Intermolecular Forces Bonding Unit Part C Intermolecular Forces
Intermolecular Forces Bonding Unit Part C) Intermolecular Forces
INTER vs. INTRA �Intramolecular forces = attractive forces that hold particles together in bonds �Bonds between atoms in a compound �Intra - within �Intermolecular forces = attractive forces between individual molecules �Inter – between or among �Intermolecular forces are weaker than intramolecular

London Dispersion Forces �Weak forces that result from temporary shifts in the density of electrons in electron clouds � When 2 molecules are in close contact, electron cloud of one molecule repels the electron cloud of other � So electron density is greater in one region than other �Weak dispersion force exists b/w oppositely charged regions �Exist between all particles �Strength of force increases with mass of molecule

London Dispersion Forces VIDEO
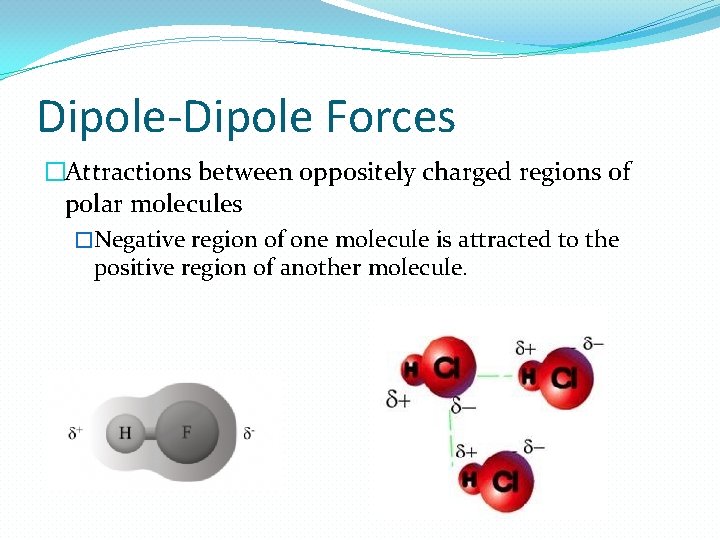
Dipole-Dipole Forces �Attractions between oppositely charged regions of polar molecules �Negative region of one molecule is attracted to the positive region of another molecule.
Dipole-Dipole Forces VIDEO
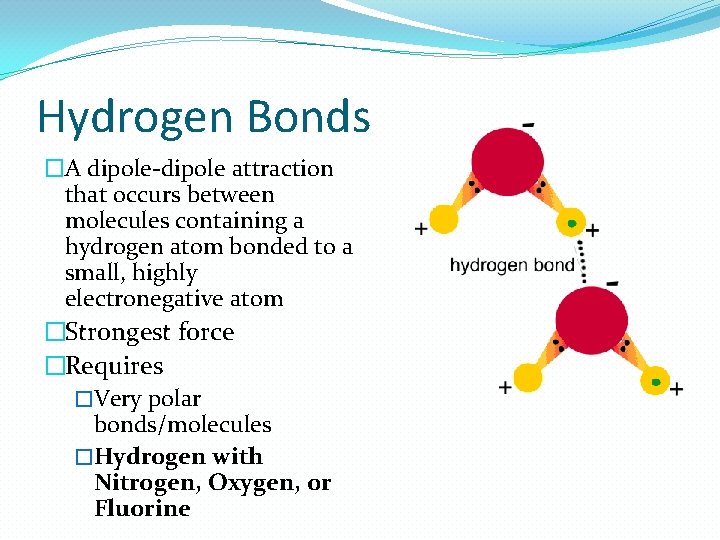
Hydrogen Bonds �A dipole-dipole attraction that occurs between molecules containing a hydrogen atom bonded to a small, highly electronegative atom �Strongest force �Requires �Very polar bonds/molecules �Hydrogen with Nitrogen, Oxygen, or Fluorine
