Intermolecular Forces a Particles in solid b Particles
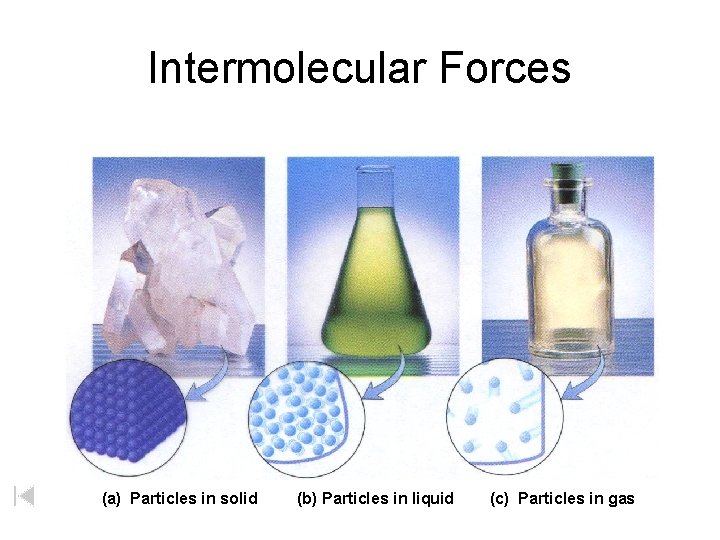
Intermolecular Forces (a) Particles in solid (b) Particles in liquid (c) Particles in gas
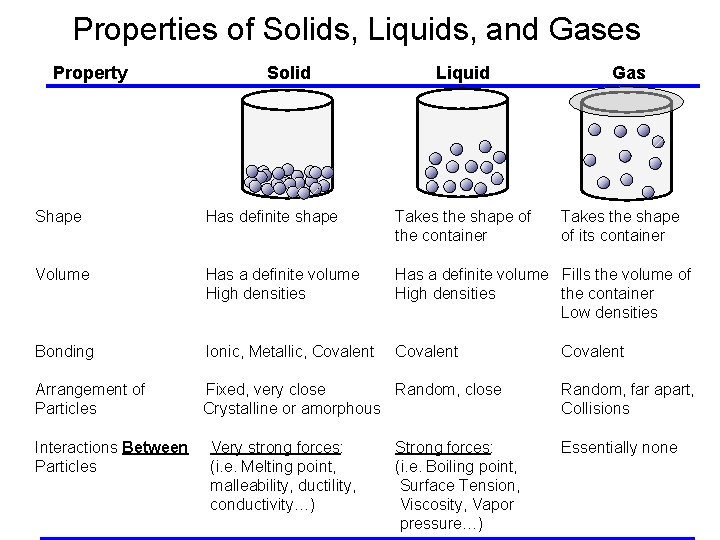
Properties of Solids, Liquids, and Gases Property Solid Liquid Gas Shape Has definite shape Takes the shape of the container Volume Has a definite volume High densities Has a definite volume Fills the volume of High densities the container Low densities Bonding Ionic, Metallic, Covalent Arrangement of Particles Fixed, very close Random, close Crystalline or amorphous Interactions Between Particles Very strong forces: (i. e. Melting point, malleability, ductility, conductivity…) Strong forces: (i. e. Boiling point, Surface Tension, Viscosity, Vapor pressure…) Takes the shape of its container Covalent Random, far apart, Collisions Essentially none
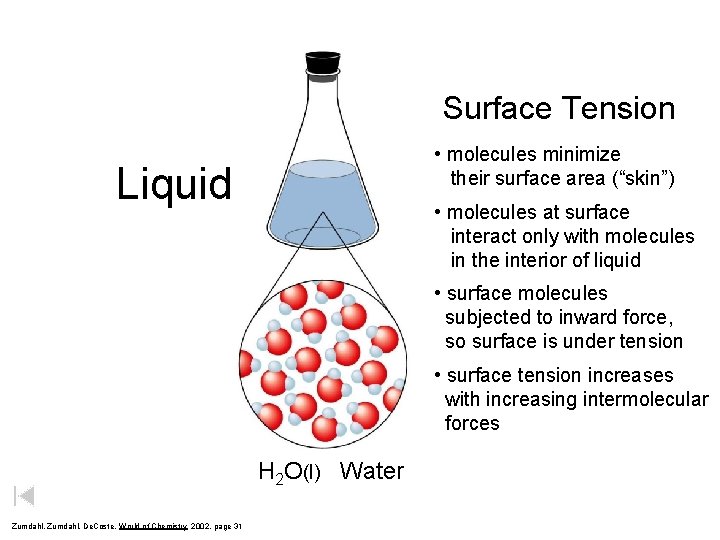
