Integrated Rate Laws How to solve Integrated Rate

![First Order form Rate = D[A] / Dt = k[A] �ln[A] = - kt First Order form Rate = D[A] / Dt = k[A] �ln[A] = - kt](https://slidetodoc.com/presentation_image_h/83ddd8ffaadd2969b5ffe2af135c387e/image-4.jpg)


![Second Order �Rate = -D[A]/Dt = k[A]2 �integrated rate law � 1/[A] = kt Second Order �Rate = -D[A]/Dt = k[A]2 �integrated rate law � 1/[A] = kt](https://slidetodoc.com/presentation_image_h/83ddd8ffaadd2969b5ffe2af135c387e/image-8.jpg)
![Second Order Half Life �[A] = [A]0 /2 at t = t 1/2 Second Order Half Life �[A] = [A]0 /2 at t = t 1/2](https://slidetodoc.com/presentation_image_h/83ddd8ffaadd2969b5ffe2af135c387e/image-9.jpg)
![Zero Order Rate Law �Rate = k[A]0 = k �Rate does not change with Zero Order Rate Law �Rate = k[A]0 = k �Rate does not change with](https://slidetodoc.com/presentation_image_h/83ddd8ffaadd2969b5ffe2af135c387e/image-10.jpg)

![Rate = k[Br. O 3 -][Br-][H+]2 �We set up the experiment so that two Rate = k[Br. O 3 -][Br-][H+]2 �We set up the experiment so that two](https://slidetodoc.com/presentation_image_h/83ddd8ffaadd2969b5ffe2af135c387e/image-13.jpg)
![Rate = k[Br. O 3 -][Br-][H+]2 �This rate law can be rewritten �Rate = Rate = k[Br. O 3 -][Br-][H+]2 �This rate law can be rewritten �Rate =](https://slidetodoc.com/presentation_image_h/83ddd8ffaadd2969b5ffe2af135c387e/image-14.jpg)
- Slides: 14

Integrated Rate Laws How to solve

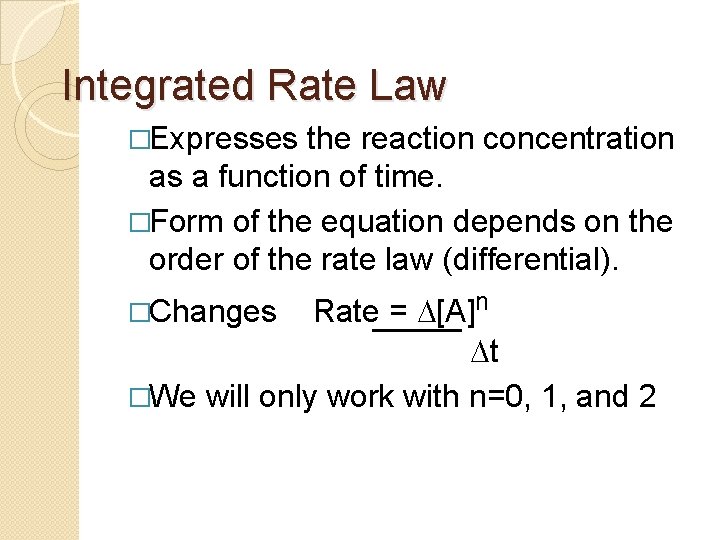
Integrated Rate Law �Expresses the reaction concentration as a function of time. �Form of the equation depends on the order of the rate law (differential). Rate = D[A]n Dt �We will only work with n=0, 1, and 2 �Changes

First Order �For the reaction 2 N 2 O 5 4 NO 2 + O 2 �We found the Rate = k[N 2 O 5]1 �If concentration doubles rate doubles. �If we integrate this equation with respect to time we get the Integrated Rate Law �ln[N 2 O 5] = - kt + ln[N 2 O 5]0 �ln is the natural ln �[N 2 O 5]0 is the initial concentration.
![First Order form Rate DA Dt kA lnA kt First Order form Rate = D[A] / Dt = k[A] �ln[A] = - kt](https://slidetodoc.com/presentation_image_h/83ddd8ffaadd2969b5ffe2af135c387e/image-4.jpg)
First Order form Rate = D[A] / Dt = k[A] �ln[A] = - kt + ln[A]0 �In the form y = mx + b �y = ln[A] m = -k �x = t b = ln[A]0 �A graph of ln[A] vs time is a straight line with a negative slope. �General

