Intensification with INSTI REALITY REALITY Study raltegravirintensified quadruple

- Slides: 6

Intensification with INSTI § REALITY

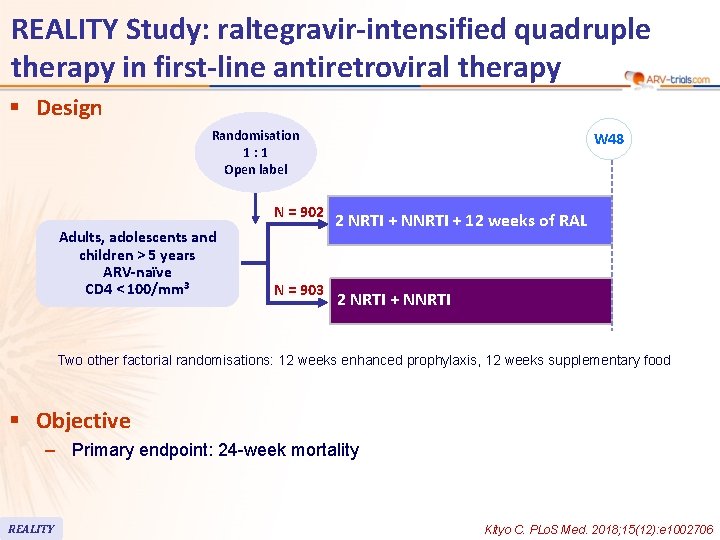
REALITY Study: raltegravir-intensified quadruple therapy in first-line antiretroviral therapy § Design Randomisation 1: 1 Open label N = 902 Adults, adolescents and children > 5 years ARV-naïve CD 4 < 100/mm 3 N = 903 W 48 2 NRTI + NNRTI + 12 weeks of RAL 2 NRTI + NNRTI Two other factorial randomisations: 12 weeks enhanced prophylaxis, 12 weeks supplementary food § Objective – Primary endpoint: 24 -week mortality REALITY Kityo C. PLo. S Med. 2018; 15(12): e 1002706

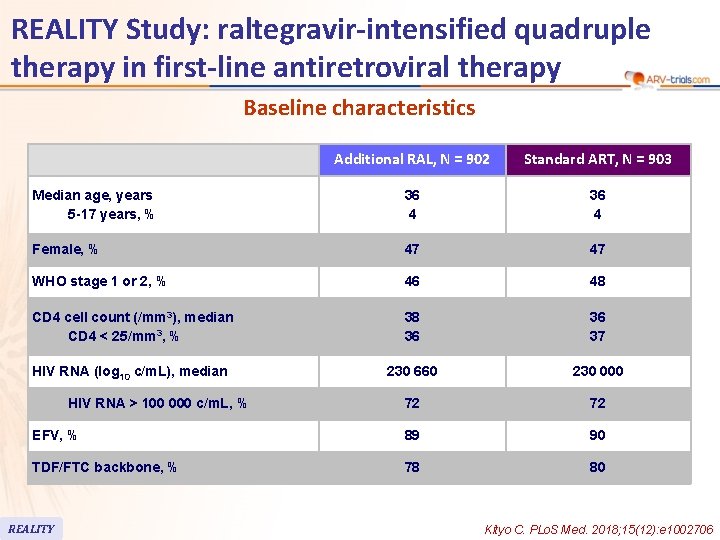
REALITY Study: raltegravir-intensified quadruple therapy in first-line antiretroviral therapy Baseline characteristics Additional RAL, N = 902 Standard ART, N = 903 Median age, years 5 -17 years, % 36 4 Female, % 47 47 WHO stage 1 or 2, % 46 48 CD 4 cell count (/mm 3), median CD 4 < 25/mm 3, % 38 36 36 37 HIV RNA (log 10 c/m. L), median 230 660 230 000 72 72 EFV, % 89 90 TDF/FTC backbone, % 78 80 HIV RNA > 100 000 c/m. L, % REALITY Kityo C. PLo. S Med. 2018; 15(12): e 1002706