Surface Tension • molecules minimize their surface area (“skin”) Liquid • molecules at surface interact only with molecules in the interior of liquid • surface molecules subjected to inward force, so surface is under tension • surface tension increases with increasing intermolecular forces H 2 O(l) Water Zumdahl, De. Coste, World of Chemistry 2002, page 31
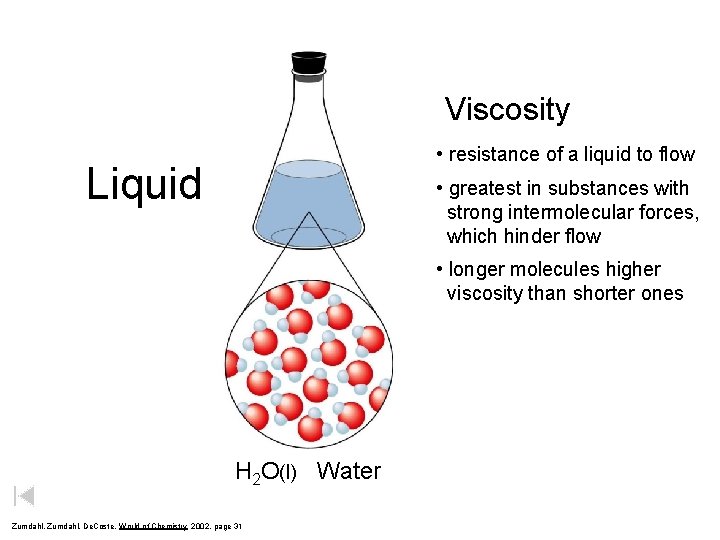
Viscosity • resistance of a liquid to flow Liquid • greatest in substances with strong intermolecular forces, which hinder flow • longer molecules higher viscosity than shorter ones H 2 O(l) Water Zumdahl, De. Coste, World of Chemistry 2002, page 31
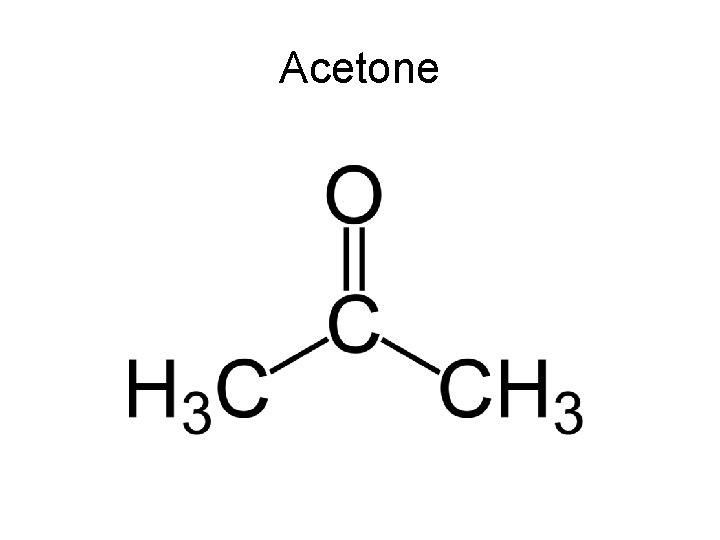
Acetone
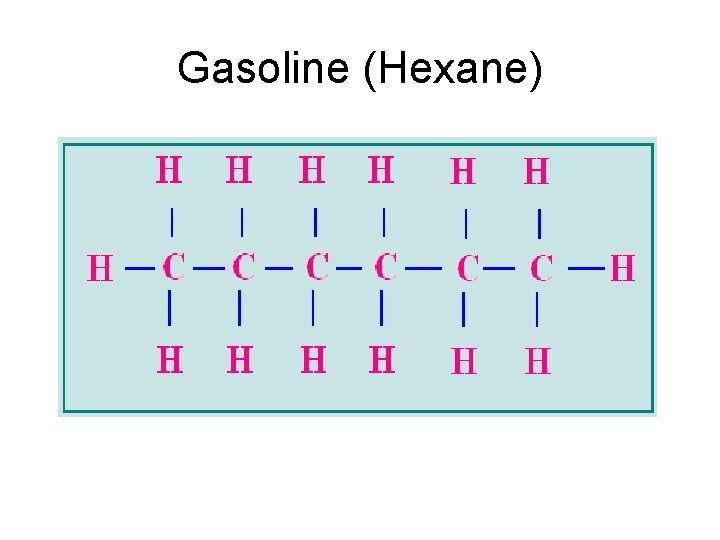
Gasoline (Hexane)
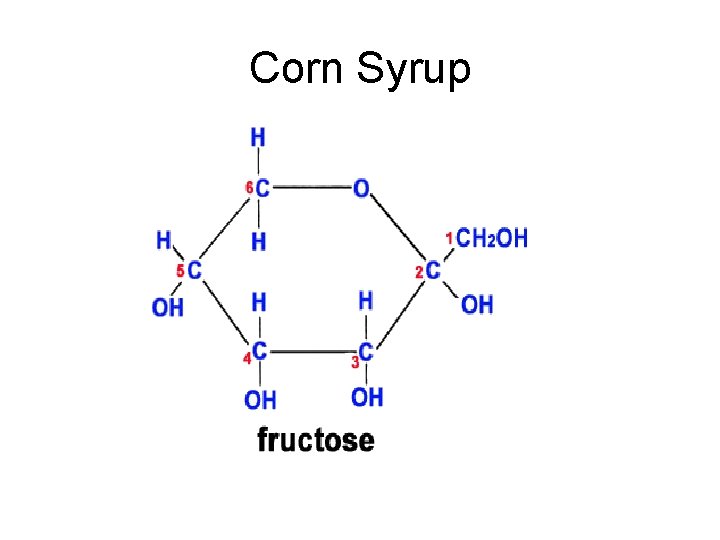
Corn Syrup
Motor Oil
Molasses
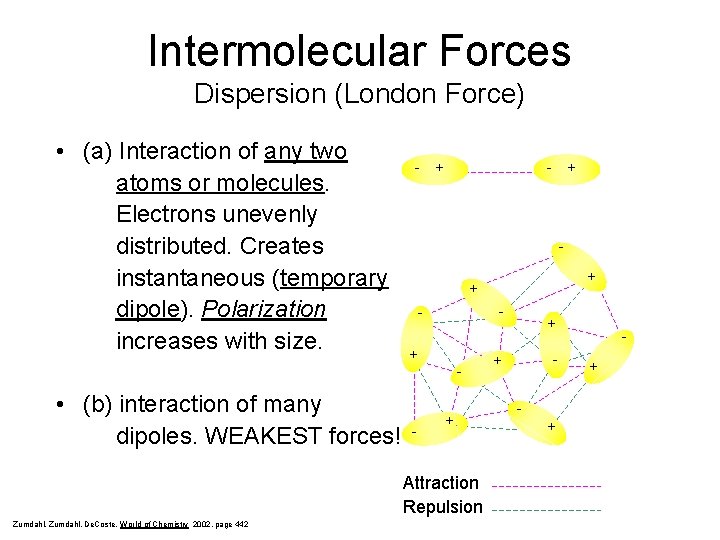
Intermolecular Forces Dispersion (London Force) • (a) Interaction of any two atoms or molecules. Electrons unevenly distributed. Creates instantaneous (temporary dipole). Polarization increases with size. - + - - - + Attraction Repulsion Zumdahl, De. Coste, World of Chemistry 2002, page 442 + + - • (b) interaction of many dipoles. WEAKEST forces! + + - + +
