Intermittent vs Continuous Infusion Of Vancomycin In Neonates







- Slides: 20

Intermittent vs Continuous Infusion Of Vancomycin In Neonates Anisa Patel – Neonatal Pharmacist Yorkhill Hospital, Glasgow Anand D 1 Lucas C 3, Thomson AH, 2, 4

Background ¢ Coagulase negative Staph (Co. NS) is a major cause of late onset sepsis in NICU ¢ Vancomycin is usually 1 st choice antibiotic ¢ Efficacy depends on achieving and maintaining optimal concentrations throughout the treatment period ¢ Avoid toxic / sub-therapeutic levels

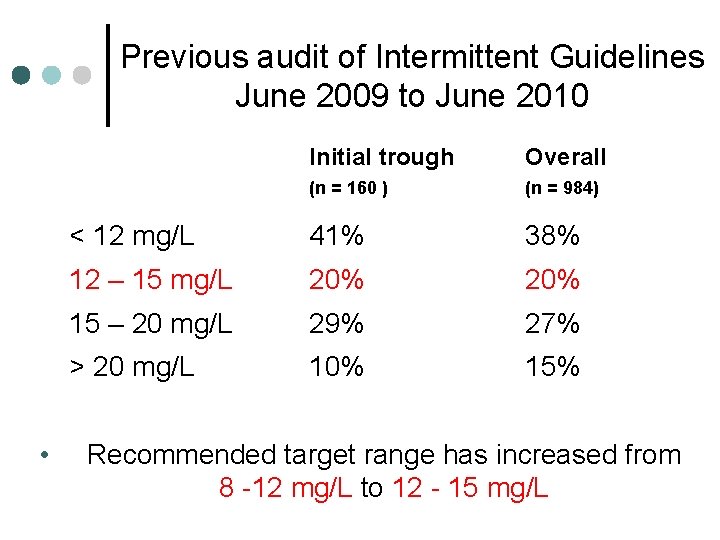
Previous audit of Intermittent Guidelines June 2009 to June 2010 • Initial trough Overall (n = 160 ) (n = 984) < 12 mg/L 41% 38% 12 – 15 mg/L 20% 15 – 20 mg/L 29% 27% > 20 mg/L 10% 15% Recommended target range has increased from 8 -12 mg/L to 12 - 15 mg/L

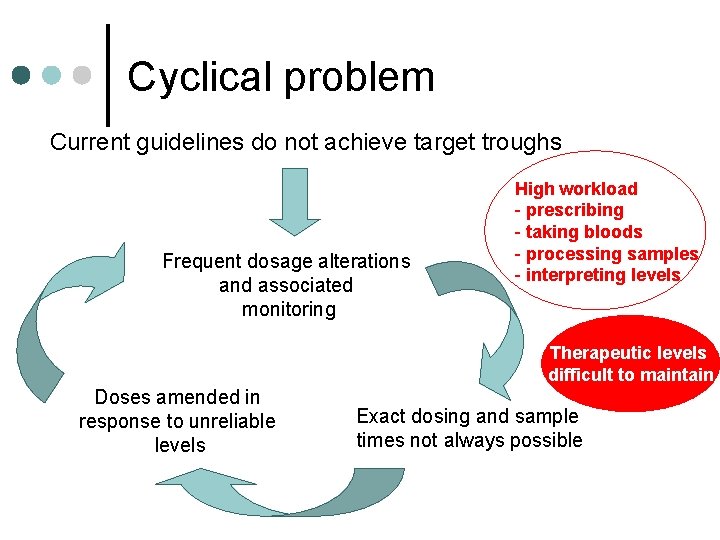
Cyclical problem Current guidelines do not achieve target troughs Frequent dosage alterations and associated monitoring High workload - prescribing - taking bloods - processing samples - interpreting levels Therapeutic levels difficult to maintain Doses amended in response to unreliable levels Exact dosing and sample times not always possible

