Integration of Good Engineering Practice GEP into the



- Slides: 13

Integration of Good Engineering Practice (GEP) into the Pharmaceutical Quality System

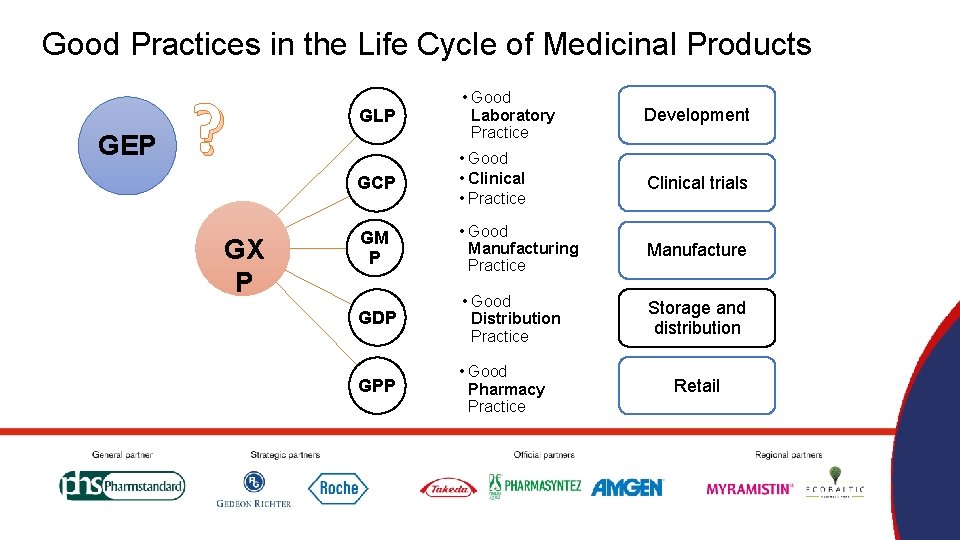
Good Practices in the Life Cycle of Medicinal Products GEP ? GX P GLP • Good Laboratory Practice Development GCP • Good • Clinical • Practice Clinical trials GM P • Good Manufacturing Practice Manufacture GDP • Good Distribution Practice Storage and distribution GPP • Good Pharmacy Practice Retail

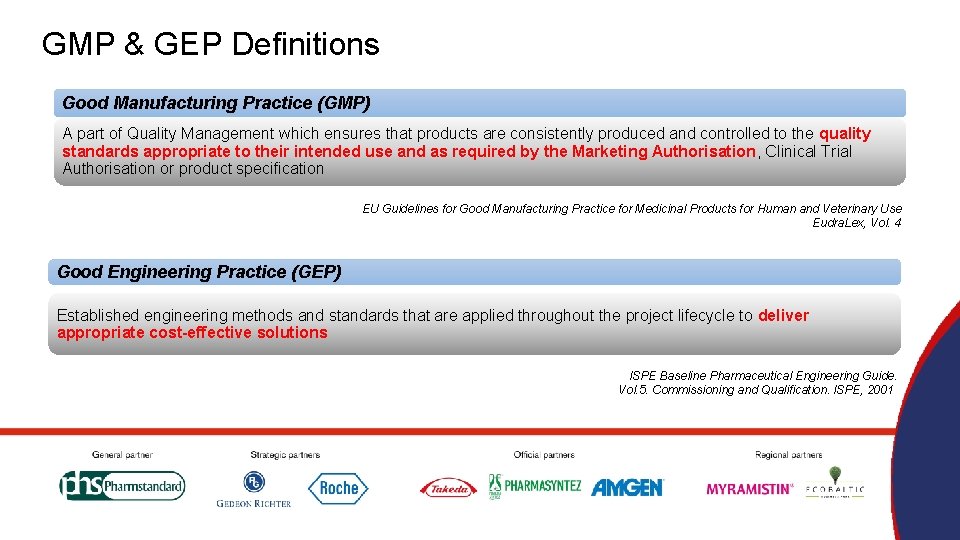
GMP & GEP Definitions Good Manufacturing Practice (GMP) A part of Quality Management which ensures that products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the Marketing Authorisation, Clinical Trial Authorisation or product specification EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use Eudra. Lex, Vol. 4 Good Engineering Practice (GEP) Established engineering methods and standards that are applied throughout the project lifecycle to deliver appropriate cost-effective solutions ISPE Baseline Pharmaceutical Engineering Guide. Vol. 5. Commissioning and Qualification. ISPE, 2001