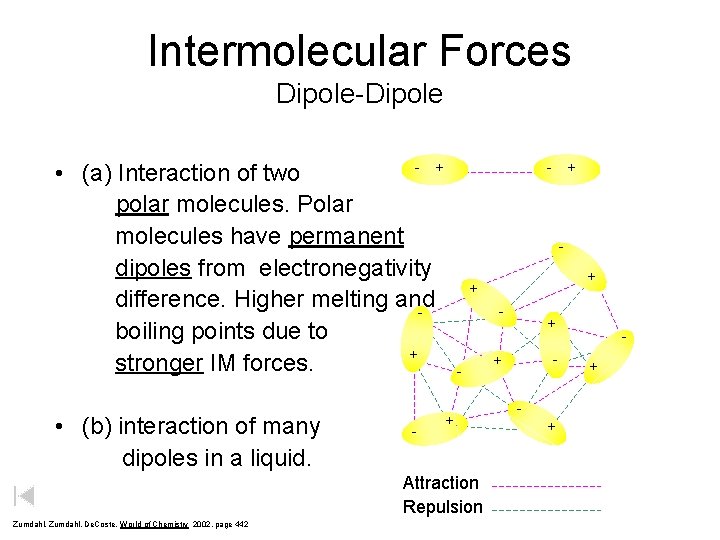
Intermolecular Forces Dipole-Dipole - + • (a) Interaction of two polar molecules. Polar molecules have permanent dipoles from electronegativity difference. Higher melting and boiling points due to + stronger IM forces. • (b) interaction of many dipoles in a liquid. - - + + - + Attraction Repulsion Zumdahl, De. Coste, World of Chemistry 2002, page 442 + + - + +
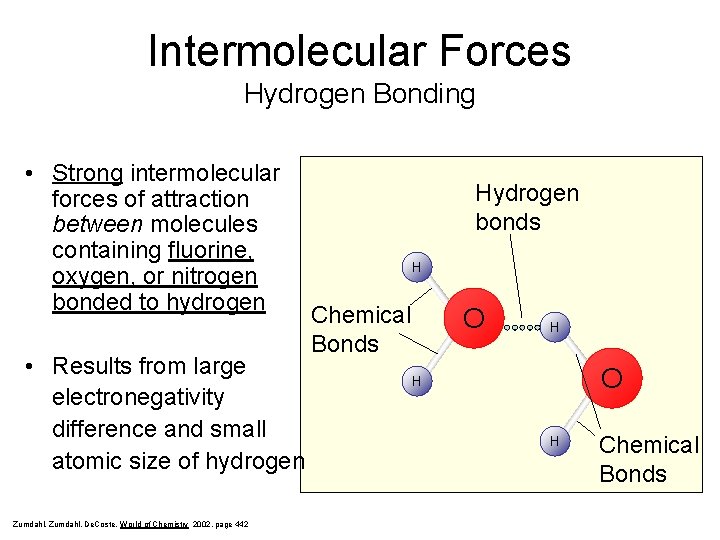
Intermolecular Forces Hydrogen Bonding • Strong intermolecular forces of attraction between molecules containing fluorine, oxygen, or nitrogen bonded to hydrogen • Results from large electronegativity difference and small atomic size of hydrogen Zumdahl, De. Coste, World of Chemistry 2002, page 442 Hydrogen bonds H Chemical Bonds O H H Chemical Bonds
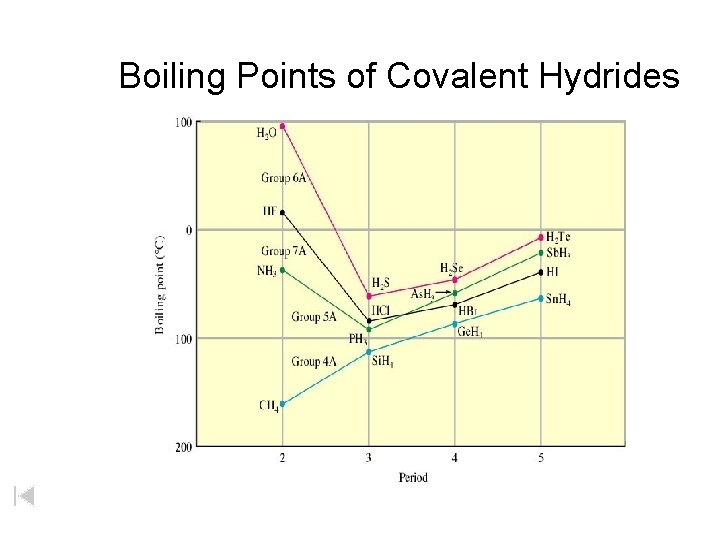
Boiling Points of Covalent Hydrides H 2 O 0 H 2 Te H 2 S Sn. H 4 Ge. H 4 Si. H 4 CH 4 50 100
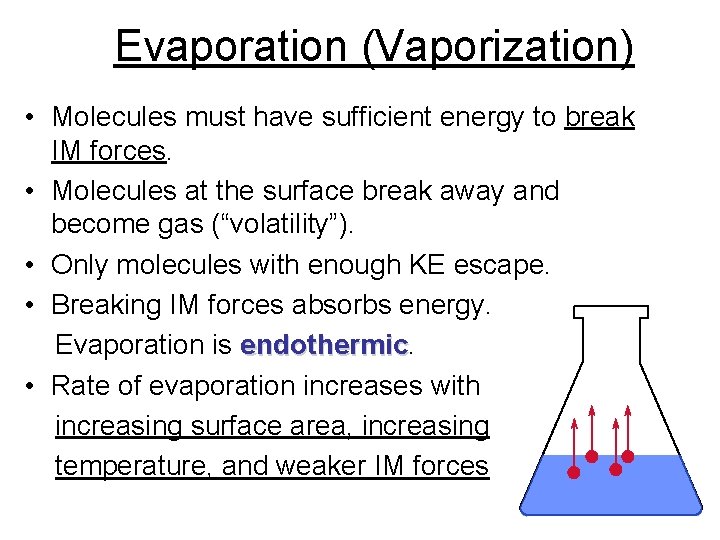