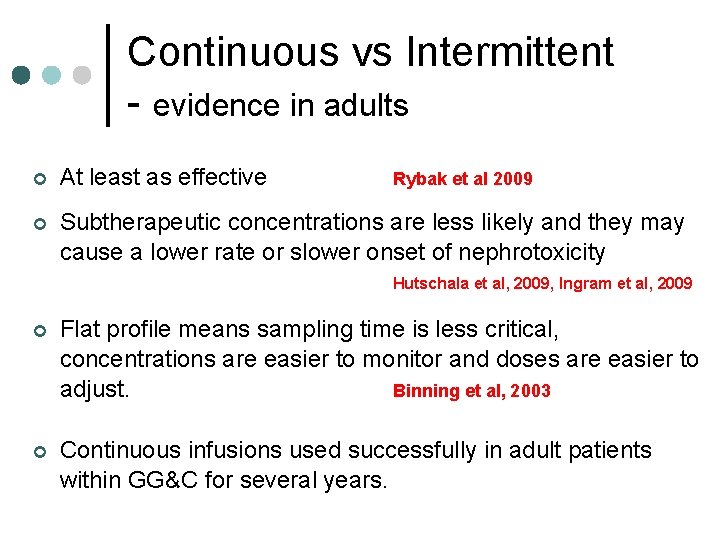
Continuous vs Intermittent - evidence in adults ¢ At least as effective ¢ Subtherapeutic concentrations are less likely and they may cause a lower rate or slower onset of nephrotoxicity Rybak et al 2009 Hutschala et al, 2009, Ingram et al, 2009 ¢ Flat profile means sampling time is less critical, concentrations are easier to monitor and doses are easier to adjust. Binning et al, 2003 ¢ Continuous infusions used successfully in adult patients within GG&C for several years.

Additional potential benefits ¢ Time dependent antibiotic so flat profile is ideal. ¢ New guideline which aims for current recommended levels ¢ Wider target range due to flat profile. ¢ Levels with routine morning bloods - fewer blood samples from patients. ¢ Standard strength syringes from pharmacy

Objectives ¢ To compare the effectiveness of intermittent and continuous vancomycin infusions in achieving target concentrations in neonates ¢ To assess the implications for clinical practice

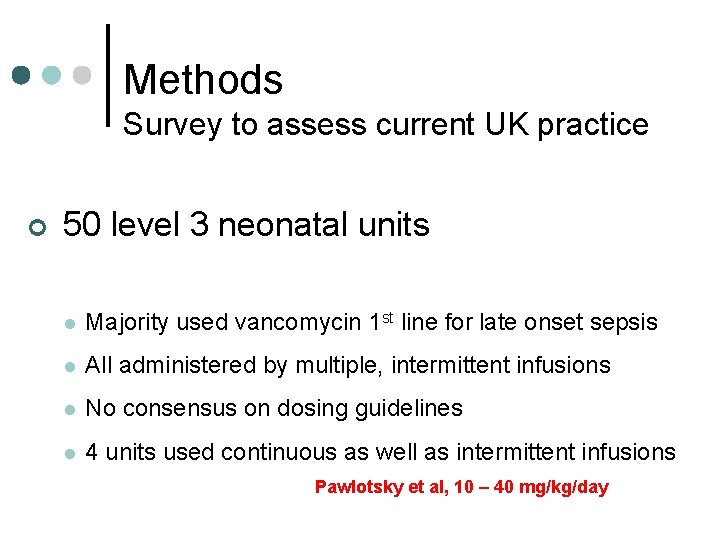
Methods Survey to assess current UK practice ¢ 50 level 3 neonatal units l Majority used vancomycin 1 st line for late onset sepsis l All administered by multiple, intermittent infusions l No consensus on dosing guidelines l 4 units used continuous as well as intermittent infusions Pawlotsky et al, 10 – 40 mg/kg/day

Methods ¢ Audit comparing intermittent and continuous infusion guidelines over a 4 month period. ¢ Included all patients admitted to NICU RHSC between March and June 2011 treated with vancomycin - Month 1: Data collection on intermittent guideline Month 2: Training on new continuous infusion guideline Month 3: Implementation of guideline Month 4: Data collection on continuous infusions

