Intermolecular Forces Chemistry Warm Up Draw the Lewis








- Slides: 19

Intermolecular Forces Chemistry

Warm Up. Draw the Lewis structure for PI 3. • 2. What is the VSEPR shape? • 3. How do you know? • 1

Quick Checks 1. What is an oxidation number? 2. Do metals have a positive or negative oxidation number? How do you know? 3. What is the difference between an ionic bond and covalent bond? 4. How do you form an ionic compound? 5. What happens when the charges are equal but opposite charge. ( +1 and -1) 6. Which elements do not form bonds? 7. How many electrons can hydrogen hold? 8. How many electrons can fluorine hold?

QUICK CHECKS • How many electrons are in each bond? • What do you add if you don’t have enough electrons to make an octet? • Which shape has 4 atoms attached to the central atom? • What does VSEPR stand for? • Which element ALWAYS goes in the center? • Which element NEVER goes in the center? • Which element does not follow the octet rule?

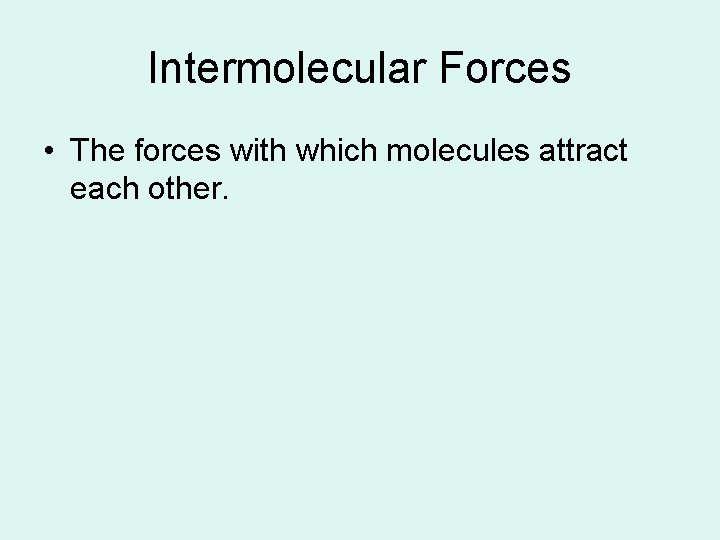
Intermolecular Forces • The forces with which molecules attract each other.

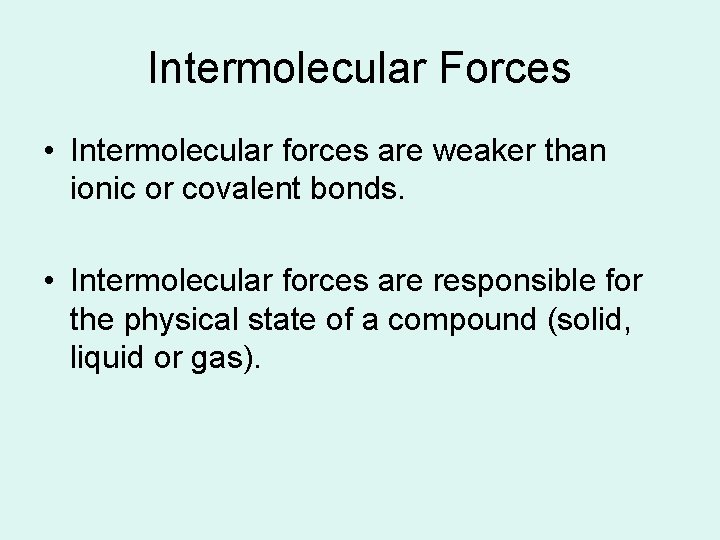
Intermolecular Forces • Intermolecular forces are weaker than ionic or covalent bonds. • Intermolecular forces are responsible for the physical state of a compound (solid, liquid or gas).

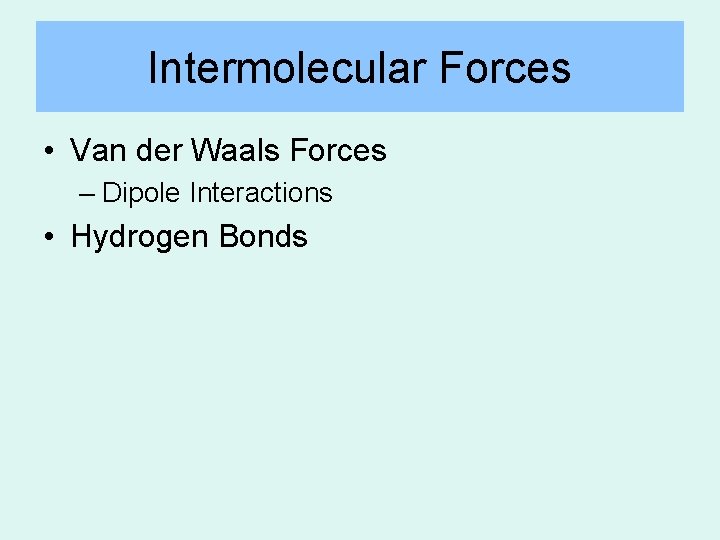
Intermolecular Forces • Van der Waals Forces – Dipole Interactions • Hydrogen Bonds

Van der Waals Forces • They are the weakest attractions between molecules.

