forces list Type of intermolecular forces
Type of intermolecular forces Ch2cl intermolecular forces
Ch2cl intermolecular forces Van der waals attraction
Van der waals attraction London dispersion force
London dispersion force Nonpolar functional groups
Nonpolar functional groups Hbr intermolecular forces
Hbr intermolecular forces Intermolecular forces
Intermolecular forces Type of intermolecular forces
Type of intermolecular forces Hybrid bonding
Hybrid bonding 2-butanone functional group
2-butanone functional group What is induced dipole
What is induced dipole Poem about intermolecular forces
Poem about intermolecular forces Intermolecular forces present in hbr
Intermolecular forces present in hbr Intermolecular forces symbol
Intermolecular forces symbol Carbonyl sulfide intermolecular forces
Carbonyl sulfide intermolecular forces Reading forces you to be quiet
Reading forces you to be quiet Hydrogen bonding intermolecular force
Hydrogen bonding intermolecular force Coh2 intermolecular forces
Coh2 intermolecular forces Intermolecular forces present in hbr
Intermolecular forces present in hbr Ion induced dipole example
Ion induced dipole example Intermolecular forces review
Intermolecular forces review Summary of intermolecular forces
Summary of intermolecular forces 3 types of intermolecular forces
3 types of intermolecular forces Unit 3 intermolecular forces and properties
Unit 3 intermolecular forces and properties Hco2h intermolecular forces
Hco2h intermolecular forces Molecules attraction
Molecules attraction Intermolecular forces from strongest to weakest
Intermolecular forces from strongest to weakest Adhesive force
Adhesive force Intermolecular forces
Intermolecular forces Surface tension intermolecular forces
Surface tension intermolecular forces Ch2cl intermolecular forces
Ch2cl intermolecular forces Which of the following imfas is considered as the weakest
Which of the following imfas is considered as the weakest Mind mapping of intermolecular forces
Mind mapping of intermolecular forces Viscosity and intermolecular forces
Viscosity and intermolecular forces Intermolecular forces
Intermolecular forces Strongest to weakest intermolecular forces
Strongest to weakest intermolecular forces Types of intermolecular forces
Types of intermolecular forces Pvc intermolecular forces
Pvc intermolecular forces Dipole induced dipole forces examples
Dipole induced dipole forces examples No+ 루이스구조
No+ 루이스구조 Intramolecular vs intermolecular forces
Intramolecular vs intermolecular forces Ap chemistry intermolecular forces
Ap chemistry intermolecular forces Similarities of intermolecular and intramolecular forces
Similarities of intermolecular and intramolecular forces Intermolecular forces strength
Intermolecular forces strength Electronegativity intermolecular forces
Electronegativity intermolecular forces Network covalent
Network covalent Liquid properties
Liquid properties Webquest types of forces answers
Webquest types of forces answers Whats the study of matter and energy
Whats the study of matter and energy Phases changes of matter
Phases changes of matter Phases of matter
Phases of matter 3 phases of matter
3 phases of matter Matter concept map
Matter concept map Four states of matter
Four states of matter Why isn't it a good idea to classify matter by its phases
Why isn't it a good idea to classify matter by its phases 4 phases of matter
4 phases of matter What are constructive and destructive forces
What are constructive and destructive forces The forces shown above are pushing/pulling forces
The forces shown above are pushing/pulling forces Contact and noncontact forces
Contact and noncontact forces Net force
Net force Resultant of parallel forces
Resultant of parallel forces Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Rhinencephalon
Rhinencephalon Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter