Interchange vs Interoperability Syntax Structure Semantics Meaning Main
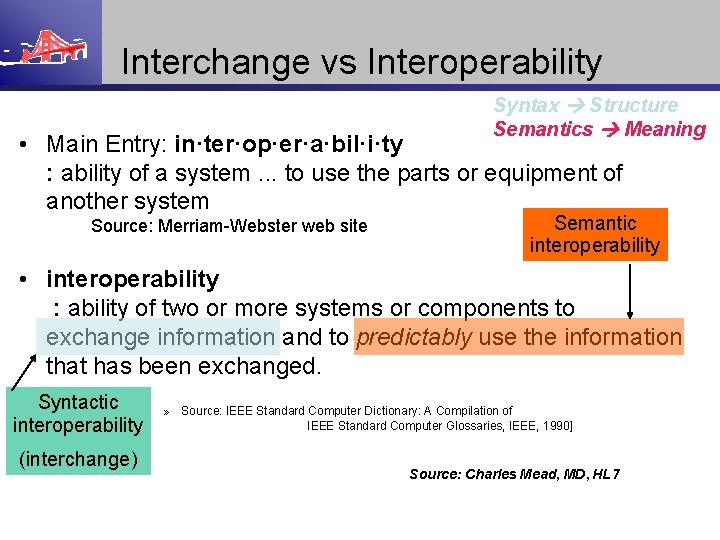
Interchange vs Interoperability Syntax Structure Semantics Meaning • Main Entry: in·ter·op·er·a·bil·i·ty : ability of a system. . . to use the parts or equipment of another system Source: Merriam-Webster web site Semantic interoperability • interoperability : ability of two or more systems or components to exchange information and to predictably use the information that has been exchanged. Syntactic interoperability (interchange) » Source: IEEE Standard Computer Dictionary: A Compilation of IEEE Standard Computer Glossaries, IEEE, 1990] Source: Charles Mead, MD, HL 7

Computerized doesn’t mean syntactic interoperability 6 1 2 5 3 4
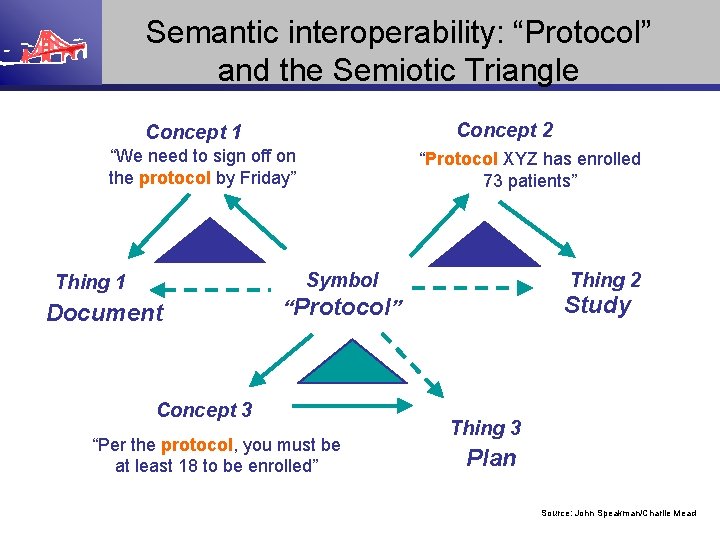
Semantic interoperability: “Protocol” and the Semiotic Triangle Concept 2 Concept 1 “We need to sign off on the protocol by Friday” “Protocol XYZ has enrolled 73 patients” Symbol Thing 1 Document Thing 2 Study “Protocol” Concept 3 “Per the protocol, you must be at least 18 to be enrolled” Thing 3 Plan Source: John Speakman/Charlie Mead