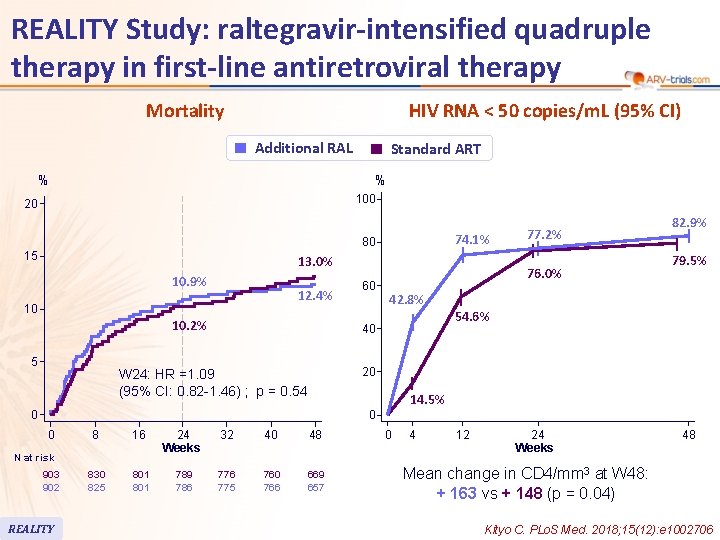
REALITY Study: raltegravir-intensified quadruple therapy in first-line antiretroviral therapy Mortality HIV RNA < 50 copies/m. L (95% CI) Additional RAL % Standard ART % 100 20 74. 1% 80 15 13. 0% 10. 9% 12. 4% 10 10. 2% 5 60 77. 2% 76. 0% 82. 9% 79. 5% 42. 8% 54. 6% 40 20 W 24: HR =1. 09 (95% CI: 0. 82 -1. 46) ; p = 0. 54 14. 5% 0 0 0 8 16 24 Weeks 32 40 48 830 825 801 789 786 775 760 766 669 657 N at risk 903 902 REALITY 0 4 12 24 Weeks 48 Mean change in CD 4/mm 3 at W 48: + 163 vs + 148 (p = 0. 04) Kityo C. PLo. S Med. 2018; 15(12): e 1002706

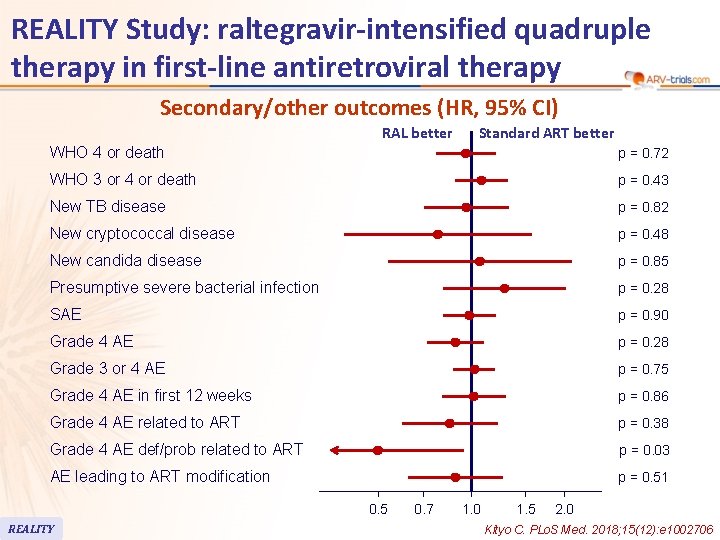
REALITY Study: raltegravir-intensified quadruple therapy in first-line antiretroviral therapy Secondary/other outcomes (HR, 95% CI) RAL better Standard ART better WHO 4 or death p = 0. 72 WHO 3 or 4 or death p = 0. 43 New TB disease p = 0. 82 New cryptococcal disease p = 0. 48 New candida disease p = 0. 85 Presumptive severe bacterial infection p = 0. 28 SAE p = 0. 90 Grade 4 AE p = 0. 28 Grade 3 or 4 AE p = 0. 75 Grade 4 AE in first 12 weeks p = 0. 86 Grade 4 AE related to ART p = 0. 38 Grade 4 AE def/prob related to ART p = 0. 03 AE leading to ART modification p = 0. 51 0. 5 REALITY 0. 7 1. 0 1. 5 2. 0 Kityo C. PLo. S Med. 2018; 15(12): e 1002706

REALITY Study: raltegravir-intensified quadruple therapy in first-line antiretroviral therapy § Conclusion – Standard triple ART (FTC/TDF + EFV) intensified with raltegravir for 12 weeks • Was well tolerated • Resulted in faster HIV RNA reduction through 24 weeks, and higher increase in CD 4 at 48 weeks • But did not reduce mortality or WHO 3/4 events through either week 24 or 48 REALITY Kityo C. PLo. S Med. 2018; 15(12): e 1002706