First Order �By getting the straight line you can prove it is first order �Often expressed in a ratio �ln([A]/[A]0) = -kt

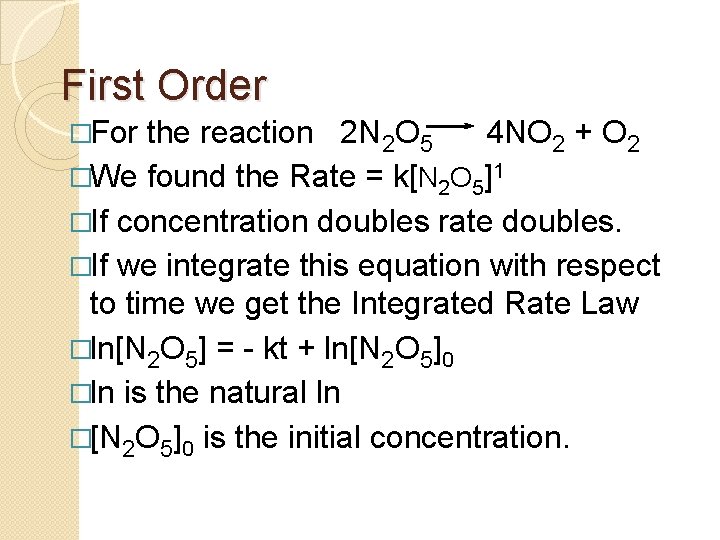
Half Life • The time required to reach half the original concentration. • If the reaction is first order • [A] = [A]0/2 when t = t 1/2 � ln(2) = kt 1/2

Half Life �t 1/2 = 0. 693 / k �The time to reach half the original concentration does not depend on the starting concentration. �An easy way to find k
![Second Order Rate DADt kA2 integrated rate law 1A kt Second Order �Rate = -D[A]/Dt = k[A]2 �integrated rate law � 1/[A] = kt](https://slidetodoc.com/presentation_image_h/83ddd8ffaadd2969b5ffe2af135c387e/image-8.jpg)
Second Order �Rate = -D[A]/Dt = k[A]2 �integrated rate law � 1/[A] = kt + 1/[A]0 �y= 1/[A] m=k �x= t b = 1/[A]0 �A straight line if 1/[A] vs t is graphed �Knowing k and [A]0 you can calculate [A] at any time t
![Second Order Half Life A A0 2 at t t 12 Second Order Half Life �[A] = [A]0 /2 at t = t 1/2](https://slidetodoc.com/presentation_image_h/83ddd8ffaadd2969b5ffe2af135c387e/image-9.jpg)
Second Order Half Life �[A] = [A]0 /2 at t = t 1/2
![Zero Order Rate Law Rate kA0 k Rate does not change with Zero Order Rate Law �Rate = k[A]0 = k �Rate does not change with](https://slidetodoc.com/presentation_image_h/83ddd8ffaadd2969b5ffe2af135c387e/image-10.jpg)
Zero Order Rate Law �Rate = k[A]0 = k �Rate does not change with concentration. �Integrated [A] = -kt + [A]0 � y = mx + b �When �t 1/2 [A] = [A]0 /2 t = t 1/2 = [A]0 /2 k

Zero Order Rate Law �Most often when reaction happens on a surface because the surface area stays constant. �Also applies to enzyme chemistry.