Evaporation (Vaporization) • Molecules must have sufficient energy to break IM forces. • Molecules at the surface break away and become gas (“volatility”). • Only molecules with enough KE escape. • Breaking IM forces absorbs energy. Evaporation is endothermic • Rate of evaporation increases with increasing surface area, increasing temperature, and weaker IM forces
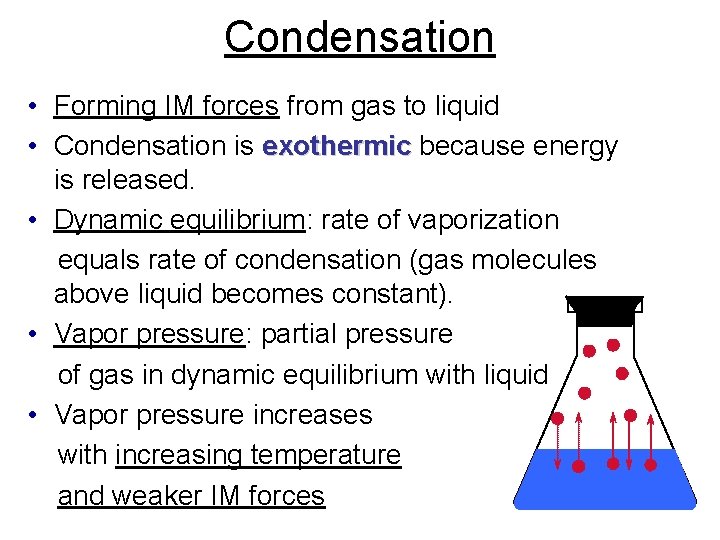
Condensation • Forming IM forces from gas to liquid • Condensation is exothermic because energy is released. • Dynamic equilibrium: rate of vaporization equals rate of condensation (gas molecules above liquid becomes constant). • Vapor pressure: partial pressure of gas in dynamic equilibrium with liquid • Vapor pressure increases with increasing temperature and weaker IM forces
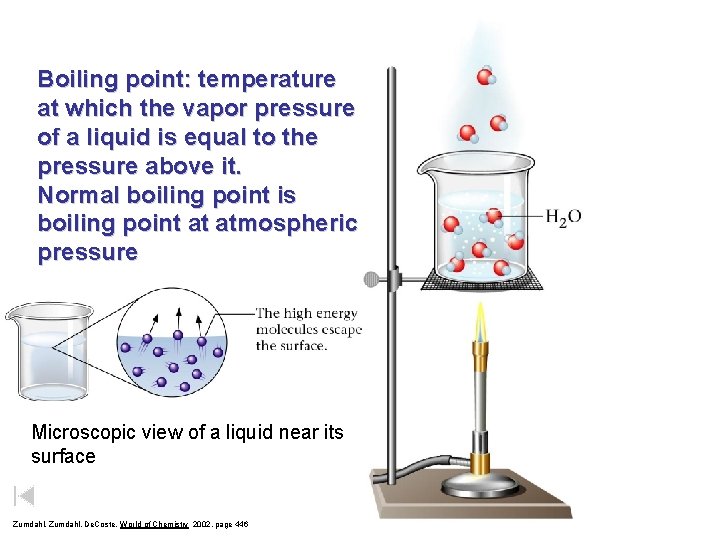
Boiling point: temperature at which the vapor pressure of a liquid is equal to the pressure above it. Normal boiling point is boiling point at atmospheric pressure Microscopic view of a liquid near its surface Zumdahl, De. Coste, World of Chemistry 2002, page 446
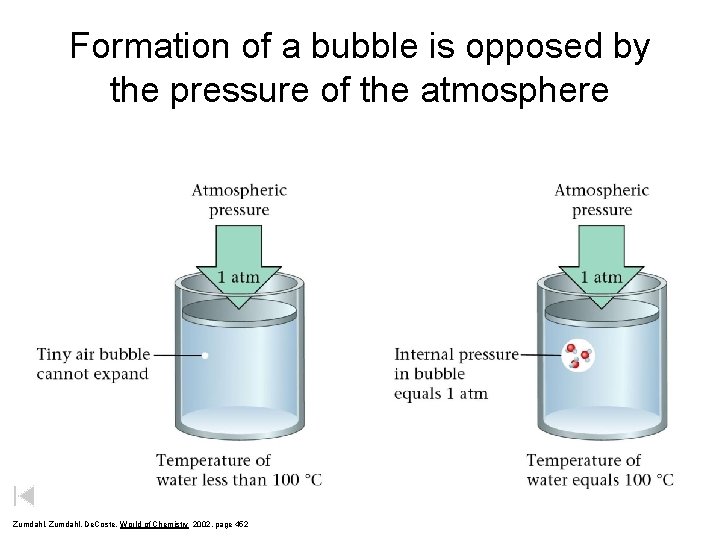
Formation of a bubble is opposed by the pressure of the atmosphere Zumdahl, De. Coste, World of Chemistry 2002, page 452