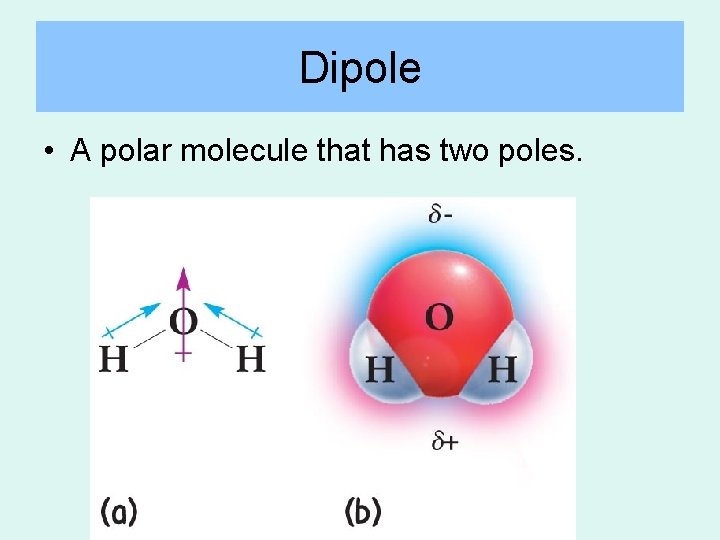
Dipole • A polar molecule that has two poles.

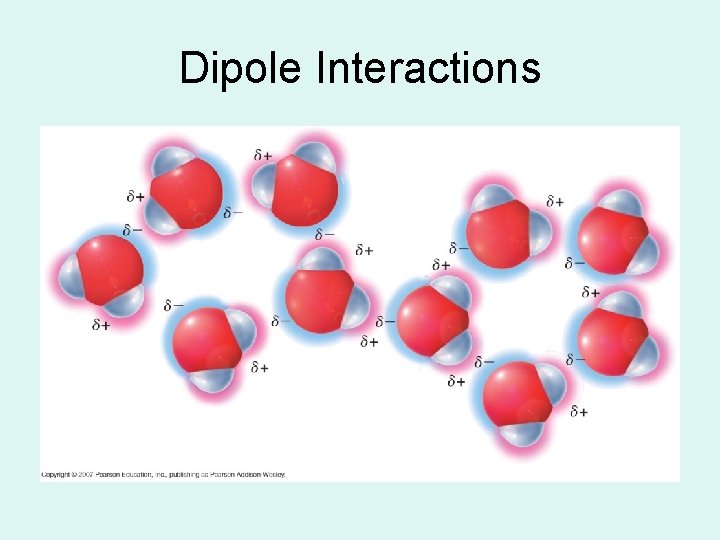
Van der Waals-Dipole Interactions • Electrostatic interaction between the oppositely charged regions of polar molecules (dipoles).

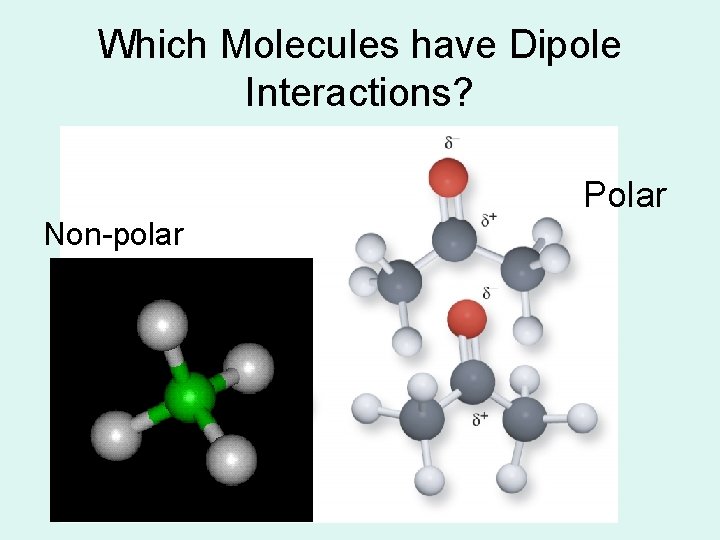
Which Molecules have Dipole Interactions? Polar Non-polar

