Intermolecular Forces How do Molecules Stay Together Intermolecular






- Slides: 16

Intermolecular Forces How do Molecules Stay Together?

Intermolecular Forces of Attraction Intermolecular forces of attraction are weak electrostatic forces of attraction between molecules Electrostatic: attraction between positive and negative charges

Three Types of Intermolecular Forces Dispersion forces (Van der Waals) Dipole-Dipole Hydrogen interactions bonding

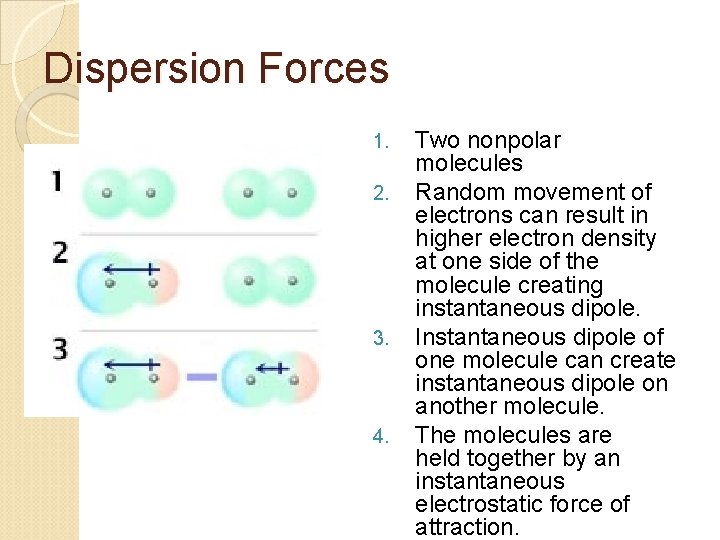
A. Dispersion Forces Only IMF for nonpolar molecules Found in ALL molecules, whether polar or nonpolar Very weak electrostatic forces Weakest intermolecular force Caused by formation of “instantaneous dipole” on a molecule. The dipolar molecule can then attract another dipolar molecule. Magnitude of force increases with increasing molecular weight, size, and number of electrons.

Dispersion Forces 1. 2. 3. 4. Two nonpolar molecules Random movement of electrons can result in higher electron density at one side of the molecule creating instantaneous dipole. Instantaneous dipole of one molecule can create instantaneous dipole on another molecule. The molecules are held together by an instantaneous electrostatic force of attraction.

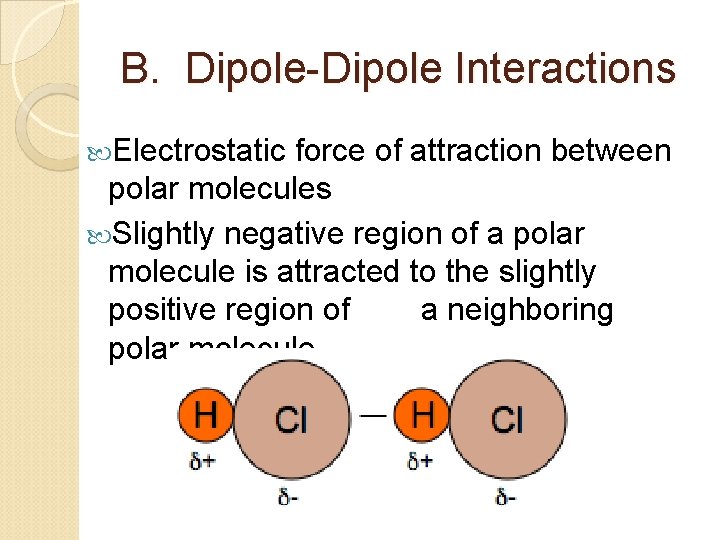
B. Dipole-Dipole Interactions Electrostatic force of attraction between polar molecules Slightly negative region of a polar molecule is attracted to the slightly positive region of a neighboring polar molecule.

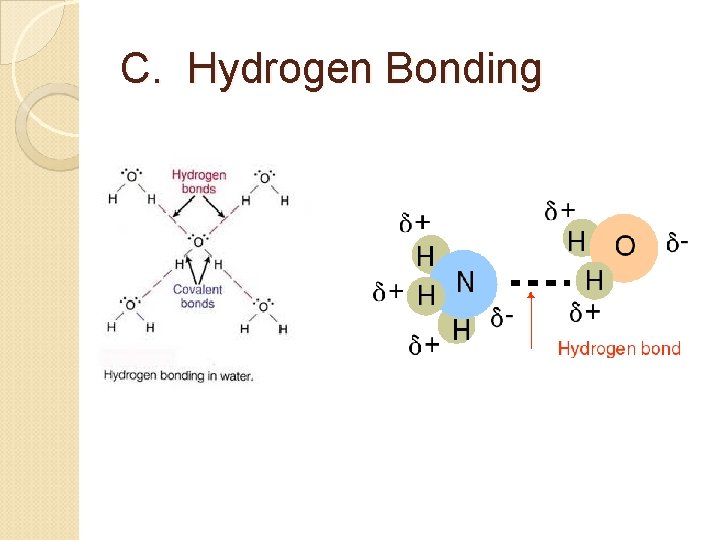
C. Hydrogen Bonding Strongest intermolecular force of attraction Occurs only when H is bonded to N, O, or F because N, O, and F are the most electronegative elements on the periodic table. The highly electronegative N, O, or F attract the shared electron pair. N, O, or F will have a negative charge. Hydrogen is positive enough from a strong dipole to attract a lone e- pair on another N, O, or F.