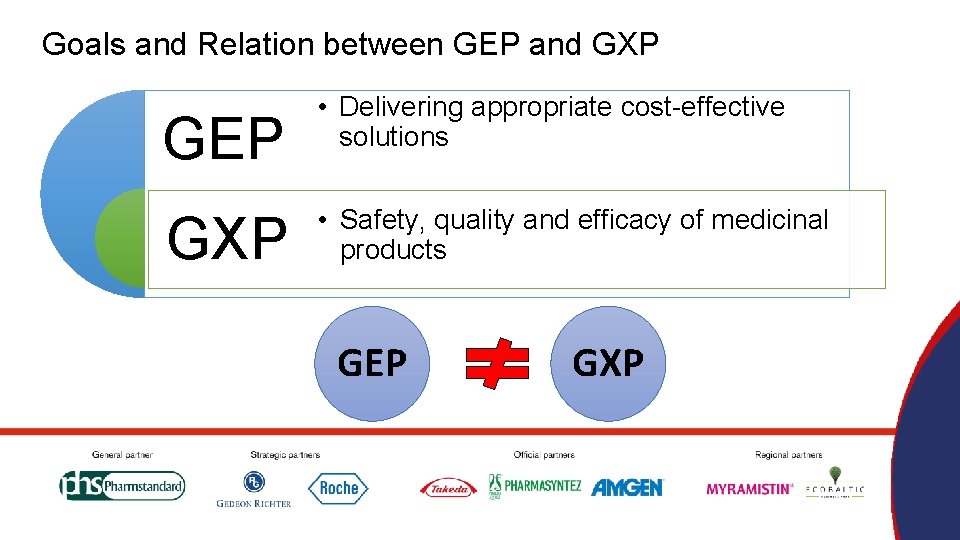
Goals and Relation between GEP and GXP GEP GXP • Delivering appropriate cost-effective solutions • Safety, quality and efficacy of medicinal products GEP GXP

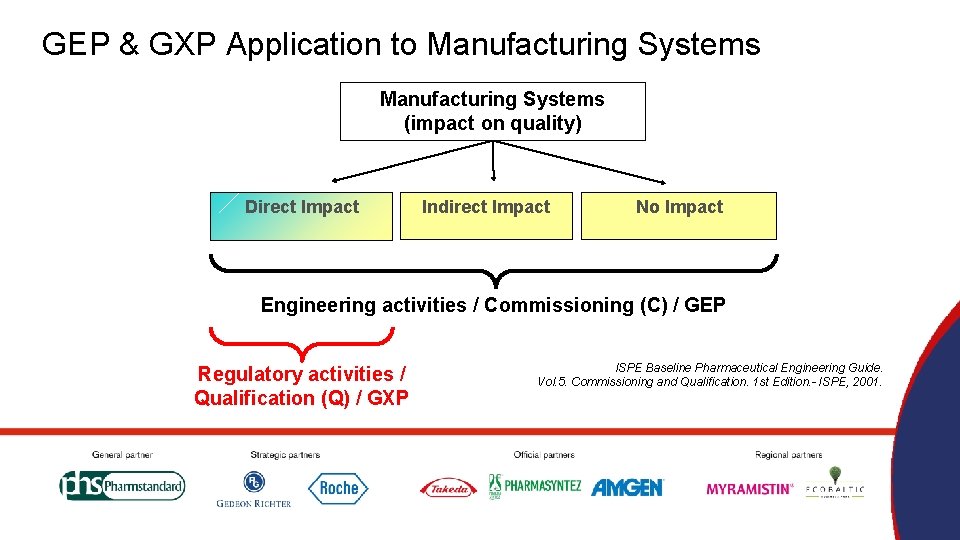
GEP & GXP Application to Manufacturing Systems (impact on quality) Direct Impact Indirect Impact No Impact Engineering activities / Commissioning (С) / GEP Regulatory activities / Qualification (Q) / GXP ISPE Baseline Pharmaceutical Engineering Guide. Vol. 5. Commissioning and Qualification. 1 st Edition. - ISPE, 2001.