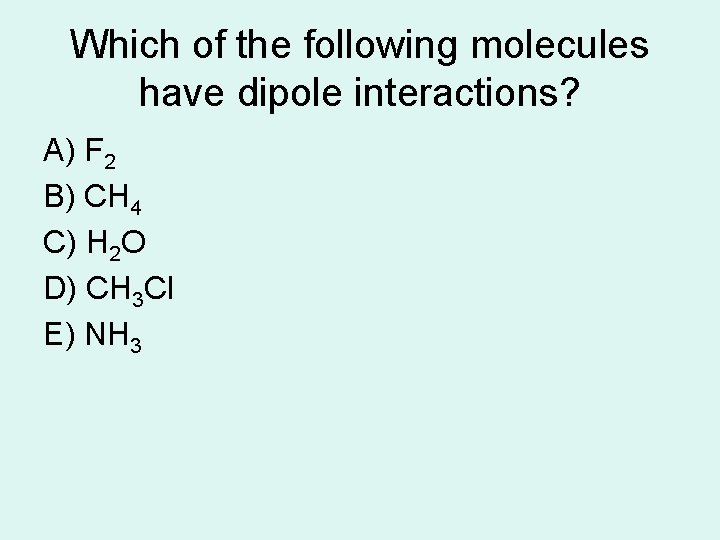
Which of the following molecules have dipole interactions? A) F 2 B) CH 4 C) H 2 O D) CH 3 Cl E) NH 3

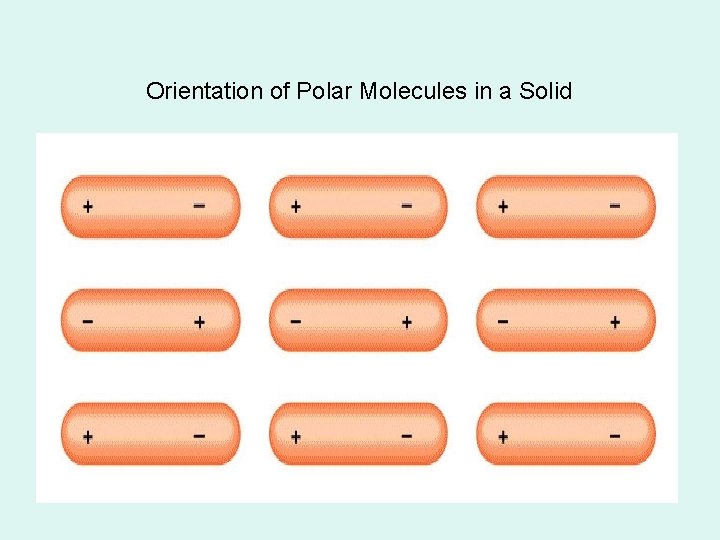
Orientation of Polar Molecules in a Solid

Hydrogen Bonding • Hydrogen bonding is the attraction between a hydrogen atom of a molecule to an unshared pair of electrons in another molecule. • Hydrogen bonding occurs in molecules where hydrogen is covalently bonded to a very electronegative element. • Hydrogen bonding occurs in molecules containing N, O, F.

WATER • https: //www. youtube. com/watch? v=Uuk. Rg qzk-KE

Hydrogen Bonding, Continued • Hydrogen bonds are the strongest of all intermolecular forces. • Hydrogen bonds are possible because in hydrogen atoms there is no “shielding” of the nucleus. • Hydrogen bonds are responsible for the physical properties of many biological substances and, more importantly, water.

Which of the following molecules can have hydrogen bonding? A) F 2 B) CH 4 C) H 2 O D) CH 3 Cl E) NH 3

Dipole Interactions

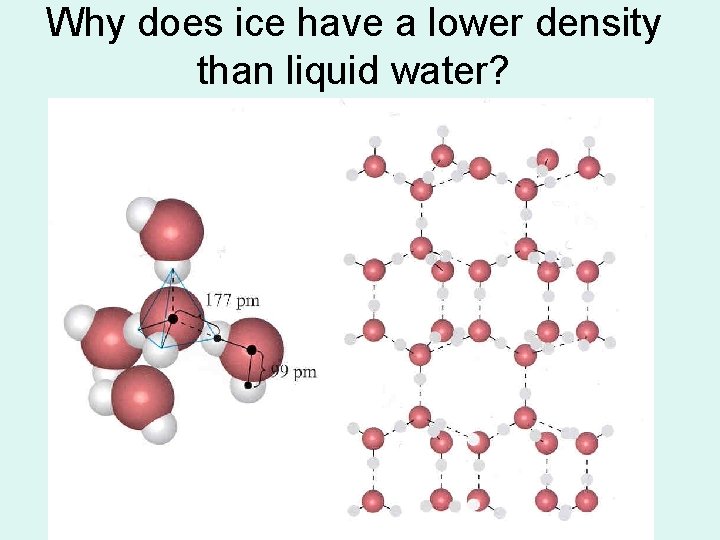
Why does ice have a lower density than liquid water?