Integration of Metabolism Review of Roles of Systems
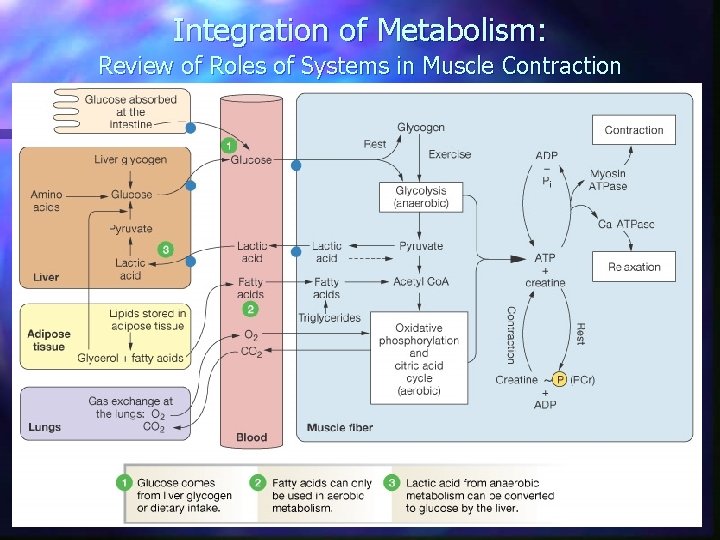
Integration of Metabolism: Review of Roles of Systems in Muscle Contraction
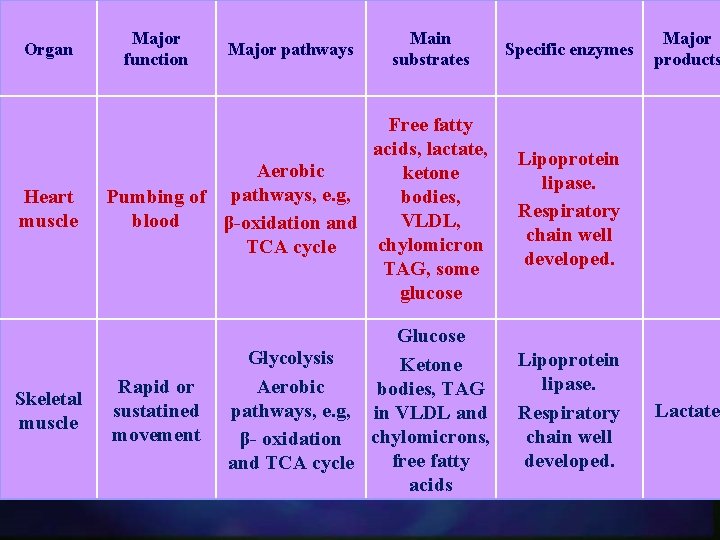
Organ Major function Major pathways Main substrates Specific enzymes Heart muscle Free fatty acids, lactate, Aerobic ketone Pumbing of pathways, e. g, bodies, blood VLDL, β-oxidation and chylomicron TCA cycle TAG, some glucose Lipoprotein lipase. Respiratory chain well developed. Skeletal muscle Glucose Glycolysis Ketone Aerobic bodies, TAG pathways, e. g, in VLDL and β- oxidation chylomicrons, free fatty and TCA cycle acids Lipoprotein lipase. Respiratory chain well developed. Rapid or sustatined movement Major products Lactate

CARDIAC AND SKELETAL MUSCLE Cardiac muscle is continuously active (always contracting) n Cardiac muscle has completely aerobic metabolism n Cardiac muscle contains negligible energy stores, e. g. glycogen and lipid) n

CARBOHYDRATE METABOLISM Glucose transport into muscle is increased after a carbohydrate-rich meal providing that insulin is secreted properly. Glucose is then readily phosphorylated to G-6 phosphate by hexokinase and subsequently is metabolized to provide energy needs. n During fasting (starvation) ketone bodies and fatty acids become the major fuels. n

n Increased glycogen synthesis is more significant for the skeletal muscle rather than for the cardiac muscle.