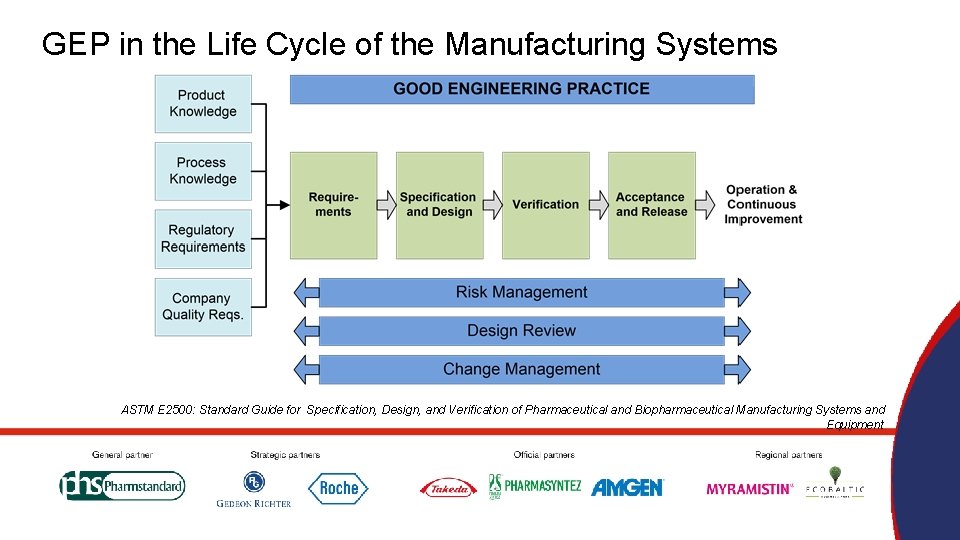
GEP in the Life Cycle of the Manufacturing Systems ASTM E 2500: Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment

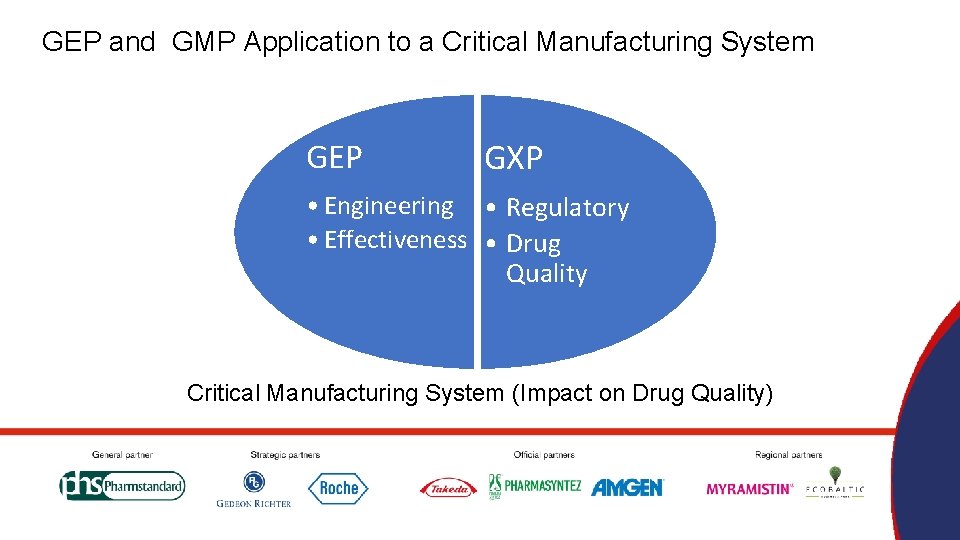
GEP and GMP Application to a Critical Manufacturing System GEP GXP • Engineering • Regulatory • Effectiveness • Drug Quality Critical Manufacturing System (Impact on Drug Quality)

Key Elements for GEP Confirmation • • • GEP Guide Description of the requirements to facilities in URS Application of Project Management tools Written Procedures for execution of engineering activities Records for executed engineering activities Maintenance Programs and Plans, based on the assessment of all applicable engineering aspects of the facilities (Design, Criticality, Operation, etc. ) Monitoring of Effective Use of the manufacturing systems, maintenance and other engineering activities including trend analysis Cost Management for engineering activities Management throughout the life cycle phases of the manufacturing systems Internal Audits of engineering management Suppliers Managements for equipment, spare parts and services