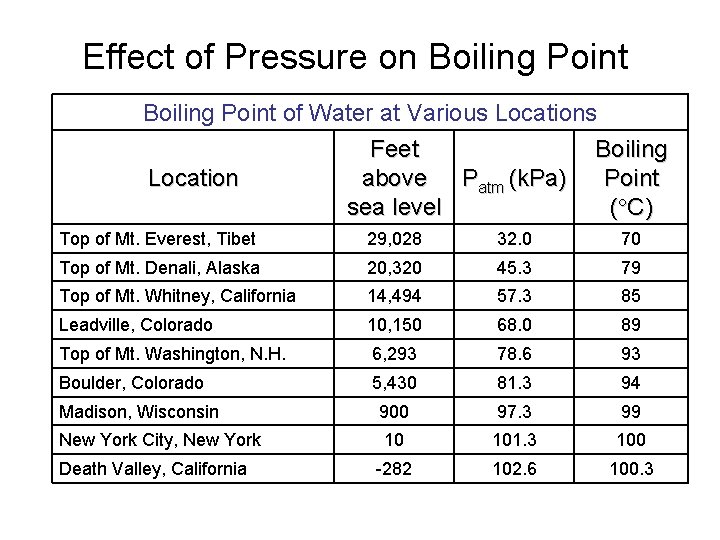
Effect of Pressure on Boiling Point of Water at Various Location Feet above Patm (k. Pa) sea level Boiling Point ( C) Top of Mt. Everest, Tibet 29, 028 32. 0 70 Top of Mt. Denali, Alaska 20, 320 45. 3 79 Top of Mt. Whitney, California 14, 494 57. 3 85 Leadville, Colorado 10, 150 68. 0 89 Top of Mt. Washington, N. H. 6, 293 78. 6 93 Boulder, Colorado 5, 430 81. 3 94 Madison, Wisconsin 900 97. 3 99 New York City, New York 10 101. 3 100 -282 102. 6 100. 3 Death Valley, California
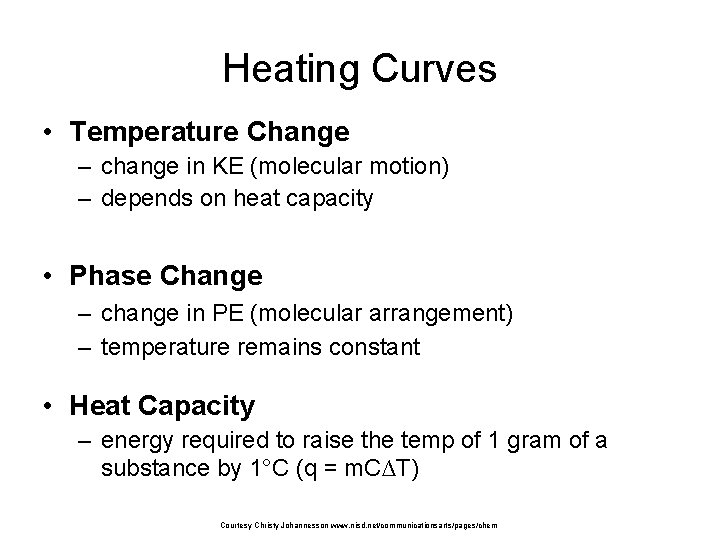
Heating Curves • Temperature Change – change in KE (molecular motion) – depends on heat capacity • Phase Change – change in PE (molecular arrangement) – temperature remains constant • Heat Capacity – energy required to raise the temp of 1 gram of a substance by 1°C (q = m. C∆T) Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
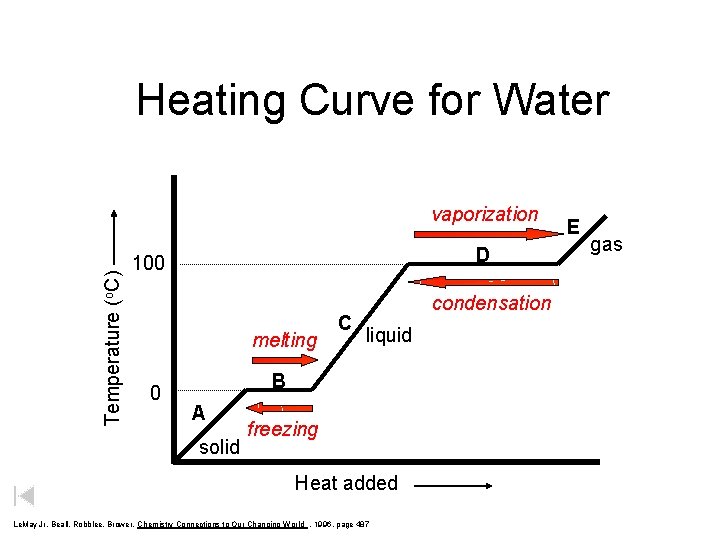
Heating Curve for Water Temperature (o. C) vaporization D 100 melting 0 C condensation liquid B A solid freezing Heat added Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 487 E gas