Semantic Interoperability • To understand the data being received you must know both: • The definition of each element of data, and its relationship with each of the other elements – you must have a semantic model of the data and – The terminology to be used to represent coded elements, including the definitions, and relationships within the terminology Source: HL 7
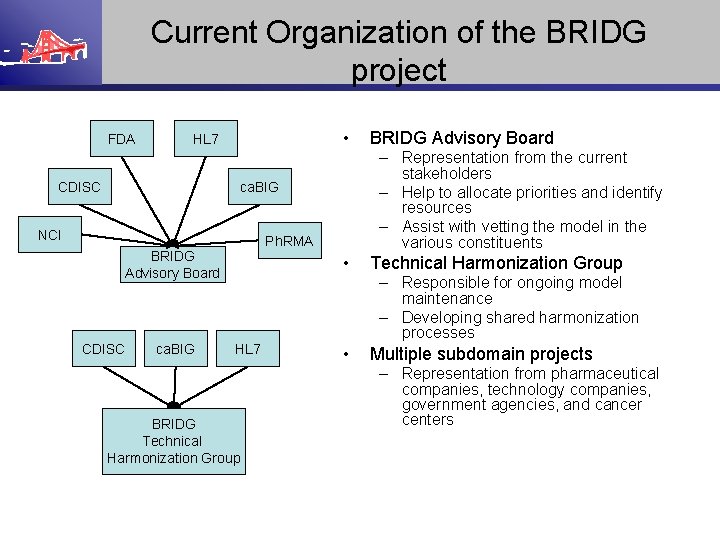
Current Organization of the BRIDG project FDA • HL 7 CDISC – Representation from the current stakeholders – Help to allocate priorities and identify resources – Assist with vetting the model in the various constituents ca. BIG NCI Ph. RMA BRIDG Advisory Board CDISC ca. BIG • HL 7 BRIDG Technical Harmonization Group BRIDG Advisory Board Technical Harmonization Group – Responsible for ongoing model maintenance – Developing shared