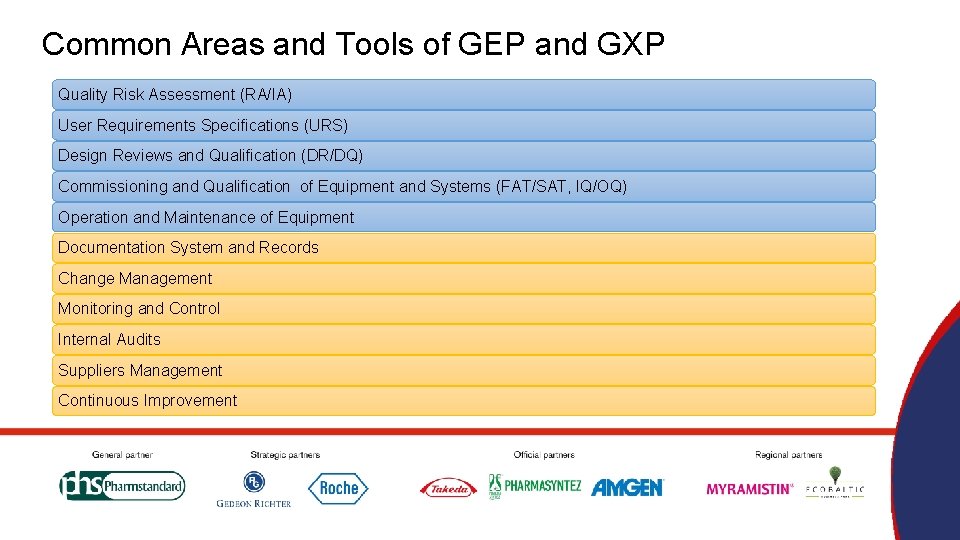
Common Areas and Tools of GEP and GXP Quality Risk Assessment (RA/IA) User Requirements Specifications (URS) Design Reviews and Qualification (DR/DQ) Commissioning and Qualification of Equipment and Systems (FAT/SAT, IQ/OQ) Operation and Maintenance of Equipment Documentation System and Records Change Management Monitoring and Control Internal Audits Suppliers Management Continuous Improvement

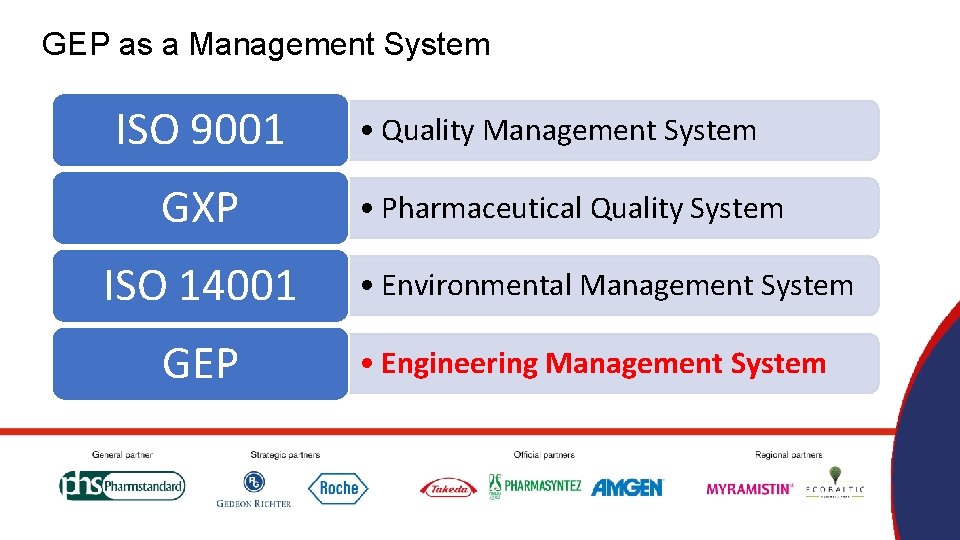
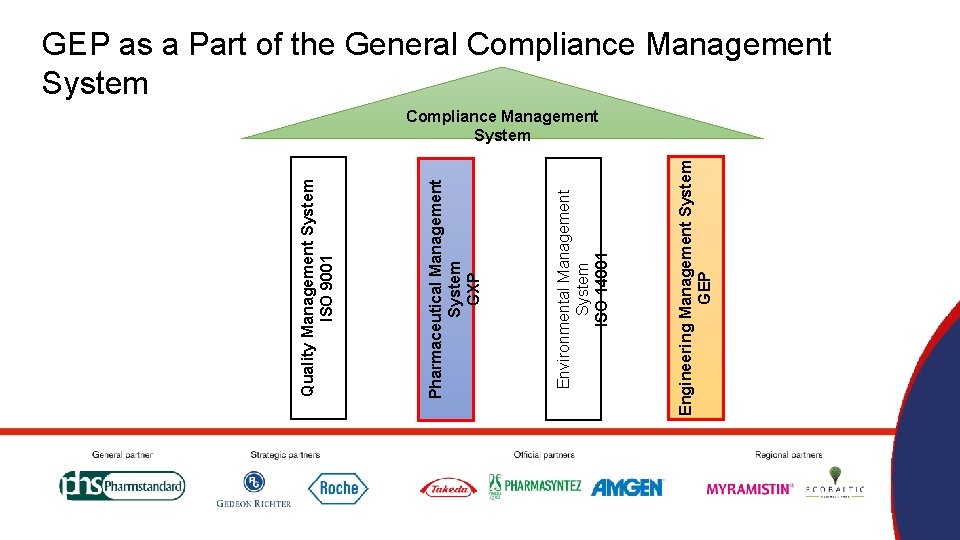
GEP as a Management System ISO 9001 GXP ISO 14001 GEP • Quality Management System • Pharmaceutical Quality System • Environmental Management System • Engineering Management System