FAT METABOLISM n Ketone bodies and fatty acids released from chylomicrons and VLDL by lipoprotein lipase (in the well-fed state) become the major sources for fuels only when glucose levels and/or insulin release is/are inadequate.
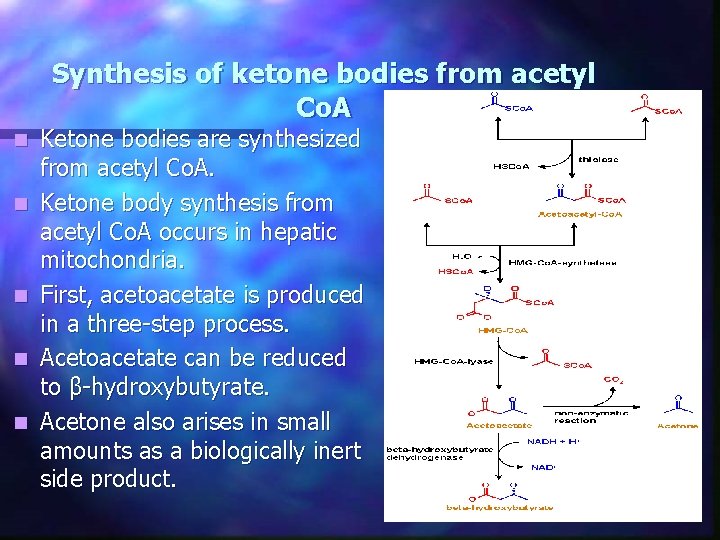
Synthesis of ketone bodies from acetyl Co. A n n n Ketone bodies are synthesized from acetyl Co. A. Ketone body synthesis from acetyl Co. A occurs in hepatic mitochondria. First, acetoacetate is produced in a three-step process. Acetoacetate can be reduced to β-hydroxybutyrate. Acetone also arises in small amounts as a biologically inert side product.
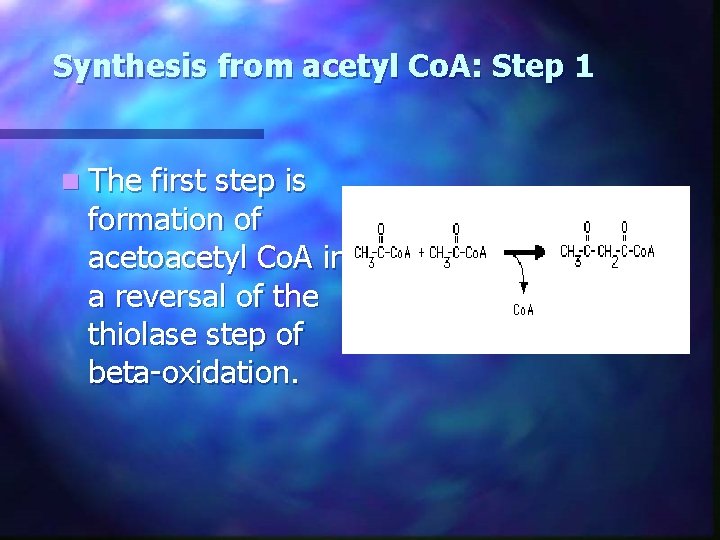