Hydrogen Bonding VIDEO
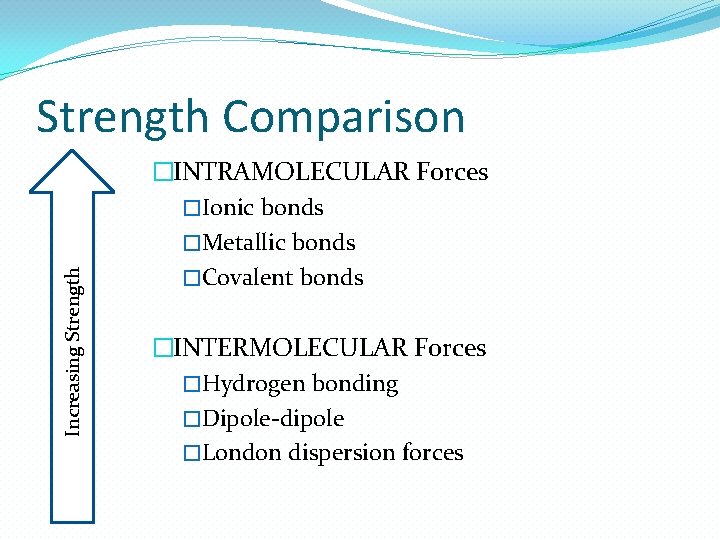
Increasing Strength Comparison �INTRAMOLECULAR Forces �Ionic bonds �Metallic bonds �Covalent bonds �INTERMOLECULAR Forces �Hydrogen bonding �Dipole-dipole �London dispersion forces

Identifying the strongest IMF in a molecule �Given a molecule �Look at atoms & Lewis dot structure �If has H bonded to O, N, or F = hydrogen bonding �If it is a polar molecule = dipole-dipole forces � Need to calculate bond polarity and look at shape � If arrows cancel out/no dipole = nonpolar � If arrows don’t cancel out/dipole present = polar �If neither = London dispersion forces
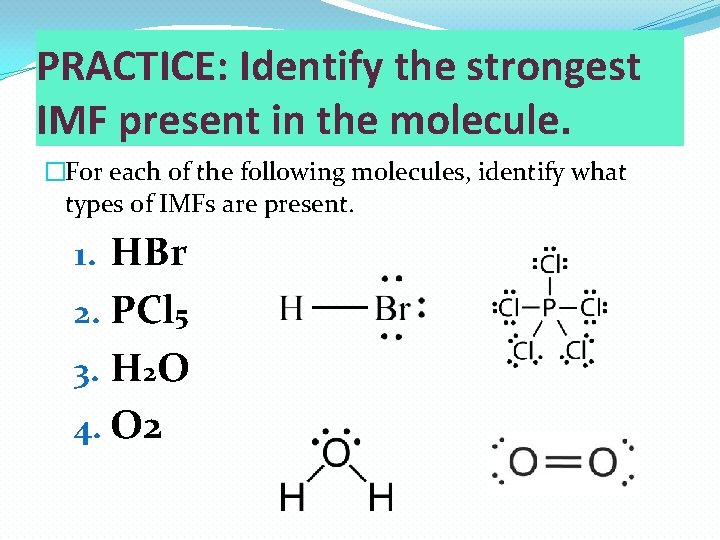
PRACTICE: Identify the strongest IMF present in the molecule. �For each of the following molecules, identify what types of IMFs are present. 1. HBr 2. PCl 5 3. H 2 O 4. O 2
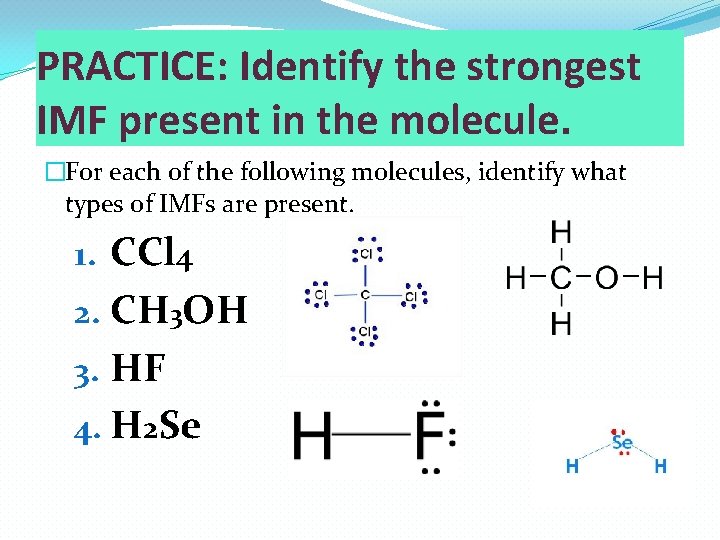
PRACTICE: Identify the strongest IMF present in the molecule. �For each of the following molecules, identify what types of IMFs are present. 1. CCl 4 2. CH 3 OH 3. HF 4. H 2 Se
IMFs and Physical Properties �IMFs control how well molecules stick together �If the IMFs between molecules are strong, it will take more energy to pull those molecules apart �IMFs impact many physical properties of molecules �Melting point/boiling point �Surface tension �Viscosity �Evaporation/Vapor pressure �Solubility