harmonization processes • Multiple subdomain projects – Representation from pharmaceutical companies, technology companies, government agencies, and cancer centers
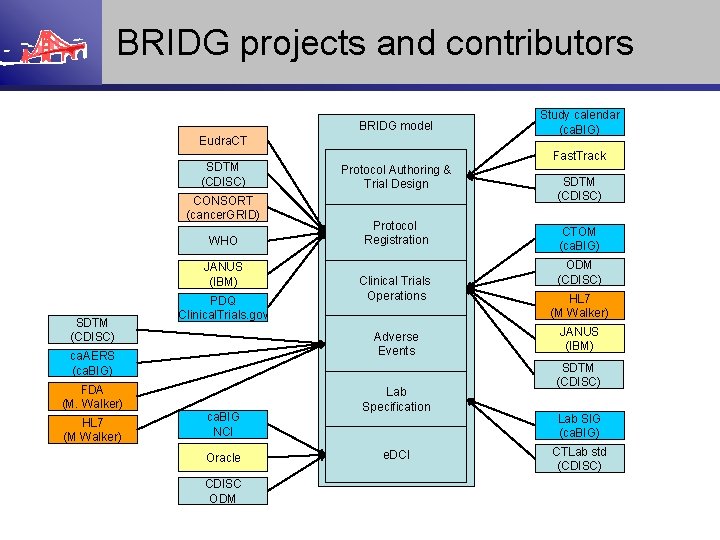
BRIDG projects and contributors BRIDG model Eudra. CT SDTM (CDISC) CONSORT (cancer. GRID) WHO JANUS (IBM) SDTM (CDISC) PDQ Clinical. Trials. gov FDA (M. Walker) HL 7 (M Walker) Fast. Track Protocol Authoring & Trial Design Protocol Registration Clinical Trials Operations Adverse Events ca. AERS (ca. BIG) ca. BIG NCI Oracle CDISC ODM Study calendar (ca. BIG) Lab Specification e. DCI SDTM (CDISC) CTOM (ca. BIG) ODM (CDISC) HL 7 (M Walker) JANUS (IBM) SDTM (CDISC) Lab SIG (ca. BIG) CTLab std (CDISC)
Model organization • Dynamic View – Captures the business process decomposition of the lifecycle of clinical trials research

Behavioral Aspects of BRIDG
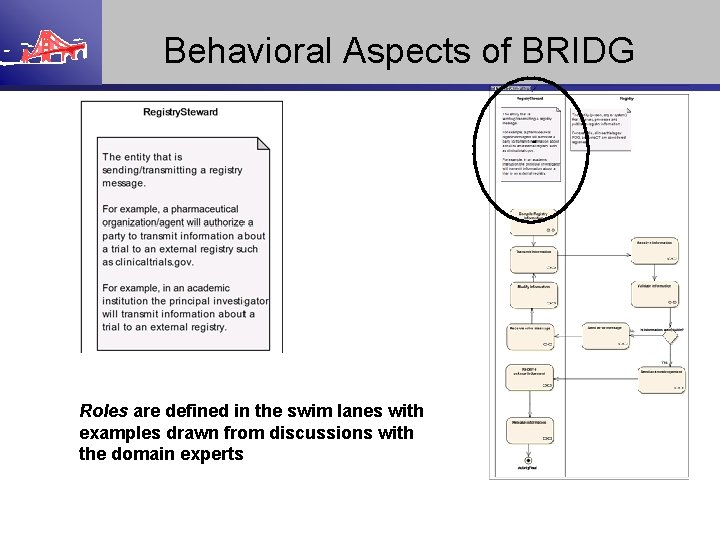
Behavioral Aspects of BRIDG Roles are defined in the swim lanes with examples drawn from discussions with the domain experts