Synthesis from acetyl Co. A: Step 1 n The first step is formation of acetoacetyl Co. A in a reversal of the thiolase step of beta-oxidation.
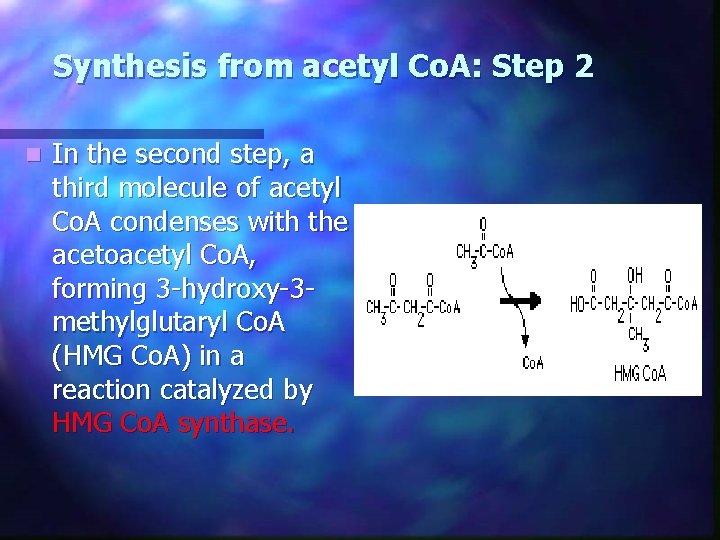
Synthesis from acetyl Co. A: Step 2 n In the second step, a third molecule of acetyl Co. A condenses with the acetoacetyl Co. A, forming 3 -hydroxy-3 methylglutaryl Co. A (HMG Co. A) in a reaction catalyzed by HMG Co. A synthase.
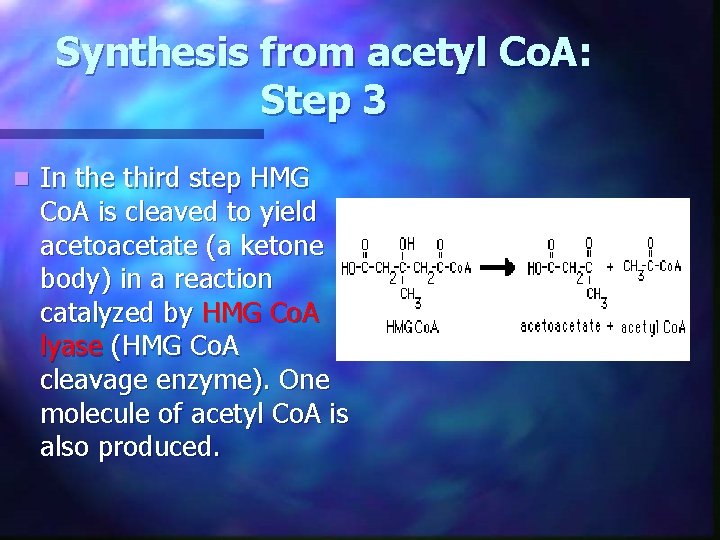
Synthesis from acetyl Co. A: Step 3 n In the third step HMG Co. A is cleaved to yield acetoacetate (a ketone body) in a reaction catalyzed by HMG Co. A lyase (HMG Co. A cleavage enzyme). One molecule of acetyl Co. A is also produced.