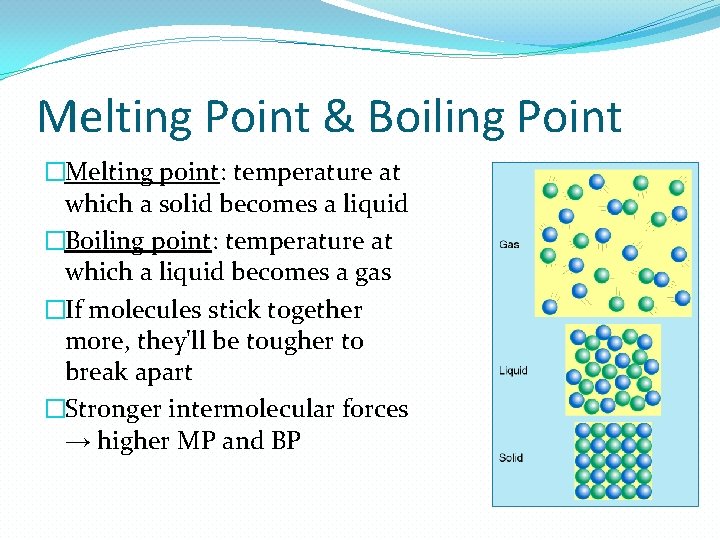
Melting Point & Boiling Point �Melting point: temperature at which a solid becomes a liquid �Boiling point: temperature at which a liquid becomes a gas �If molecules stick together more, they'll be tougher to break apart �Stronger intermolecular forces → higher MP and BP
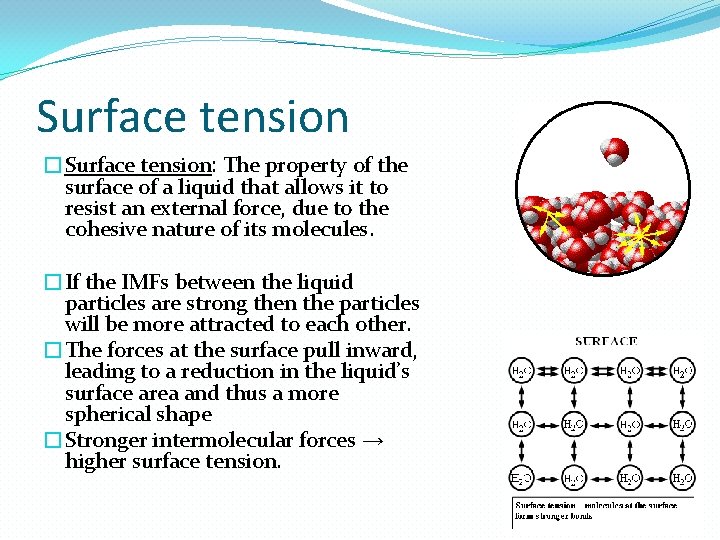
Surface tension �Surface tension: The property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of its molecules. �If the IMFs between the liquid particles are strong then the particles will be more attracted to each other. �The forces at the surface pull inward, leading to a reduction in the liquid’s surface area and thus a more spherical shape �Stronger intermolecular forces → higher surface tension.
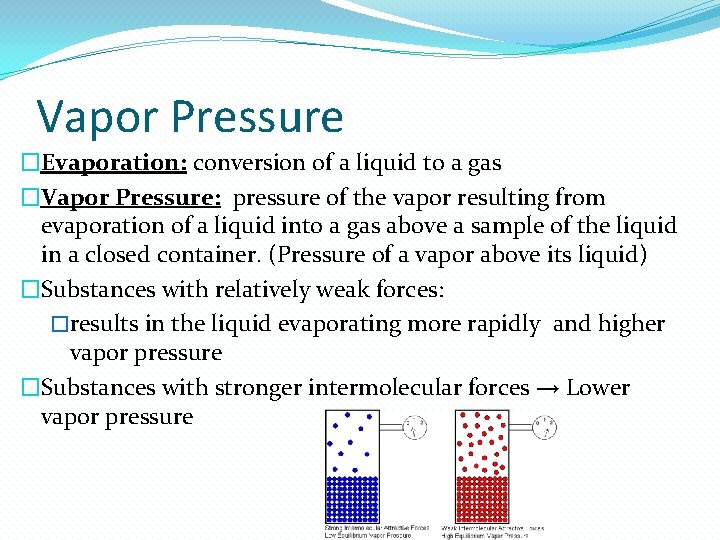
Vapor Pressure �Evaporation: conversion of a liquid to a gas �Vapor Pressure: pressure of the vapor resulting from evaporation of a liquid into a gas above a sample of the liquid in a closed container. (Pressure of a vapor above its liquid) �Substances with relatively weak forces: �results in the liquid evaporating more rapidly and higher vapor pressure �Substances with stronger intermolecular forces → Lower vapor pressure
- Slides: 16