Intermolecular forces attraction between one molecule and a
Intermolecular forces “attraction between one molecule and a neighbouring molecule”

Intermolecular forces n Van Der Waal’s n n London forces Dipole-dipole Dipole- induced polarity Hydrogen bonding

London forces n n n Between non-polar molecules. Instantaneous polarity caused by the random. movement of electrons within a non-polar molecule (=temporary dipole). Instantaneous polarity induces polarity in neighbouring molecules. London forces is the attraction as a result of the polarity in neighbouring molecules. Weakest of the intermolecular forces and only acts over a short distance.
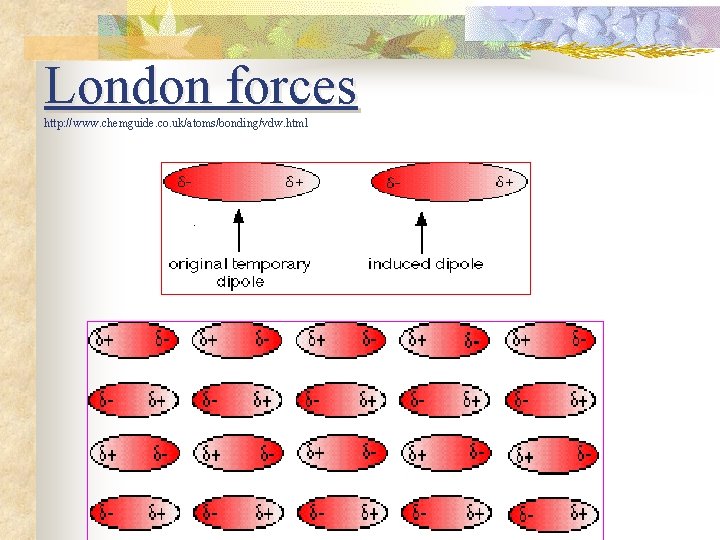
London forces http: //www. chemguide. co. uk/atoms/bonding/vdw. html .
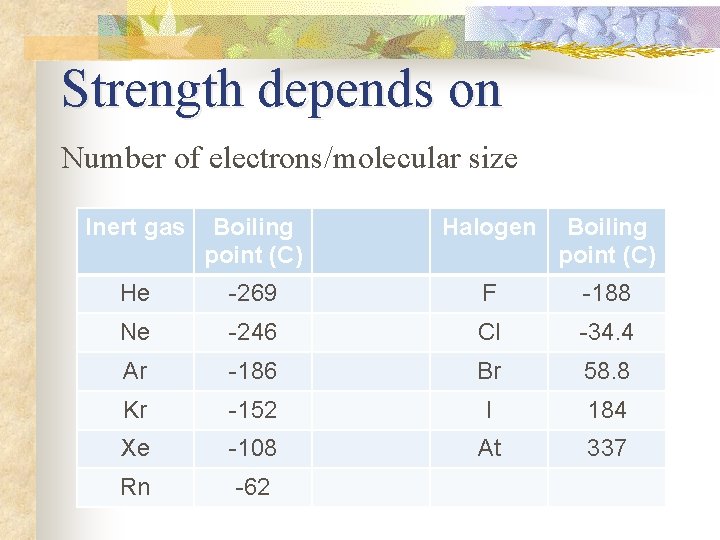
Strength depends on Number of electrons/molecular size Inert gas Boiling point (C) Halogen Boiling point (C) He -269 F -188 Ne -246 Cl -34. 4 Ar -186 Br 58. 8 Kr -152 I 184 Xe -108 At 337 Rn -62