Synthesis from acetyl Co. A: Acetoacetate Subsequently acetoacetate can be reduced to beta-hydroxybutyrate by beta-hydroxybutyrate dehydrogenase in a NADH-requiring reaction. The extent of this reaction depends on the state of the NAD pool of the cell; when it is highly reduced, most or all of the ketones can be in the form of betahydroxybutyrate. n Near-total reduction of acetoacetate to beta-hydroxybutyrate can occur in severe ethanol toxicity. n

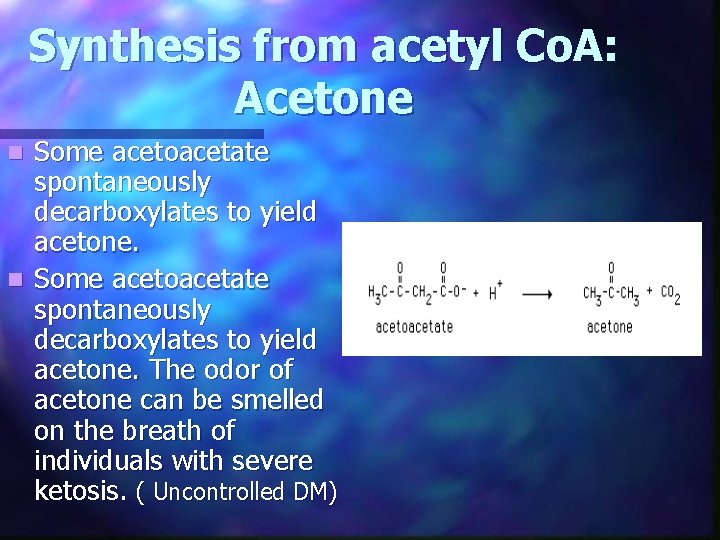
Synthesis from acetyl Co. A: Acetone Some acetoacetate spontaneously decarboxylates to yield acetone. n Some acetoacetate spontaneously decarboxylates to yield acetone. The odor of acetone can be smelled on the breath of individuals with severe ketosis. ( Uncontrolled DM) n
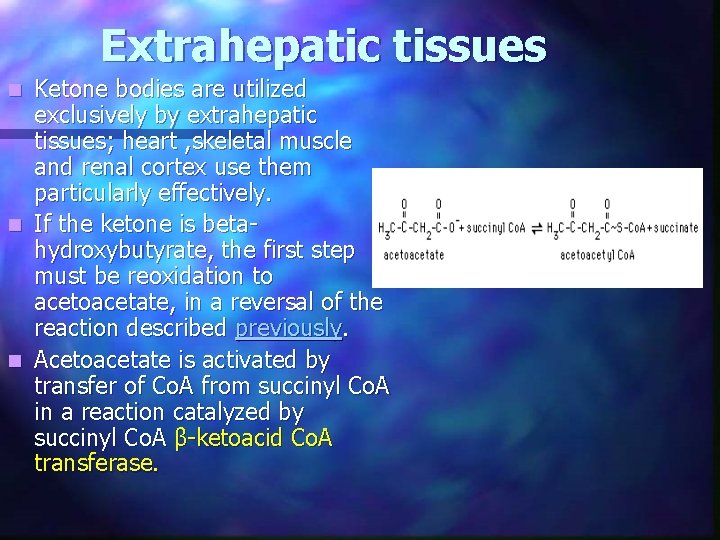
Extrahepatic tissues Ketone bodies are utilized exclusively by extrahepatic tissues; heart , skeletal muscle and renal cortex use them particularly effectively. n If the ketone is betahydroxybutyrate, the first step must be reoxidation to acetoacetate, in a reversal of the reaction described previously. n Acetoacetate is activated by transfer of Co. A from succinyl Co. A in a reaction catalyzed by succinyl Co. A β-ketoacid Co. A transferase. n