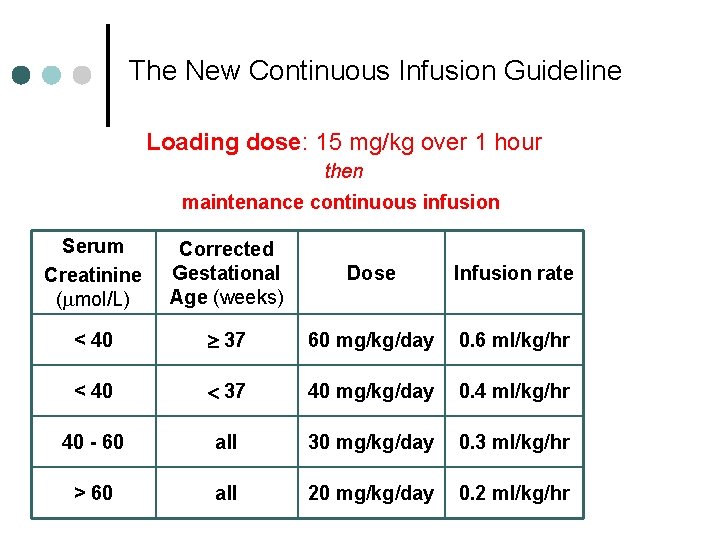
The New Continuous Infusion Guideline Loading dose: 15 mg/kg over 1 hour then maintenance continuous infusion Serum Creatinine (mmol/L) Corrected Gestational Age (weeks) Dose Infusion rate < 40 37 60 mg/kg/day 0. 6 ml/kg/hr < 40 37 40 mg/kg/day 0. 4 ml/kg/hr 40 - 60 all 30 mg/kg/day 0. 3 ml/kg/hr > 60 all 20 mg/kg/day 0. 2 ml/kg/hr

Development of the Guideline ¢ Existing published guidelines & population PK ¢ Modified using published recommendations and anecdotal experience within Glasgow and Leeds. Frymoyer et al 2009, Glover et al, 2000 ¢ Not used in practice prior to this audit ¢ Recommended target range = 15 – 25 mg/L

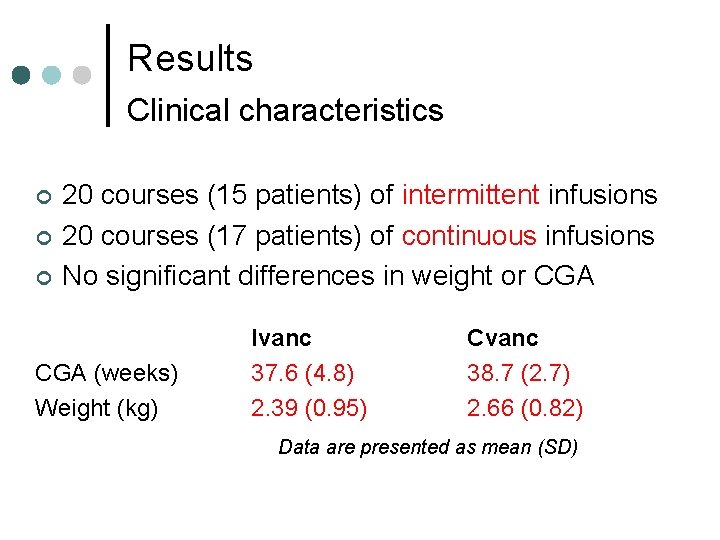
Results Clinical characteristics ¢ ¢ ¢ 20 courses (15 patients) of intermittent infusions 20 courses (17 patients) of continuous infusions No significant differences in weight or CGA (weeks) Weight (kg) Ivanc 37. 6 (4. 8) 2. 39 (0. 95) Cvanc 38. 7 (2. 7) 2. 66 (0. 82) Data are presented as mean (SD)

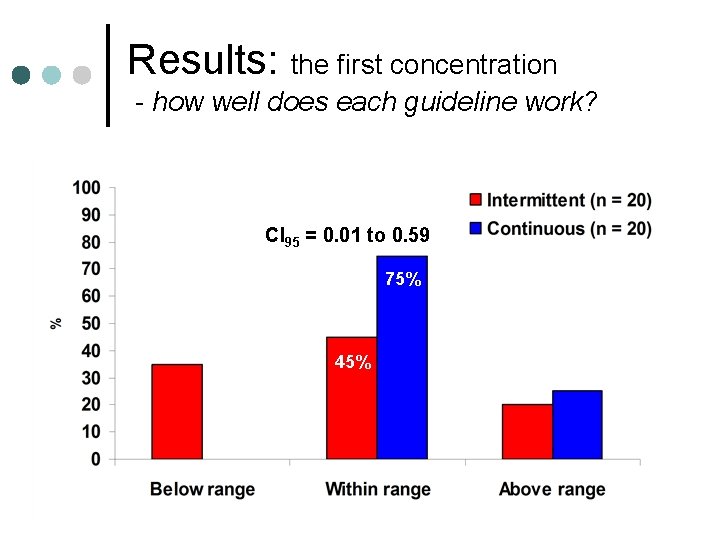
Results: the first concentration - how well does each guideline work? CI 95 = 0. 01 to 0. 59 75% 45%

