Intellectual Property Rights Investment and Transfer of Technology










- Slides: 17

Intellectual Property Rights, Investment and Transfer of Technology in the Pharmaceutical Sector Patrizia Carlevaro Head of the International Aid Unit Syria, Damascus April 25 and 26, 2005

Innovation for Better Health ØThe discovery of new drugs is the only way to address the challenges of current and emerging diseases ØIt becomes more and more costly to discover and develop new drugs (average 1 Billion USD) and, for the inventor, to recoup its costs 2

Innovation for Better Health ØIP rights encourage discovery, recognize innovation as being essential for the industry development and bring improvement to public health ØMore than 95% of the WHO Essential Drugs ED List is off patent 3

Innovation for Better Health ØBefore the creation of the IPRs System, the knowledge was not widely disseminated and societies could not benefit from this disclosed information ØThe IPRs allow science to progress and people to benefit from new discoveries Ø 10, 000 products have to be tested to eventually come up to the discovery of one 4

Innovation for Better Health ØWithin the same therapeutical class a considerable competition exists in terms of quality, efficacy and price ØThanks to IPRs, the generic industry can have full access to data related to invention and discovery ØMost of the drugs used worldwide are developed and marketed by the private industry 5

Affordable Prices Ø Access to healthcare services is not exclusively a drug price issue Ø Although a large number of generic drugs are available at very competitive prices, they are not present in certain markets and when they are, they have not been able to change or improve the population’s health profile 6

7

A Good IP System Ø Adequate standards of protection Ø System for applying the standards Ø Limited exceptions Ø A brief and effective transition period The TRIPS agreement is the result of delicate and careful negotiations, humanity needs it in order to continue to progress 8

A Good Data Exclusivity System Ø Is a key starting point for creating a core competency in life sciences, pharmaceuticals, biotechnology Ø Aims to encourage development of all data (Phase I, III) necessary to establish that a drug is both safe and efficacious for human consumption, and to fully compensate the originator for its efforts Ø Provides incentives outside of traditional forms of IPR for companies to invest in research and clinical development of drugs, drug combinations and uses Ø Spurs the development and testing of new active ingredients – particularly when patents are not available 9

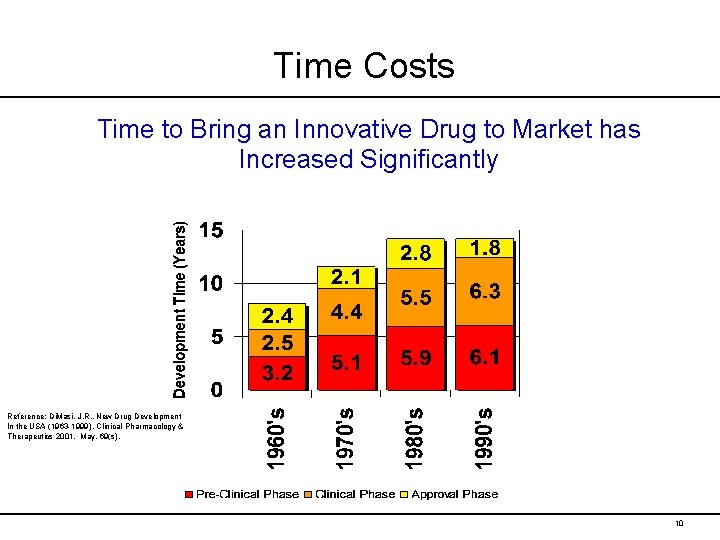
Time Costs Time to Bring an Innovative Drug to Market has Increased Significantly Reference: Di. Masi, J. R. , New Drug Development In the USA (1963 -1999), Clinical Pharmacology & Therapeutics 2001. May, 69(s). 10

Development Costs ØCompanies are challenged to reduce costs and increase productivity and efficiencies ØWithout IPR, a company is unlikely to invest in product launch , Physician education programs , Clinical research efforts , Public awareness efforts , Corporate Philanthropy 11

Potential Partnerships with the Pharmaceutical Industry Ø As much with the Public sector and as the Private sector Ø Important to better serve the local markets and create a long-term goal Ø Contribute with the Transfer of Technology and its competencies Ø Contribute to the economical and social development Ø Bring additional resources to populations most in need 12