The enzyme catalyzing this reaction is absent from liver; hence liver, which synthesizes ketone bodies, cannot use them. This places liver in the role of being a net producer of ketones. n The resulting acetoacetyl Co. A can be cleaved by thiolase to form two molecules of acetyl Co. A, which can then be oxidized by the tricarboxylic acid cycle. n
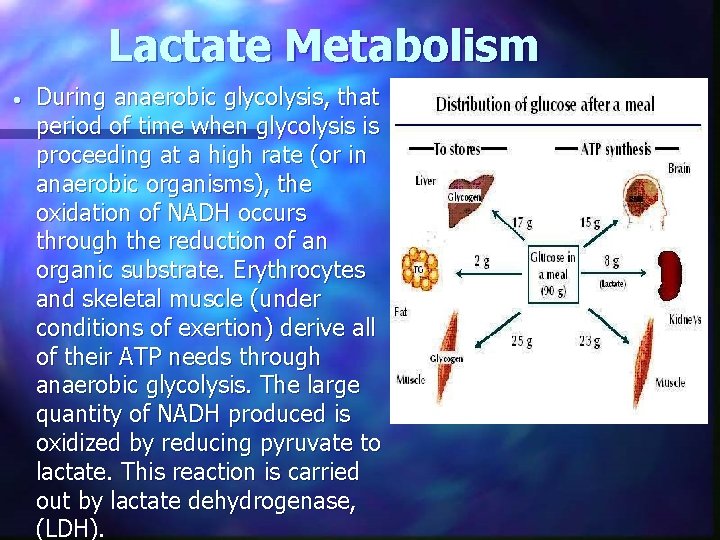
Lactate Metabolism • During anaerobic glycolysis, that period of time when glycolysis is proceeding at a high rate (or in anaerobic organisms), the oxidation of NADH occurs through the reduction of an organic substrate. Erythrocytes and skeletal muscle (under conditions of exertion) derive all of their ATP needs through anaerobic glycolysis. The large quantity of NADH produced is oxidized by reducing pyruvate to lactate. This reaction is carried out by lactate dehydrogenase, (LDH).
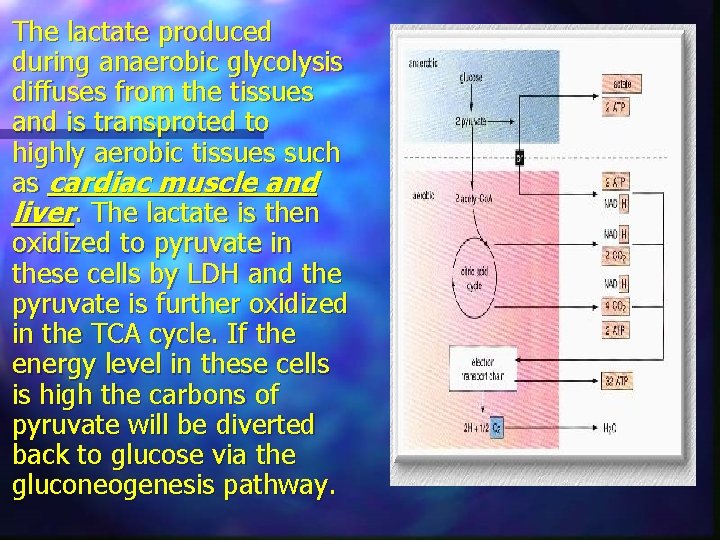
The lactate produced during anaerobic glycolysis diffuses from the tissues and is transproted to highly aerobic tissues such as cardiac muscle and liver. The lactate is then oxidized to pyruvate in these cells by LDH and the pyruvate is further oxidized in the TCA cycle. If the energy level in these cells is high the carbons of pyruvate will be diverted back to glucose via the gluconeogenesis pathway.
• Mammalian cells contain two distinct types of LDH subunits, termed M and H. Combinations of these different subunits generates LDH isoenzymes with different characteristics. The H type subunit predominates in aerobic tissues such as heart muscle (as the H 4 tetramer) while the M subunit predominates in anaerobic tissues such as skeletal muscle as the M 4 tetramer).

n H 4 LDH has a low KM for pyruvate and also is inhibited by high levels of pyruvate. The M 4 LDH enzyme has a high KM for pyruvate and is not inhibited by pyruvate. This suggests that the H-type LDH is utilized for oxidizing lactate to pyruvate and the M-type the reverse.

n Every LDH molecule consists of four subunits, where each subunit is either H or M (based on their electrophoretic properties. ) There are, therefore, five LDH isotypes: n LDH-1 (4 H) - in the heart n LDH-2 (3 H 1 M) - in the reticuloendothelial system n LDH-3 (2 H 2 M) - in the lungs n LDH-4 (1 H 3 M) - in the kidneys n LDH-5 (4 M) - in the liver and striated muscle

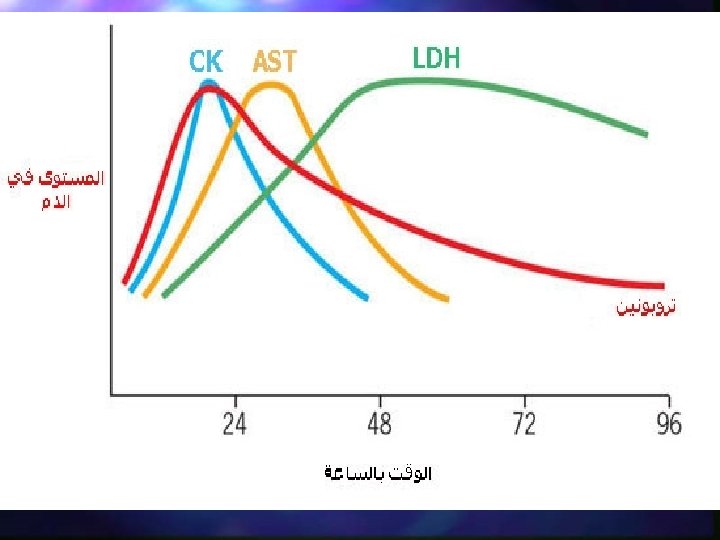
n Usually LDH-2 is the predominant form in the serum. An LDH-1 level higher than the LDH-2 level (a "flipped pattern"), suggests myocardial infarction (damage to heart tissues releases heart LDH, which is rich in LDH-1, into the bloodstream). The use of this pheonomenon to diagnose infarction has been largely superseded by the use of Troponin I or T measurement.

