Intellectual Property Rights and Pharmaceuticals Case study Novartiss





- Slides: 8

Intellectual Property Rights and Pharmaceuticals (Case study- Novartis’s claim in India) Background note prepared for PHM Vic Internet Workshop

India – Pharmaceutical Industry • Huge drug manufacturing capacity (2 nd after US in having USFDA approved manufacturing companies) – Prominent generic sector (Mueller, 2007) • Production as well as export • Generic drugs manufactured in India constitute 25% of total drug purchase of MSF and 80% of total anti-HIV drugs used to treat 80, 000 HIV patients under MSF’s AIDS projects in more than 30 countries (MSF, 2007)

Patent Legislation, WTO and India 1972: The Patent Act 1970 came in to force (Only Process Patents) (CGPDT, 2007) 1994/1995: Creation of the World Trade Organization & entry into force of the TRIPS Agreement, which obliges developing countries to grant patents on medicines no later than 2005. (WTO-TRIPS) Patents (Amendments) Act, 1999 ( EMR provision) Patents (Amendments) Act, 2002 April 2005: Amendment of India's Patents Act: medicines can now be patented in India. However, the law stipulates that only true medical innovations will be protected by patents. Section 3(d) specifies that new forms of known substances do not deserve patents. Patents (Amendments) Rules 2005 (opposition+fees etc) Patents (Amendments) Rules 2006 (Dates+fees etc) (CGPDT, 2007)

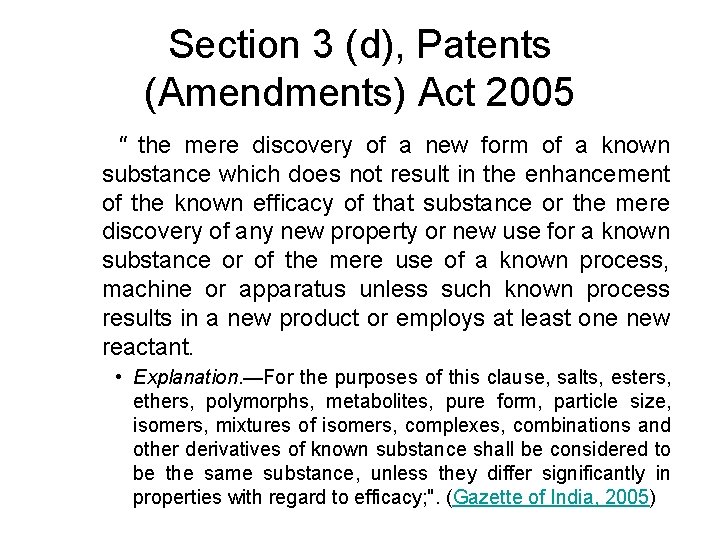
Section 3 (d), Patents (Amendments) Act 2005 " the mere discovery of a new form of a known substance which does not result in the enhancement of the known efficacy of that substance or the mere discovery of any new property or new use for a known substance or of the mere use of a known process, machine or apparatus unless such known process results in a new product or employs at least one new reactant. • Explanation. —For the purposes of this clause, salts, esters, ethers, polymorphs, metabolites, pure form, particle size, isomers, mixtures of isomers, complexes, combinations and other derivatives of known substance shall be considered to be the same substance, unless they differ significantly in properties with regard to efficacy; ". (Gazette of India, 2005)