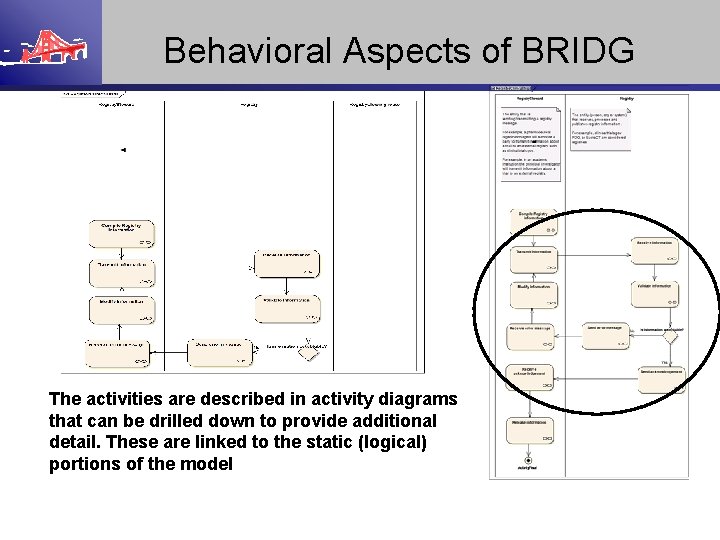
Behavioral Aspects of BRIDG The activities are described in activity diagrams that can be drilled down to provide additional detail. These are linked to the static (logical) portions of the model
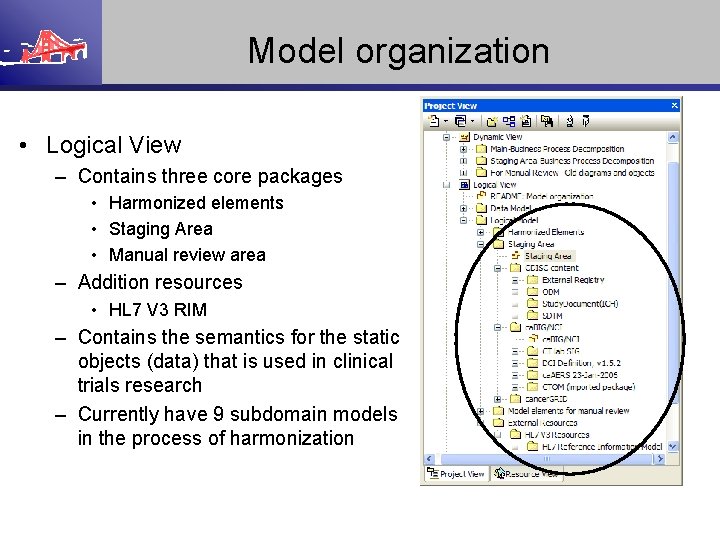
Model organization • Logical View – Contains three core packages • Harmonized elements • Staging Area • Manual review area – Addition resources • HL 7 V 3 RIM – Contains the semantics for the static objects (data) that is used in clinical trials research – Currently have 9 subdomain models in the process of harmonization
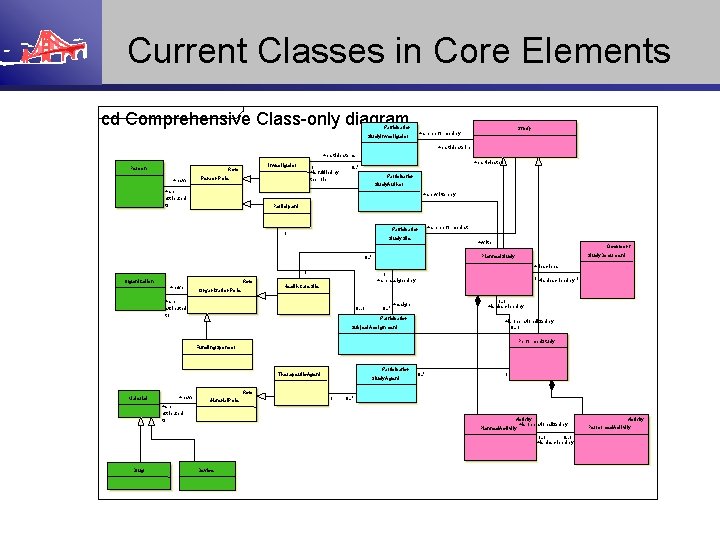
Current Classes in Core Elements cd Comprehensive Class-only diagram Participation Study +are performed by Study. Investigator +participate in +participate as Person Investigator Role +have 1 +is fulfiled by the role Person. Role +are attributed to +participate in 0. . * Participation Study. Author +are