AMINO ACID METABOLISM Following protein-rich meals, amino acid uptake and protein synthesis occur to replace protein degraded during the time before meals. n Branched-chain amino acids (leu, ile and val) escape metabolism in the liver to provide for protein synthesis and for fuels as well. n

Energy for Skeletal Muscle Contraction n ATP & ADP n Phosphocreatine n Aerobic pathways n Anaerobic pathways n (glycolytic metabolism) In resting muscle, fatty acids are the major fuel, meeting 85% of the energy needs.
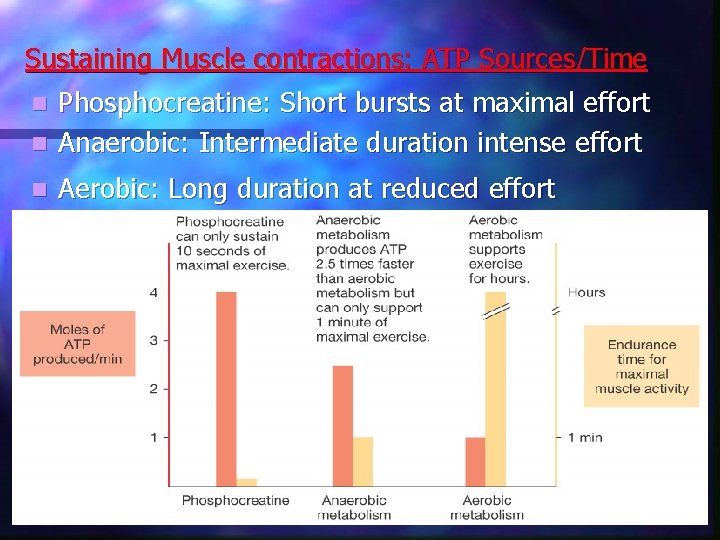
Sustaining Muscle contractions: ATP Sources/Time Phosphocreatine: Short bursts at maximal effort n Anaerobic: Intermediate duration intense effort n n Aerobic: Long duration at reduced effort