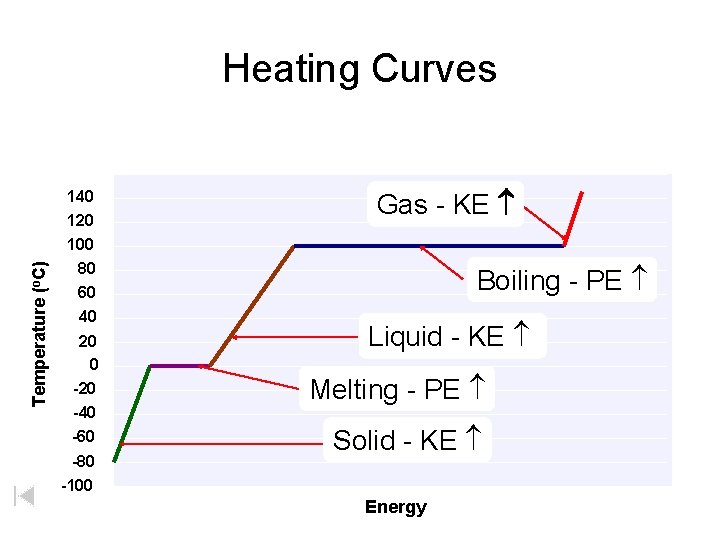
Temperature (o. C) Heating Curves 140 120 100 80 60 40 20 0 -20 -40 -60 -80 -100 Gas - KE Boiling - PE Liquid - KE Melting - PE Solid - KE Energy

Heating Curves • Heat of Fusion ( Hfus) – energy required to melt 1 gram of a substance at its melting point. Breaking intermolecular forces in the solid. Water = 6. 02 k. J/mol (@ M. P. ) • Heat of Vaporization ( Hvap) – energy required to vaporize 1 gram of a substance at its boiling point. – usually larger than Hfus (requires complete separation of molecules) – higher temperatures = lower ( Hvap) Water = 40. 7 k. J/mol (@ B. P. ) Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
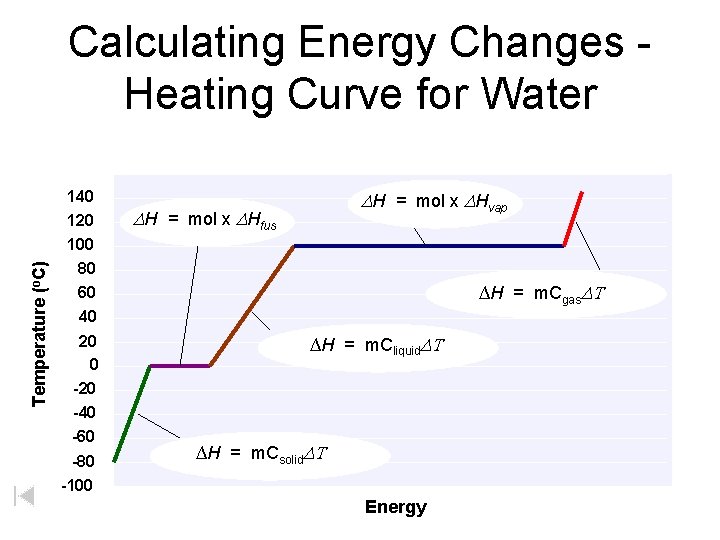
Temperature (o. C) Calculating Energy Changes Heating Curve for Water 140 120 100 80 60 40 20 0 -20 -40 -60 -80 -100 DH = mol x DHvap DH = mol x DHfus ∆H = m. Cgas. DT ∆H = m. Cliquid. DT ∆H = m. Csolid. DT Energy
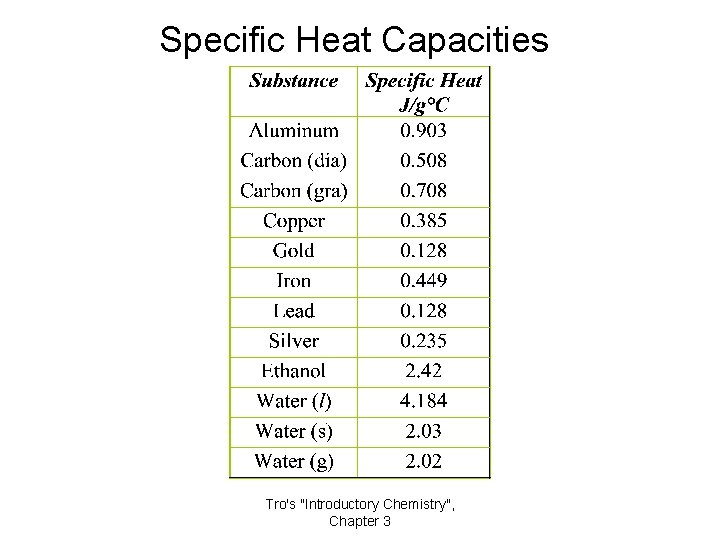