written by Participant Participation 1 Study. Site +are performed at +write Document Study. Document Planned. Study 0. . * +describes 1 Organization Role +have Organization. Role 1 +are assigned by 1 +is described by 1 Health. Care. Site +are attributed to 0. . 1 0. . * 1. . 1 +is described by +assign Participation +is operationalized by 0. . 1 Subject. Assignment Performed. Study Funding. Sponsor Participation Therapeutic. Agent Material +have Study. Agent 0. . * 1 Role Material. Role +are attributed to 1 0. . * Activity +is operationalized by Planned. Activity 1. . 1 0. . 1 +is described by Drug Device Activity Performed. Activity

Harmonized BRIDG elements
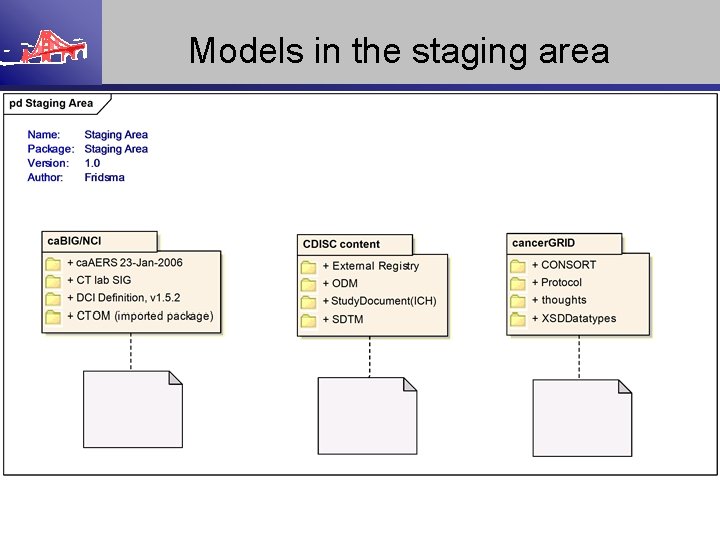
Models in the staging area
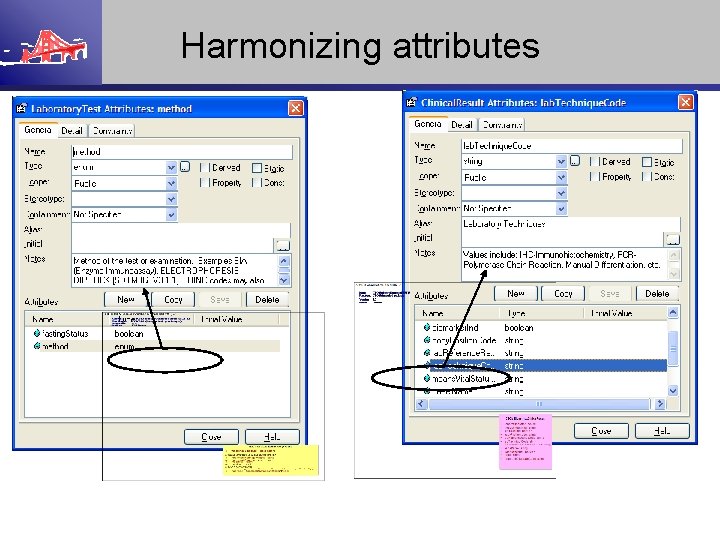
Harmonizing attributes
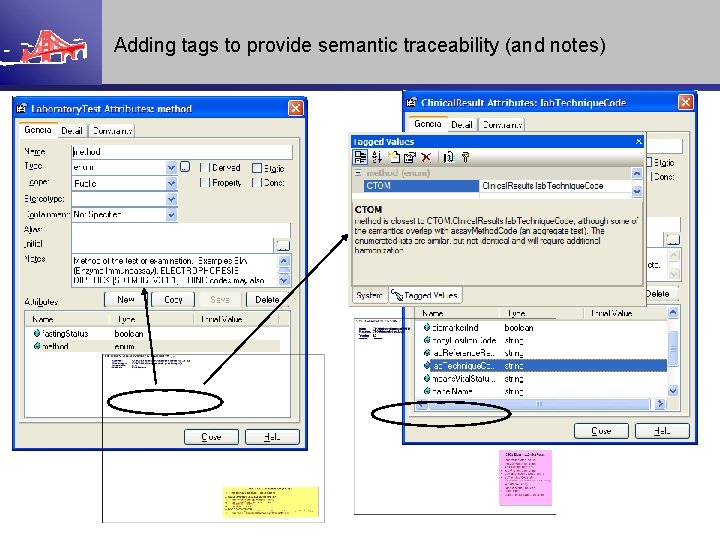
Adding tags to provide semantic traceability (and notes)
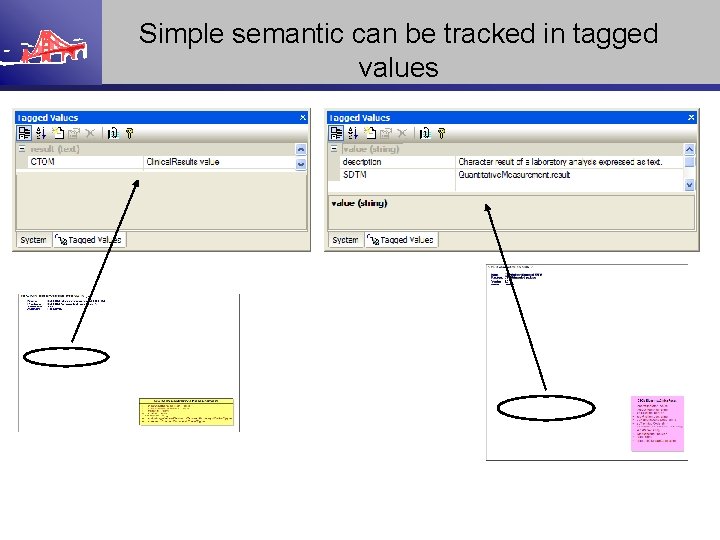
Simple semantic can be tracked in tagged values