Hormonal regulation of Energy Source for ATP Production n Metabolic Shifts Glucagon n Cortisol n Epinephrine/NE n GH n n (insulin)
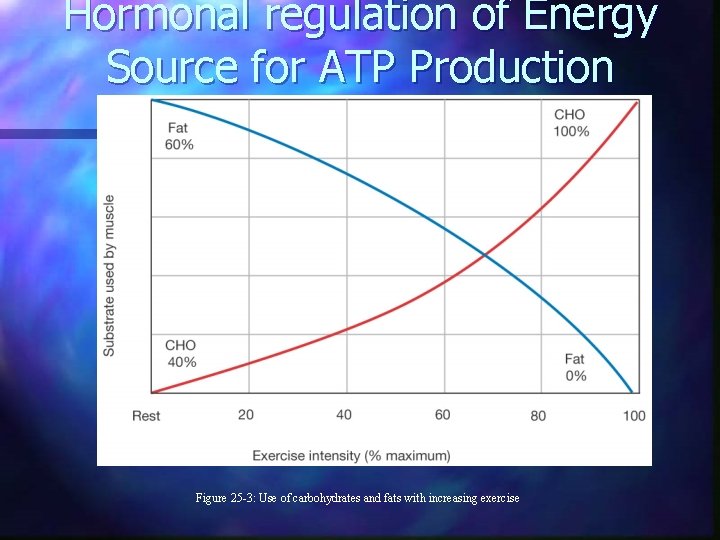
Hormonal regulation of Energy Source for ATP Production Figure 25 -3: Use of carbohydrates and fats with increasing exercise
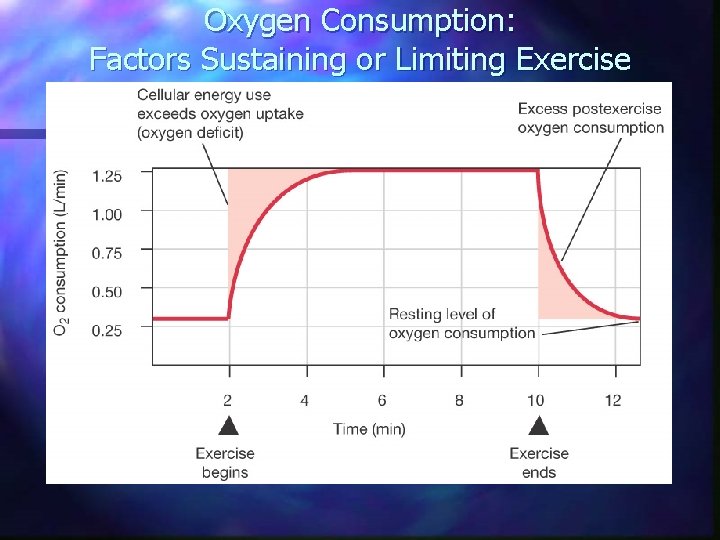
Oxygen Consumption: Factors Sustaining or Limiting Exercise
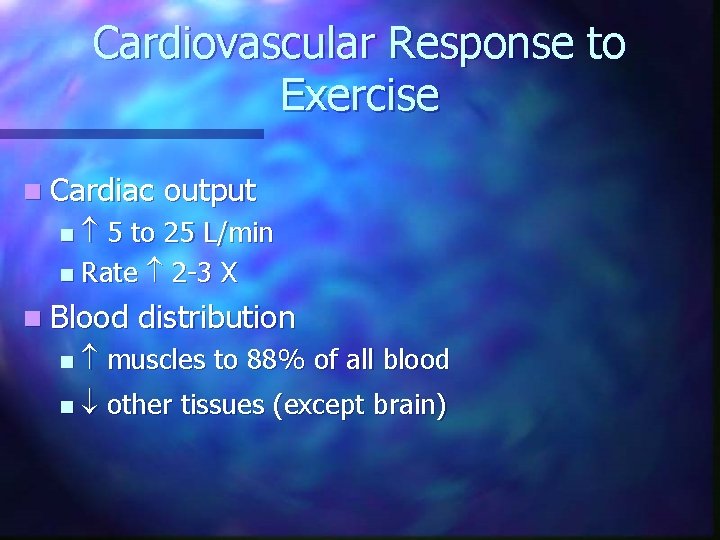
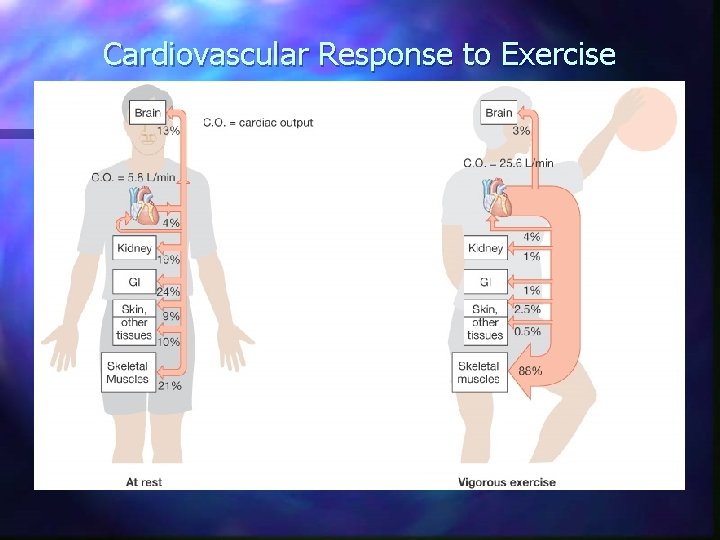
Cardiovascular Response to Exercise n Cardiac output 5 to 25 L/min n Rate 2 -3 X n n Blood distribution n muscles to 88% of all blood n other tissues (except brain)
Cardiovascular Response to Exercise

END
- Slides: 31