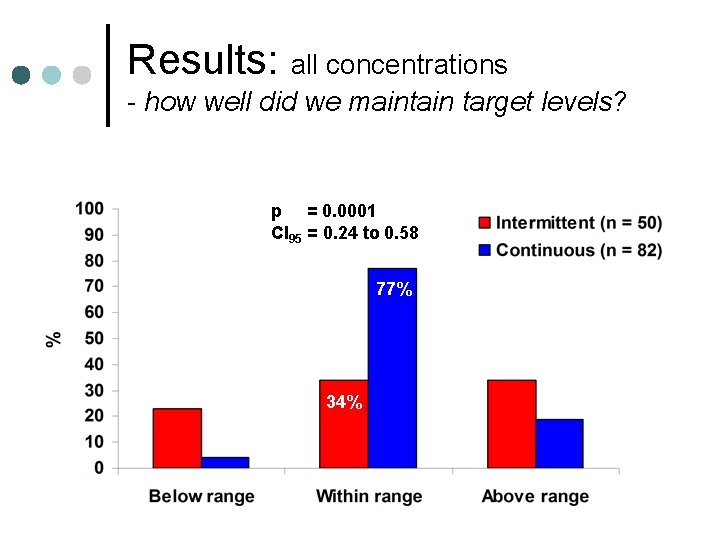
Results: all concentrations - how well did we maintain target levels? p = 0. 0001 CI 95 = 0. 24 to 0. 58 77% 34%

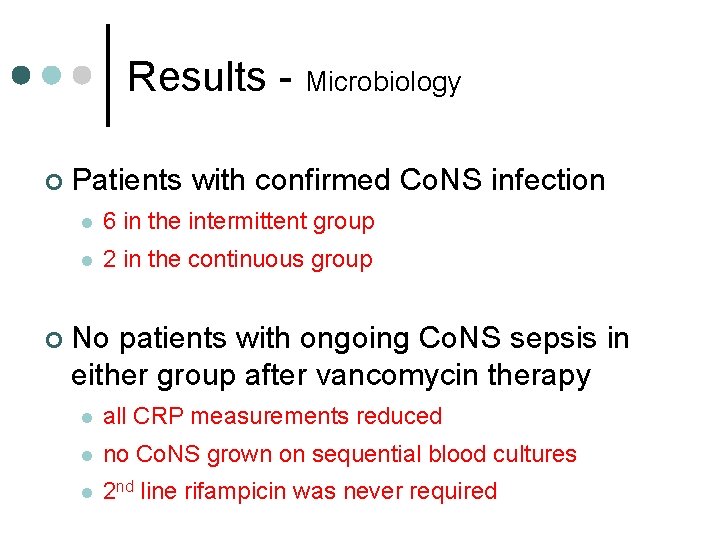
Results - Microbiology ¢ ¢ Patients with confirmed Co. NS infection l 6 in the intermittent group l 2 in the continuous group No patients with ongoing Co. NS sepsis in either group after vancomycin therapy l all CRP measurements reduced l no Co. NS grown on sequential blood cultures l 2 nd line rifampicin was never required

Results – Creatinine concentration ¢ No significant increase in creatinine concentration in either group during or after vancomycin therapy ¢ Creatinine concentrations fell between the start and end of treatment in 16 out of 20 courses in the Ivanc group l 14 out of 20 courses in the Cvanc group l