Engineering Management System GEP Environmental Management System ISO 14001 Pharmaceutical Management System GXP Quality Management System ISO 9001 GEP as a Part of the General Compliance Management System

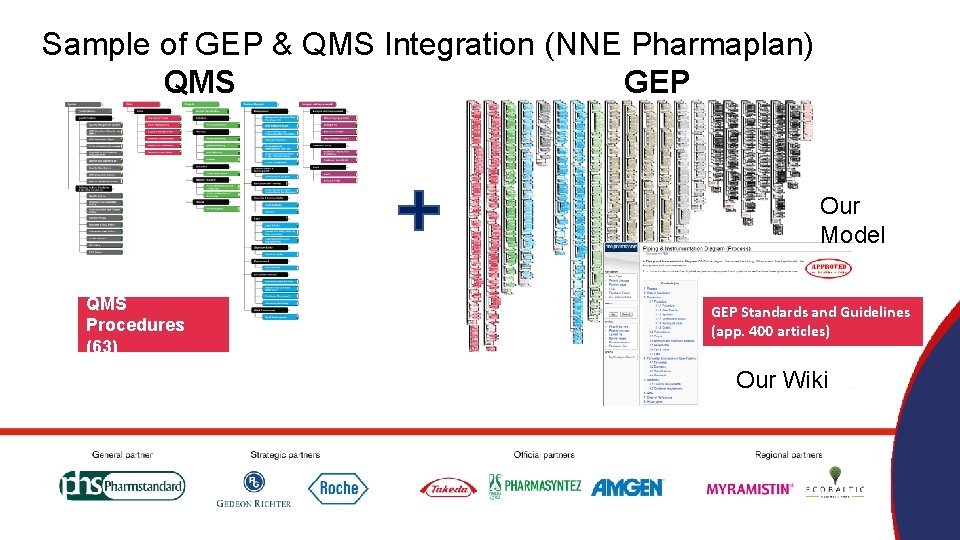
Sample of GEP & QMS Integration (NNE Pharmaplan) QMS GEP Our Model QMS Procedures (63) GEP Standards and Guidelines (app. 400 articles) Our Wiki

Benefits of GEP Implementation • «Smooth» Project Execution • Cost Optimization for Engineering Activities and Facilities • Achievement and Maintenance of appropriate Project Quality • Proper Operation and Maintenance of Manufacturing Systems • Support of Regulatory Activities (GXP) • «Synergetic» Use of available Resources in Management