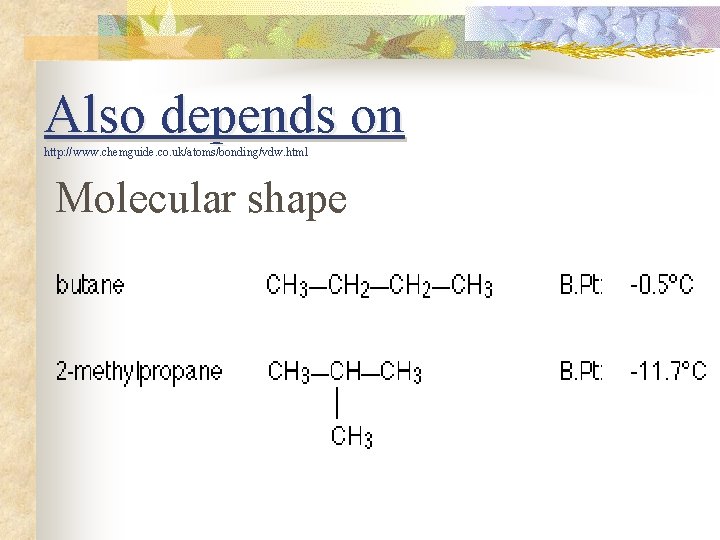
Also depends on http: //www. chemguide. co. uk/atoms/bonding/vdw. html Molecular shape
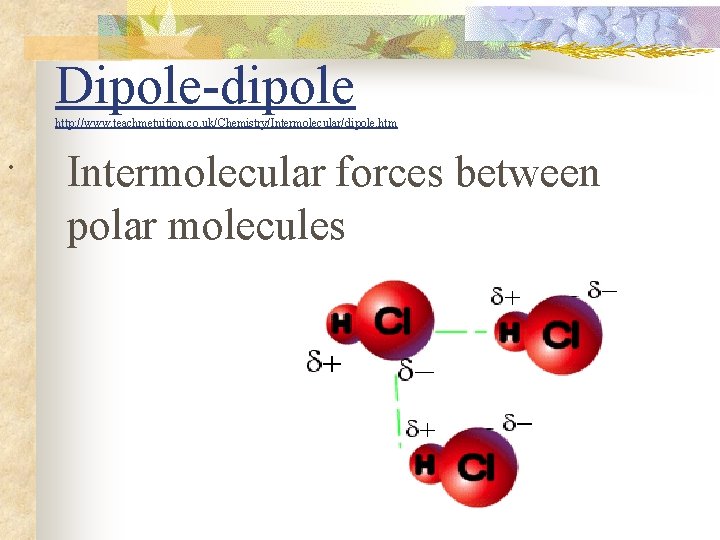
Dipole-dipole http: //www. teachmetuition. co. uk/Chemistry/Intermolecular/dipole. htm . Intermolecular forces between polar molecules
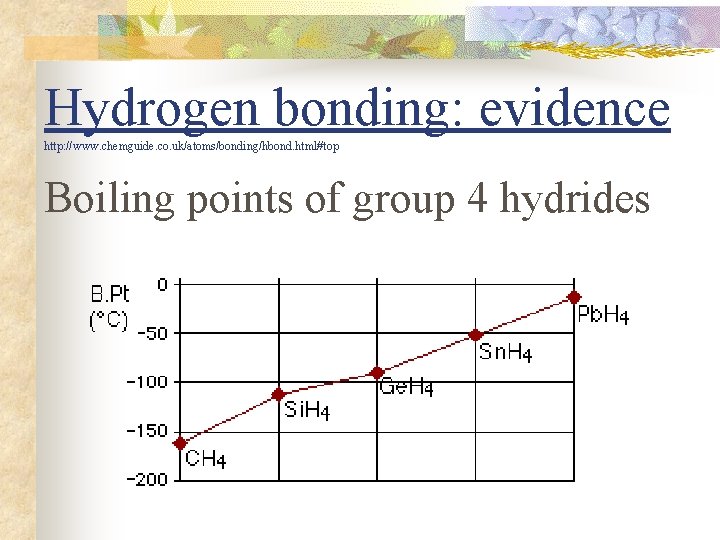
Hydrogen bonding: evidence http: //www. chemguide. co. uk/atoms/bonding/hbond. html#top Boiling points of group 4 hydrides
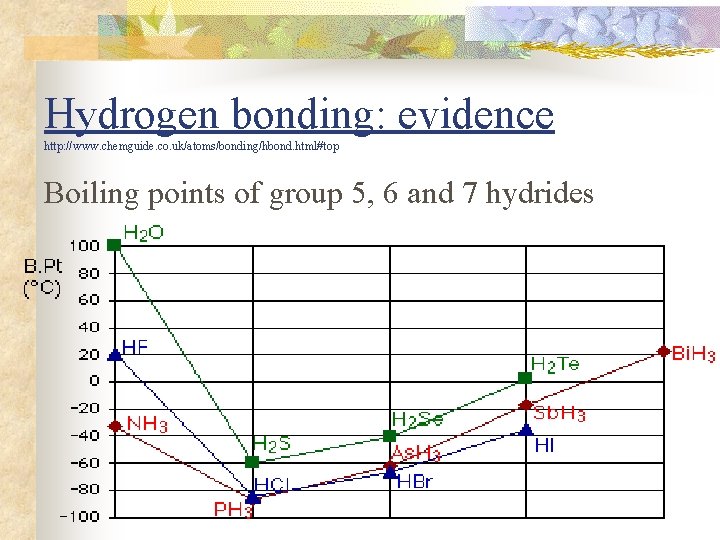
Hydrogen bonding: evidence http: //www. chemguide. co. uk/atoms/bonding/hbond. html#top Boiling points of group 5, 6 and 7 hydrides
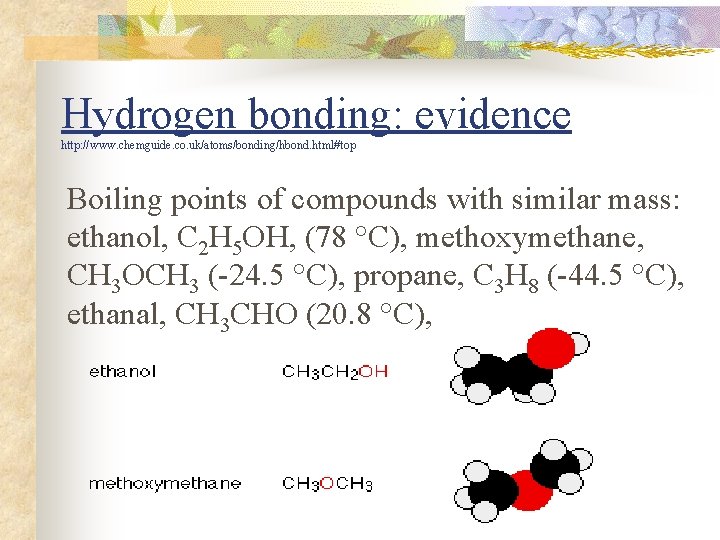
Hydrogen bonding: evidence http: //www. chemguide. co. uk/atoms/bonding/hbond. html#top Boiling points of compounds with similar mass: ethanol, C 2 H 5 OH, (78 °C), methoxymethane, CH 3 OCH 3 (-24. 5 °C), propane, C 3 H 8 (-44. 5 °C), ethanal, CH 3 CHO (20. 8 °C),