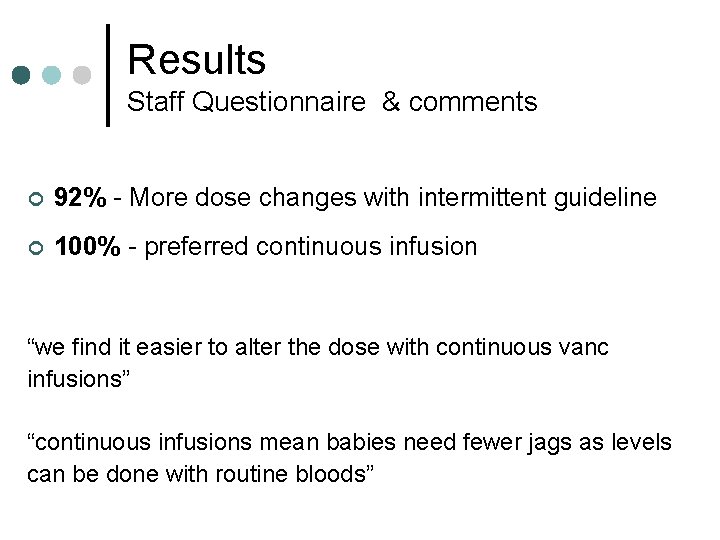
Results Staff Questionnaire & comments ¢ 92% - More dose changes with intermittent guideline ¢ 100% - preferred continuous infusion “we find it easier to alter the dose with continuous vanc infusions” “continuous infusions mean babies need fewer jags as levels can be done with routine bloods”

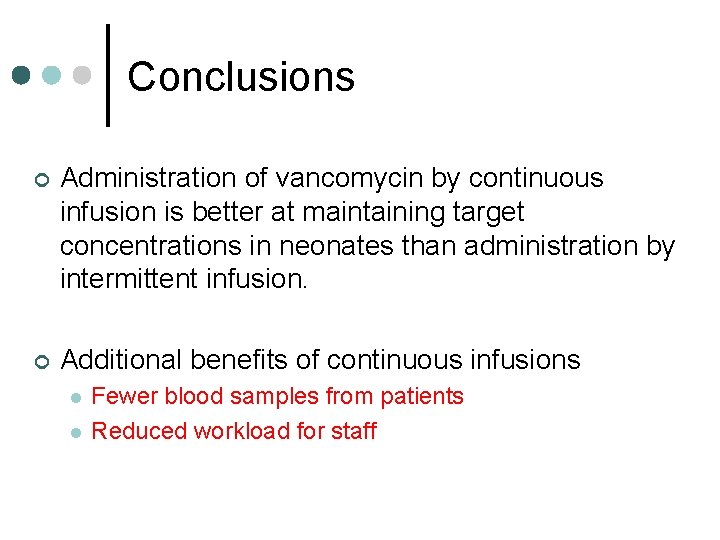
Conclusions ¢ Administration of vancomycin by continuous infusion is better at maintaining target concentrations in neonates than administration by intermittent infusion. ¢ Additional benefits of continuous infusions l l Fewer blood samples from patients Reduced workload for staff

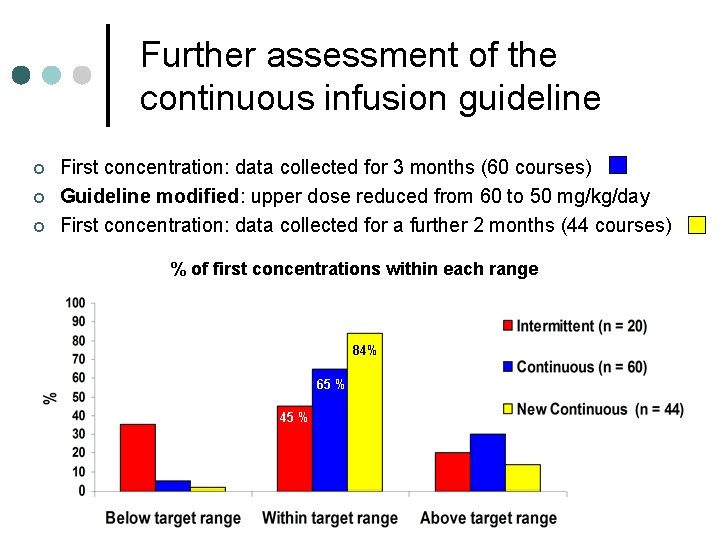
Further assessment of the continuous infusion guideline ¢ ¢ ¢ First concentration: data collected for 3 months (60 courses) Guideline modified: upper dose reduced from 60 to 50 mg/kg/day First concentration: data collected for a further 2 months (44 courses) % of first concentrations within each range 84% 65 % 45 %

Questions?