Specific Heat Capacities Tro's "Introductory Chemistry", Chapter 3
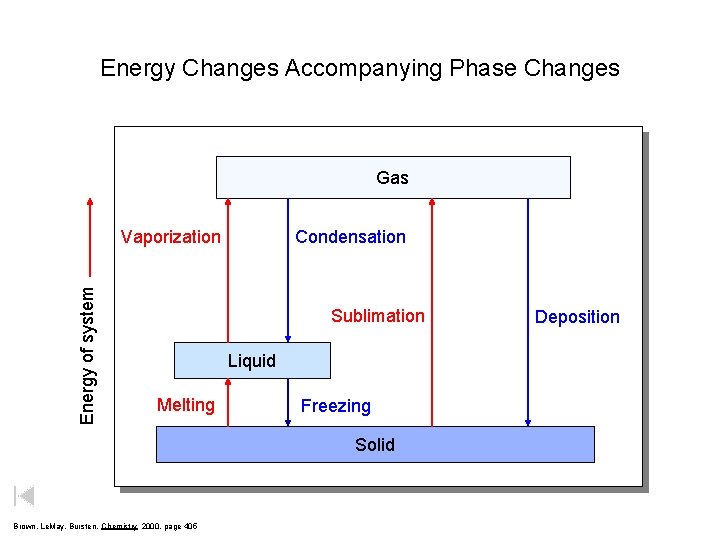
Energy Changes Accompanying Phase Changes Gas Energy of system Vaporization Condensation Sublimation Liquid Melting Freezing Solid Brown, Le. May, Bursten, Chemistry 2000, page 405 Deposition
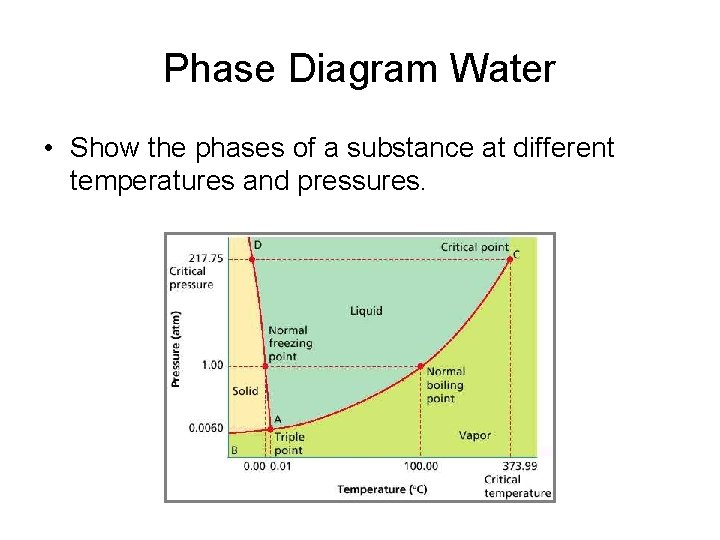
Phase Diagram Water • Show the phases of a substance at different temperatures and pressures.
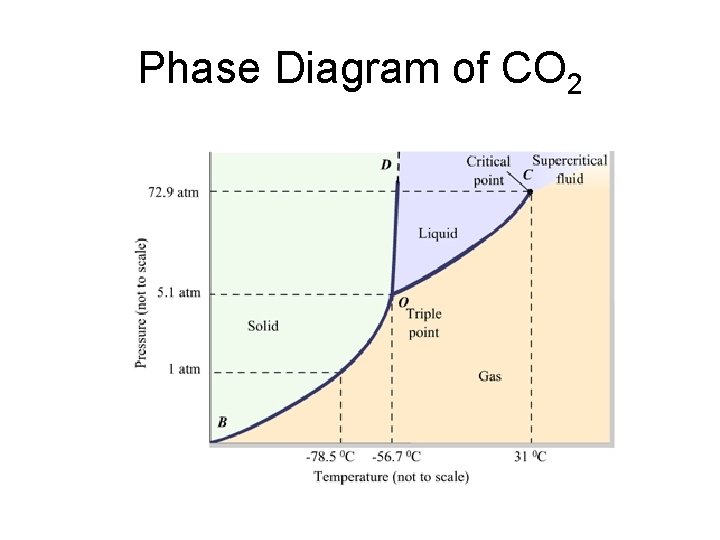
Phase Diagram of CO 2

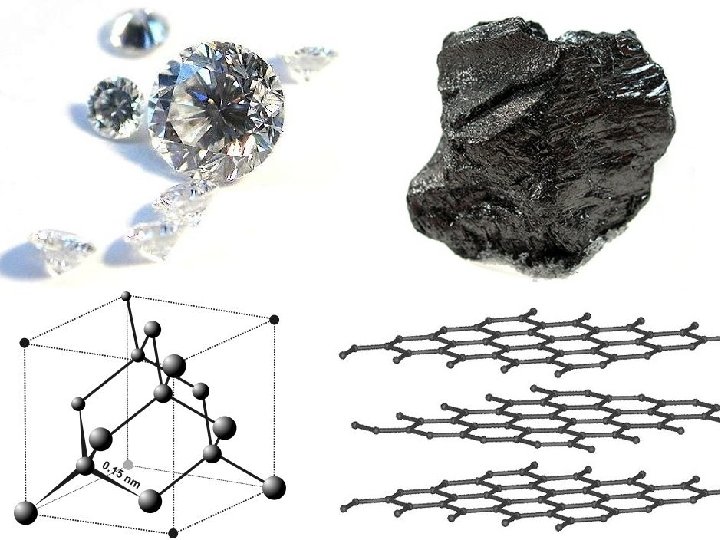
Solids • Ionic – Giant lattice of ions, strong electrostatic attractions between metal and nonmetal, strong bonds, high melting points, crystalline, poor conductors except in molten and aqueous states (Na. Cl, KBr…) • Discrete Covalent – Groups of atoms covalently bonded by sharing electrons between two nonmetals/metalloids, weaker bonds than ionic, IMFs with relatively low melting points (except very large polymers), soft, poor conductors (plastics, wax…) • Giant Covalent – Massive structures of atoms bonded through network covalent bonds, very strong