Hydrogen bonding: evidence n n n High solubility of ammonia in water (89. 9 g/100 mol of water at 0°C) as hydrogen bonding occurs between ammonia and water molecules Strong forces between polymer molecules Ethanoic acid forms dimers in non-polar solvents so it has a high melting and boiling point

Hydrogen bonding: water!! n n n high surface tension high m. p. /b. p. (water is a liquid over a wide range of temperatures) greater specific heat capacity than almost any other liquid high heat of vaporization structure of ice (lower density than water)
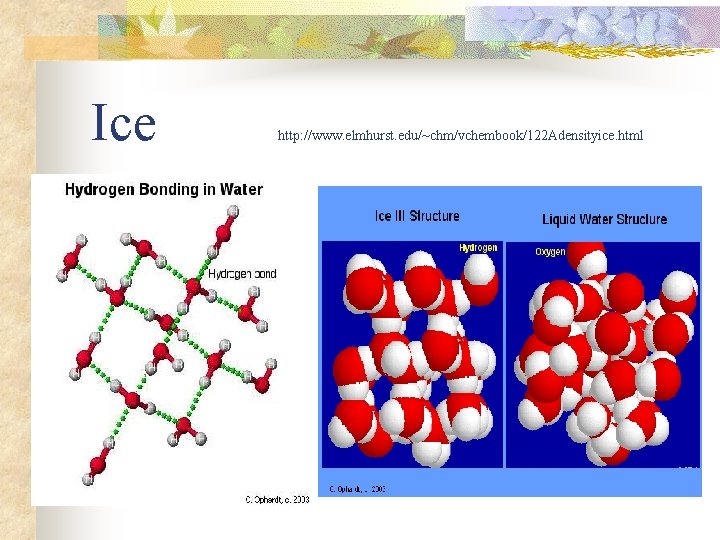
Ice http: //www. elmhurst. edu/~chm/vchembook/122 Adensityice. html
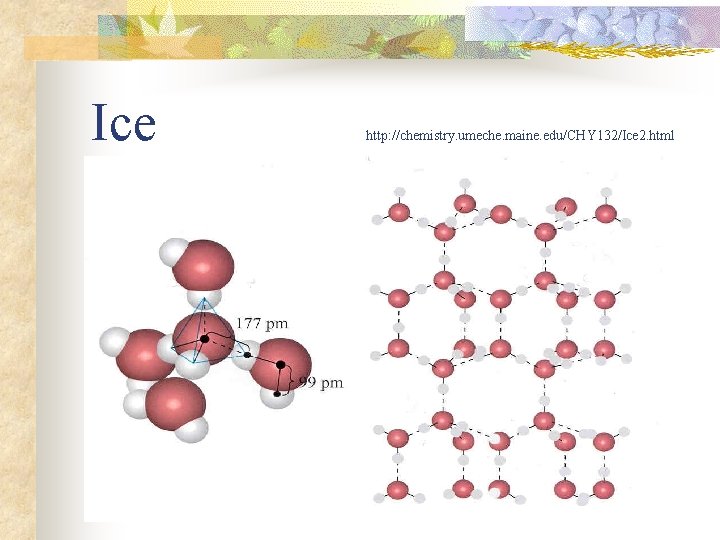
Ice http: //chemistry. umeche. maine. edu/CHY 132/Ice 2. html