Factors Pharmaceutical Companies Consider Prior to Investing ØCountry politically and economically stable ØBarriers to entry (e. g. currency restrictions, slow regulatory approvals) ØSize of local pharmaceutical market and growth’s potential ØIntellectual property protection and enforcement measures , Patents for pharmaceutical (compound protection) , Trademark and copyright laws , Data for registration protected from disclosure 13

Factors Pharmaceutical Companies Consider Prior to Investing ØWTO Member or in process of joining ØCurrent Good Manufacturing Practices (c. GMPs) available ØMultinational companies treated same as local companies ØPotential to export from country (free trade agreements) ØTransparent regulatory/pricing and tender processes ØTax - other incentives to invest 14

Investment Types / Cooperation Ø Develop research projects with local institutions both public & private Ø Build manufacturing facilities alone or with local partner Ø Use local manufacturers as 3 rd parties Ø Use local manufacturers to toll manufacturing products (package) y Multi-national or local company market or both (co-marketing) Ø Sell bulk products to local manufacturers allow them to market y With or without manufacturing know how Ø Sell or license trademarks, patents or other rights to local companies 15

Some Changes Required in Syrian Law for TRIPS Compliance ØProvide protection for Pharmaceutical compounds (product protection) ØPatent term of 20 years from filing date ØData package exclusivity – period during which another company cannot rely on innovator’s safety and efficacy data 16

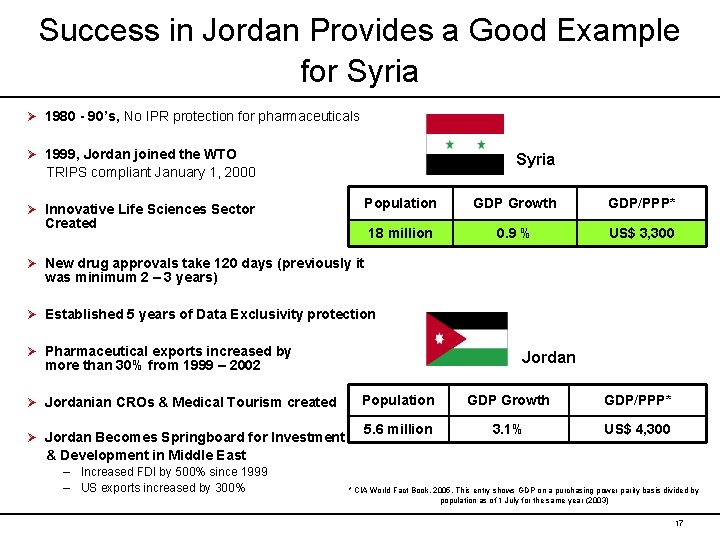
Success in Jordan Provides a Good Example for Syria Ø 1980 - 90’s, No IPR protection for pharmaceuticals Ø 1999, Jordan joined the WTO Syria TRIPS compliant January 1, 2000 Ø Innovative Life Sciences Sector Created Population GDP Growth GDP/PPP* 18 million 0. 9 % US$ 3, 300 Ø New drug approvals take 120 days (previously it was minimum 2 – 3 years) Ø Established 5 years of Data Exclusivity protection Ø Pharmaceutical exports increased by Jordan more than 30% from 1999 – 2002 Ø Jordanian CROs & Medical Tourism created Ø Jordan Becomes Springboard for Investment Population GDP Growth GDP/PPP* 5. 6 million 3. 1% US$ 4, 300 & Development in Middle East – Increased FDI by 500% since 1999 – US exports increased by 300% * CIA World Fact Book, 2005. This entry shows GDP on a purchasing power parity basis divided by population as of 1 July for the same year (2003) 17
 Trade related aspects of intellectual property rights
Trade related aspects of intellectual property rights Intellectual property in professional practices
Intellectual property in professional practices Intellectual property rights
Intellectual property rights Intellectual property rights
Intellectual property rights Fixed investment and inventory investment
Fixed investment and inventory investment Importance of intellectual property
Importance of intellectual property Intellectual property management definition
Intellectual property management definition Licensing advantages
Licensing advantages Intellectual property business plan
Intellectual property business plan Property
Property Right to intellectual property of teachers
Right to intellectual property of teachers Industrial property definition
Industrial property definition Theories of intellectual property william fisher
Theories of intellectual property william fisher Concept of intellectual property
Concept of intellectual property Valuing intangible assets
Valuing intangible assets Characteristics of intellectual property
Characteristics of intellectual property Discuss intellectual property frankly
Discuss intellectual property frankly Sfas 142
Sfas 142






