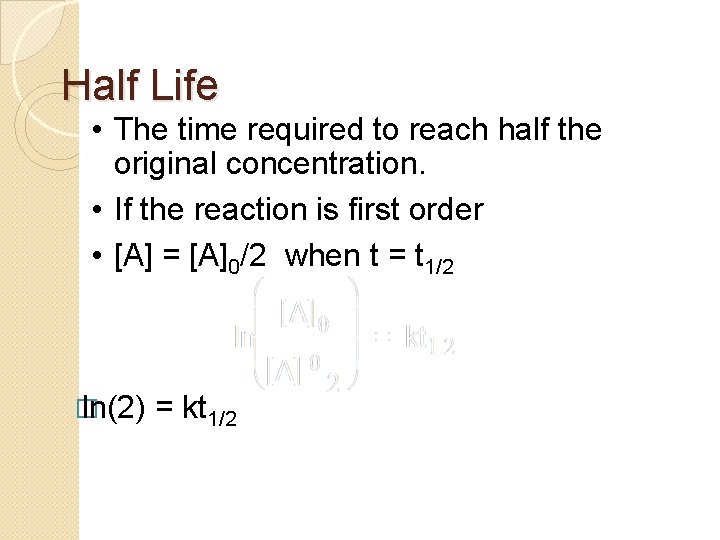
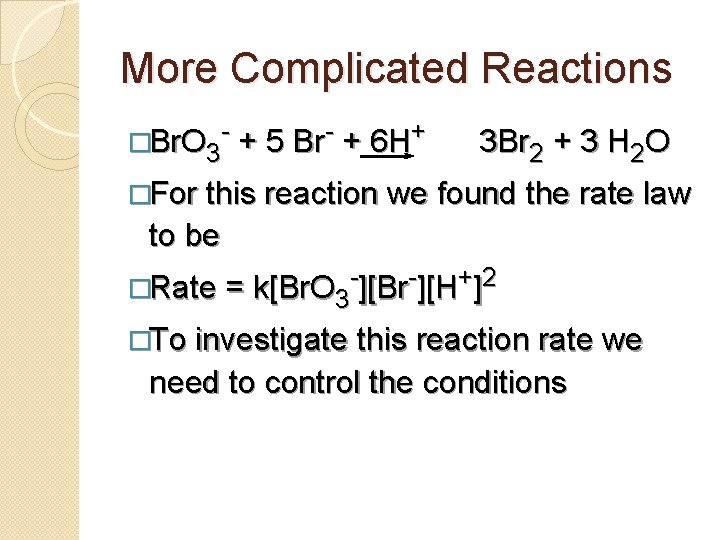
More Complicated Reactions �Br. O 3 - + 5 Br- + 6 H+ 3 Br 2 + 3 H 2 O �For this reaction we found the rate law to be �Rate = k[Br. O 3 -][Br-][H+]2 �To investigate this reaction rate we need to control the conditions
![Rate kBr O 3 BrH2 We set up the experiment so that two Rate = k[Br. O 3 -][Br-][H+]2 �We set up the experiment so that two](https://slidetodoc.com/presentation_image_h/83ddd8ffaadd2969b5ffe2af135c387e/image-13.jpg)
Rate = k[Br. O 3 -][Br-][H+]2 �We set up the experiment so that two of the reactants are in large excess. �[Br. O 3 -]0= 1. 0 x 10 -3 M �[Br-]0 = 1. 0 M �[H+]0 = 1. 0 M �As the reaction proceeds [Br. O 3 -] changes noticeably �[Br-] and [H+] don’t
![Rate kBr O 3 BrH2 This rate law can be rewritten Rate Rate = k[Br. O 3 -][Br-][H+]2 �This rate law can be rewritten �Rate =](https://slidetodoc.com/presentation_image_h/83ddd8ffaadd2969b5ffe2af135c387e/image-14.jpg)
Rate = k[Br. O 3 -][Br-][H+]2 �This rate law can be rewritten �Rate = k[Br. O 3 -][Br-]0[H+]02 �Rate = k[Br-]0[H+]02[Br. O 3 -] �Rate = k’[Br. O 3 -] �This is called a pseudo first order rate law. k’ �k = [Br-]0[H+]02