Interim UK Regulatory Route Map for Stem Cell
- Slides: 1

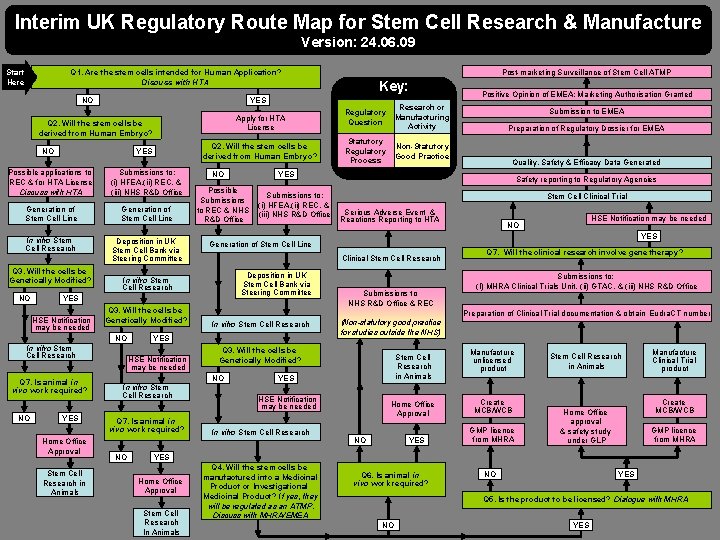
Interim UK Regulatory Route Map for Stem Cell Research & Manufacture Version: 24. 06. 09 Post-marketing Surveillance of Stem Cell ATMP Q 1. Are the stem cells intended for Human Application? Discuss with HTA Start Here NO Apply for HTA License Q 2. Will the stem cells be derived from Human Embryo? NO YES Possible applications to REC & for HTA License Discuss with HTA Submissions to: (i) HFEA, (ii) REC, & (iii) NHS R&D Office Generation of Stem Cell Line In vitro Stem Cell Research Deposition in UK Stem Cell Bank via Steering Committee Q 3. Will the cells be Genetically Modified? NO Q 7. Is animal in vivo work required? YES Home Office Approval Stem Cell Research in Animals Q 7. Is animal in vivo work required? Statutory Regulatory Process Non-Statutory Good Practice Positive Opinion of EMEA: Marketing Authorisation Granted Submission to EMEA Preparation of Regulatory Dossier for EMEA Quality, Safety & Efficacy Data Generated Safety reporting to Regulatory Agencies Stem Cell Clinical Trial Serious Adverse Event & Reactions Reporting to HTA Clinical Stem Cell Research Deposition in UK Stem Cell Bank via Steering Committee HSE Notification may be needed NO YES Generation of Stem Cell Line Submissions to NHS R&D Office & REC Q 7. Will the clinical research involve gene therapy? Submissions to: (I) MHRA Clinical Trials Unit, (ii) GTAC, & (iii) NHS R&D Office Preparation of Clinical Trial documentation & obtain Eudra. CT number In vitro Stem Cell Research (Non-statutory good practice for studies outside the NHS) Q 3. Will the cells be Genetically Modified? NO In vitro Stem Cell Research NO Submissions to: (i) HFEA, (ii) REC, & (iii) NHS R&D Office YES HSE Notification may be needed Regulatory Question Research or Manufacturing Activity YES Possible Submissions to REC & NHS R&D Office In vitro Stem Cell Research NO NO YES HSE Notification may be needed Key: YES Stem Cell Research in Animals YES HSE Notification may be needed In vitro Stem Cell Research Home Office Approval NO YES Manufacture unlicensed product Create MCB/WCB GMP licence from MHRA Stem Cell Research in Animals Manufacture Clinical Trial product Create MCB/WCB Home Office approval & safety study under GLP GMP licence from MHRA YES Home Office Approval Stem Cell Research In Animals Q 4. Will the stem cells be manufactured into a Medicinal Product or Investigational Medicinal Product? If yes, they will be regulated as an ATMP. Discuss with MHRA/EMEA Q 6. Is animal in vivo work required? NO YES Q 5. Is the product to be licensed? Dialogue with MHRA NO YES