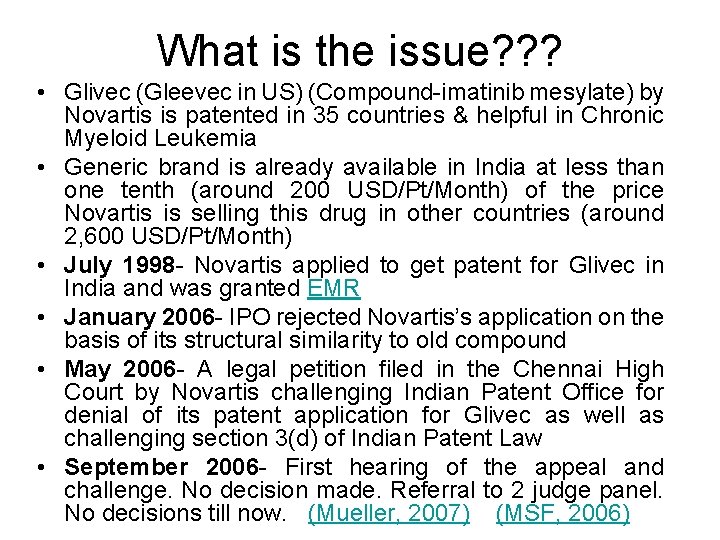
What is the issue? ? ? • Glivec (Gleevec in US) (Compound-imatinib mesylate) by Novartis is patented in 35 countries & helpful in Chronic Myeloid Leukemia • Generic brand is already available in India at less than one tenth (around 200 USD/Pt/Month) of the price Novartis is selling this drug in other countries (around 2, 600 USD/Pt/Month) • July 1998 - Novartis applied to get patent for Glivec in India and was granted EMR • January 2006 - IPO rejected Novartis’s application on the basis of its structural similarity to old compound • May 2006 - A legal petition filed in the Chennai High Court by Novartis challenging Indian Patent Office for denial of its patent application for Glivec as well as challenging section 3(d) of Indian Patent Law • September 2006 - First hearing of the appeal and challenge. No decision made. Referral to 2 judge panel. No decisions till now. (Mueller, 2007) (MSF, 2006)

What Novartis says. . • Strong IPRs + Economic incentives = Innovation • Gilvec International Patient Assistance Program (GIPAP) - Free drugs to more than 17000 patients in 83 countries, 99% of Indian patients who are getting Glivec are getting it free through Novartis’s Patient Assistance Program • Access to medicines clearly favors people in affluent societies- limited access to medicines and limited research to address disease burden in Developing world should be priority- PPPs, Govt funded health system, Strong IPRS+ Other economic incentives for R & D) • 3 PPP with WHO (Leprosy, Malaria and Tuberculosis) • ITD (Institute for Tropical Diseases) Singapore- (Dengue, Drug resistant TB, Malaria) (WHO, 2006)

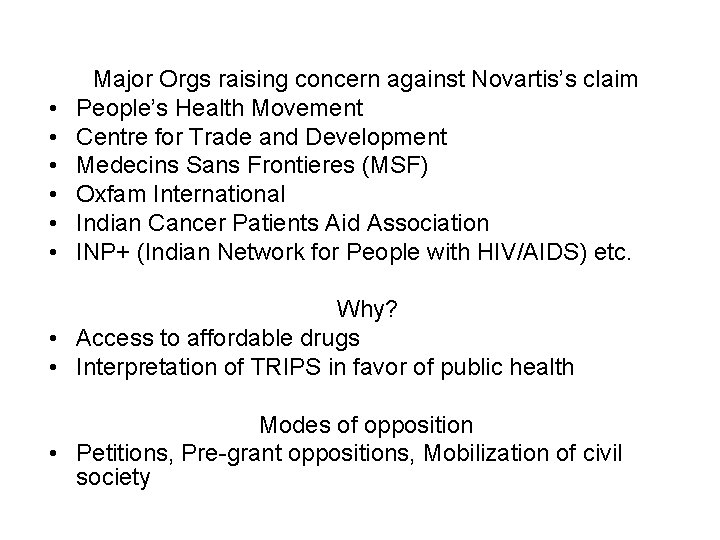
• • • Major Orgs raising concern against Novartis’s claim People’s Health Movement Centre for Trade and Development Medecins Sans Frontieres (MSF) Oxfam International Indian Cancer Patients Aid Association INP+ (Indian Network for People with HIV/AIDS) etc. Why? • Access to affordable drugs • Interpretation of TRIPS in favor of public health Modes of opposition • Petitions, Pre-grant oppositions, Mobilization of civil society

Future issues… • Judicial Interpretation of Indian Patent Act (esp. section 3 (d)) • US influence… (MOU on Bilateral Cooperation on IP) (Bilateral FTA) • Priority to public health within IPRs Framework • Use of other public health measures (US-India IP)
 Trade-related aspects of intellectual property rights
Trade-related aspects of intellectual property rights Intellectual property rights in professional practices
Intellectual property rights in professional practices Intellectual property rights
Intellectual property rights Intellectual property rights
Intellectual property rights Importance of intellectual property
Importance of intellectual property Intellectual property management definition
Intellectual property management definition Licensing advantages
Licensing advantages Intellectual property business plan example
Intellectual property business plan example Property
Property