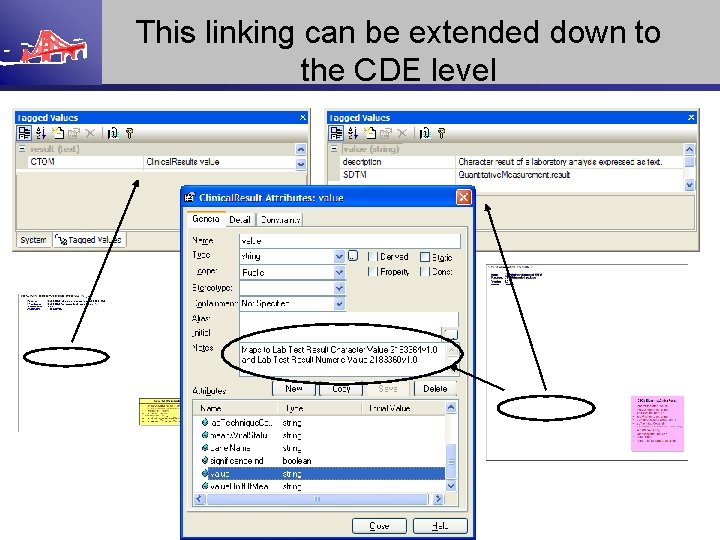
This linking can be extended down to the CDE level
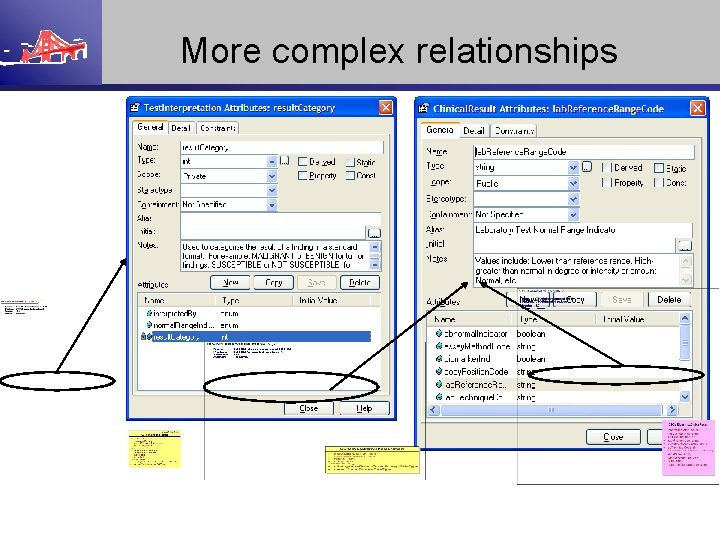
More complex relationships
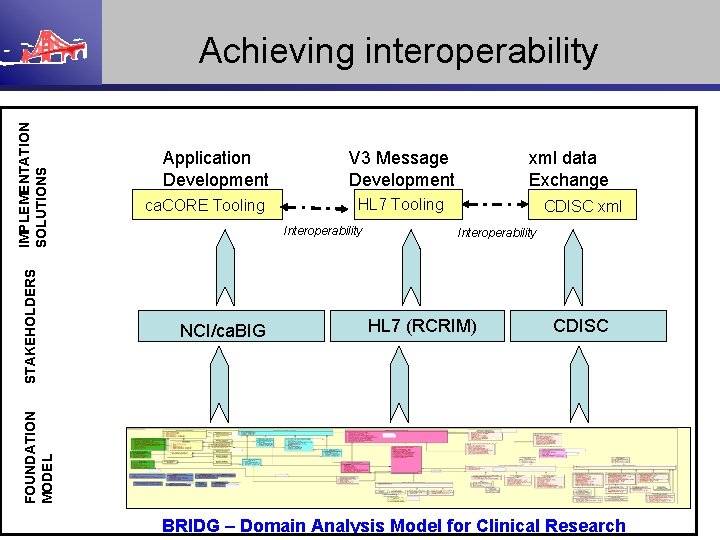
Application Development ca. CORE Tooling V 3 Message Development HL 7 Tooling Interoperability NCI/ca. BIG xml data Exchange CDISC xml Interoperability HL 7 (RCRIM) CDISC FOUNDATION MODEL STAKEHOLDERS IMPLEMENTATION SOLUTIONS Achieving interoperability BRIDG – Domain Analysis Model for Clinical Research
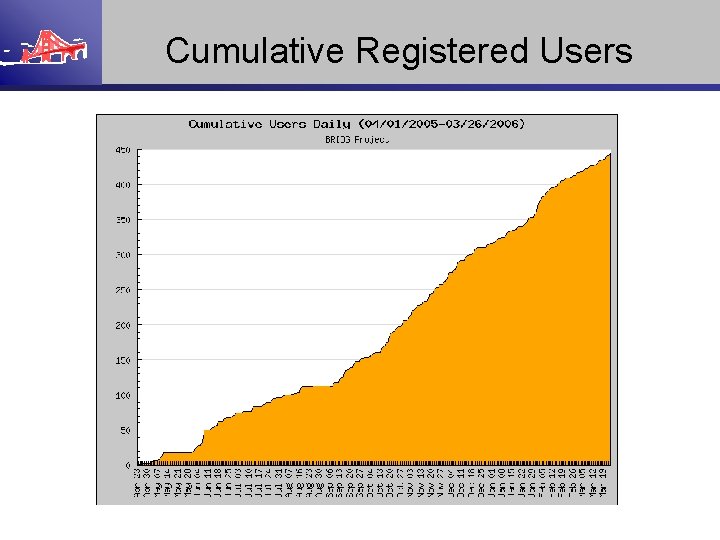
Cumulative Registered Users
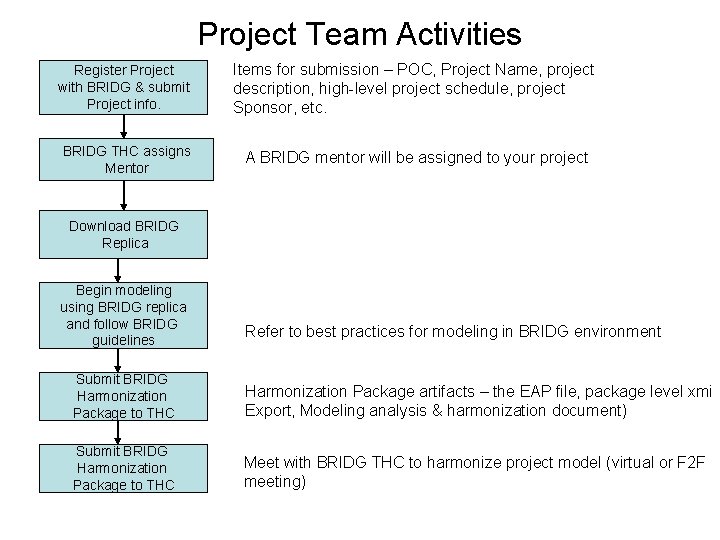
Project Team Activities Register Project with BRIDG & submit Project info. Items for submission – POC, Project Name, project description, high-level project schedule, project Sponsor, etc. BRIDG THC assigns Mentor A BRIDG mentor will be assigned to your project Download BRIDG Replica Begin modeling using BRIDG replica and follow BRIDG guidelines Refer to best practices