Hydrogen bonding n n Strong attraction between n highly positive hydrogen atom (part of a large dipole or polar bond i. e. bonded with N, O or F) n the lone pair of a highly electronegative atom (usually N, O or F) of a neighbouring molecule (=intermolecular hydrogen bonds) or the same molecule (=intramolecular hydrogen bonds as in proteins). Strongest intermolecular force
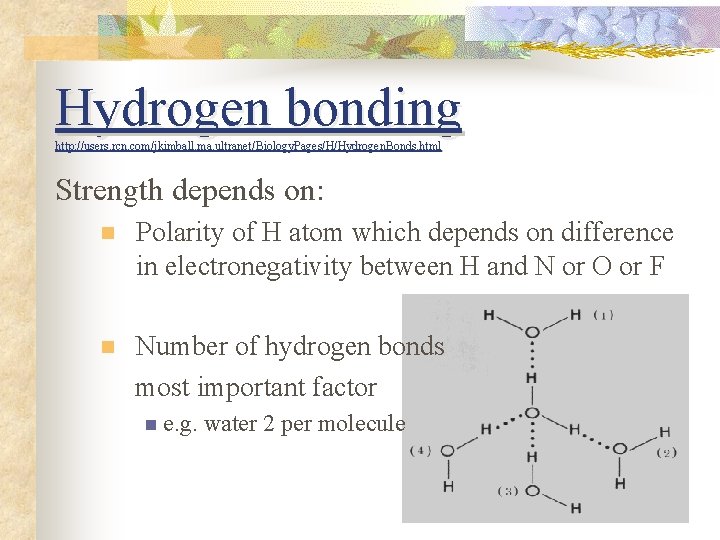
Hydrogen bonding http: //users. rcn. com Hydrogen. Bonds. html http: //users. rcn. com//jkimball. ma. ultranet/Biology. Pages/H/Hydrogen. Bonds. html Strength depends on: n Polarity of H atom which depends on difference in electronegativity between H and N or O or F n Number of hydrogen bonds most important factor n e. g. water 2 per molecule

Intermolecular forces n n n Weakest: London forces (typically around 3 k. J/mol) Strongest: hydrogen bonding (typically around 30 k. J/mol) Molecules which can make hydrogen bonds will also make London and dipole-dipole; dipoles will also have London.
- Slides: 17