bonds with very high melting points, hard/rigid, poor conductors (graphite (carbon), diamond (carbon), Si. O 2, Si…) • Metallic – Closely packed array of atoms or ions with “sea” of free moving electrons, all metals and alloys (mixtures), stronger bonds than covalent but weaker than ionic, high melting points, malleable/ductile/shiny, very good conductors (Cu, Na…)


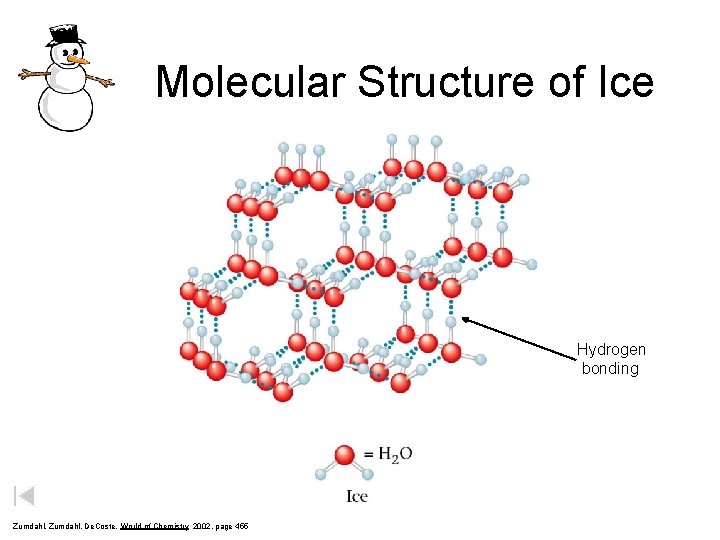
Molecular Structure of Ice Hydrogen bonding Zumdahl, De. Coste, World of Chemistry 2002, page 455
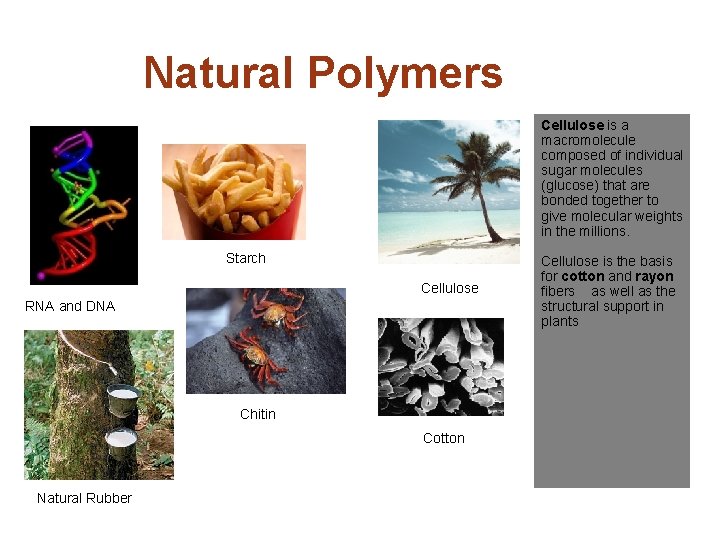
Natural Polymers Cellulose is a macromolecule composed of individual sugar molecules (glucose) that are bonded together to give molecular weights in the millions. Starch Cellulose RNA and DNA Chitin Cotton Natural Rubber Cellulose is the basis for cotton and rayon fibers as well as the structural support in plants

Synthetic Polymers “Everyday Polys” Polypropylene Polystyrene Polyvinyl Chloride Polyester Polyethylene Polyvinyl chloride Polypropylene Polystyrene Polyethylene
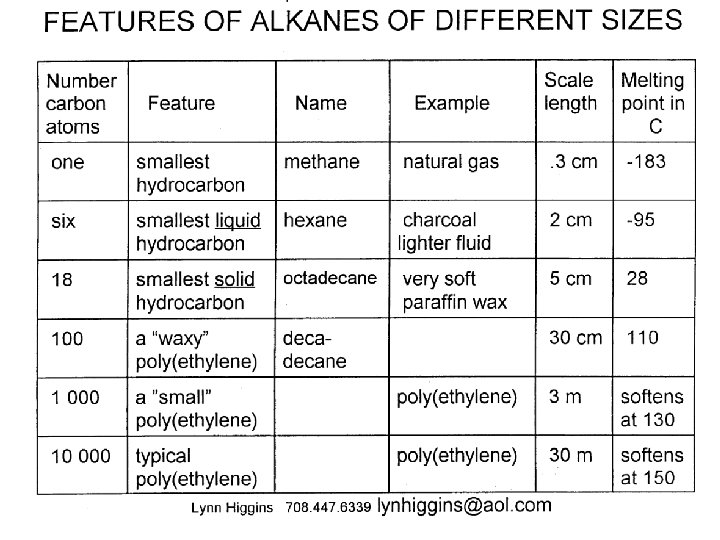
- Slides: 38