for modeling in BRIDG environment Submit BRIDG Harmonization Package to THC Harmonization Package artifacts – the EAP file, package level xmi Export, Modeling analysis & harmonization document) Submit BRIDG Harmonization Package to THC Meet with BRIDG THC to harmonize project model (virtual or F 2 F meeting)
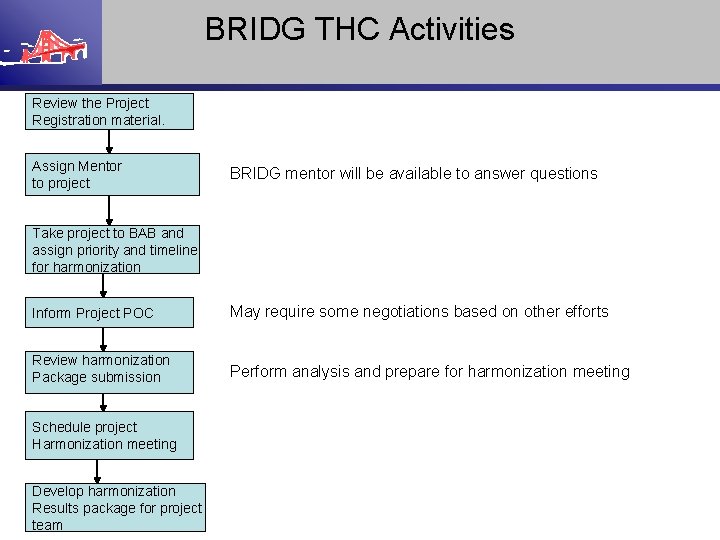
BRIDG THC Activities Review the Project Registration material. Assign Mentor to project BRIDG mentor will be available to answer questions Take project to BAB and assign priority and timeline for harmonization Inform Project POC May require some negotiations based on other efforts Review harmonization Package submission Perform analysis and prepare for harmonization meeting Schedule project Harmonization meeting Develop harmonization Results package for project team

Further Information • • • www. CDISC. org ncicb. nci. nih. gov ca. BIG. nci. nih. gov www. BRIDGproject. org fridsma@cbmi. pitt. edu jevans@cdisc. org
- Slides: 25