C. Hydrogen Bonding

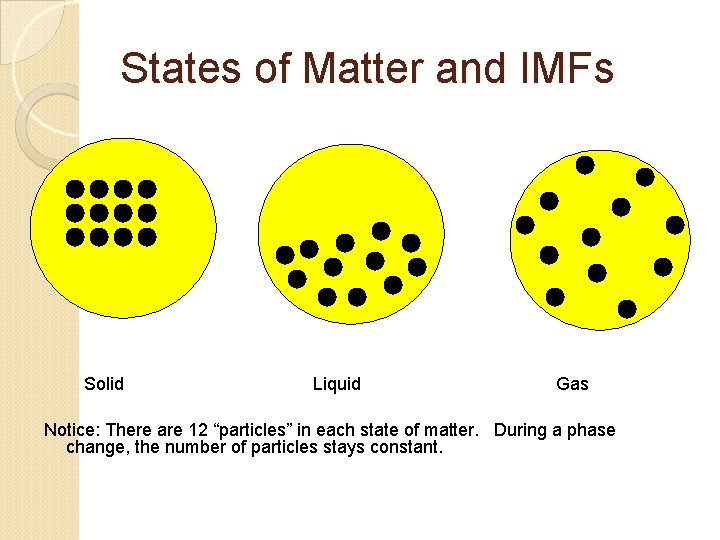
States of Matter and IMFs Solid Liquid Gas Notice: There are 12 “particles” in each state of matter. During a phase change, the number of particles stays constant.

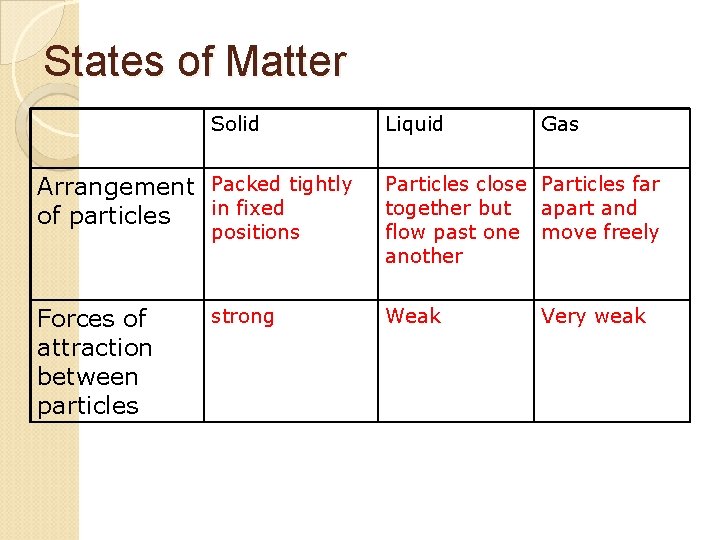
States of Matter Solid Liquid Gas Arrangement Packed tightly in fixed of particles Particles close Particles far together but apart and flow past one move freely another Forces of attraction between particles Weak positions strong Very weak

The state of matter of a substance depends on the forces that hold the particles of a substance together. Solids have strong forces holding the particles together. Liquids have weak forces holding particles together. Gases have the weakest forces holding particles together. Intermolecular forces are weak. They are much weaker than covalent and ionic bonds. This is why molecular compounds are generally liquids or gases at room temperature.

Chlorine, Iodine, and Bromine Notice from the periodic table that chlorine (Cl 2) is a gas at room temperature, bromine (Br 2) is a liquid at room temperature, and iodine (I 2) is a solid at room temperature. How would you explain this using IMFs?

Chlorine, Iodine, and Bromine Notice from the periodic table that chlorine (Cl 2) is a gas at room temperature, bromine (Br 2) is a liquid at room temperature, and iodine (I 2) is a solid at room temperature. How would you explain this using IMFs? Cl 2, Br 2, and I 2 are all nonpolar, which means that dispersion forces hold molecules to one another. The strength of the dispersion force increases with increasing number of electrons. The strength of the dispersion forces is strong enough to make Br 2 an liquid and I 2 a solid.

Phase Changes and IMFs In order to melt or boil, energy (heat) must be added in order to weaken or break intermolecular forces, which separates the particles. The stronger the forces holding the particles together, the more energy (heat) must be added to the substance. Therefore, the stronger the intermolecular forces, the higher the

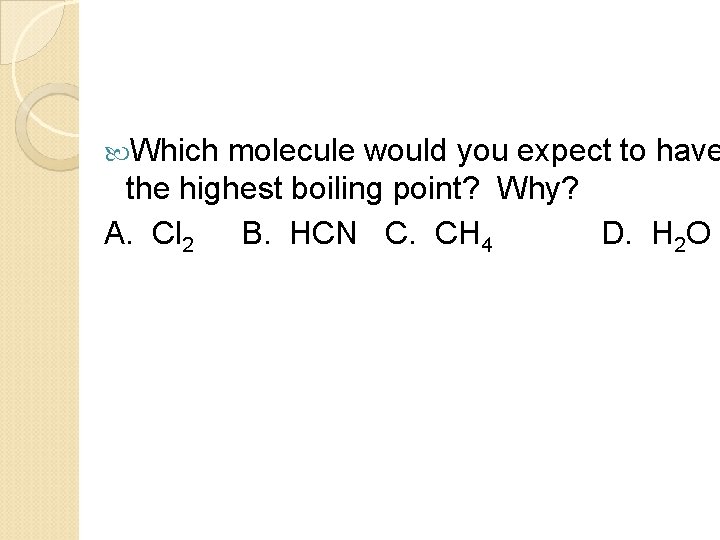
Which molecule would you expect to have the highest boiling point? Why? A. Cl 2 B. HCN C. CH 4 D. H 2 O

Which molecule would you expect to have the highest boiling point? Why? A. Cl 2 B. HCN C. CH 4 D. H 2 O has hydrogen bonding, which is the strongest IMF.