INSULIN RESISTANCE IN CHILDHOOD Dr Abdullah Al Fares

INSULIN RESISTANCE IN CHILDHOOD Dr Abdullah Al Fares Pediatric endocrinology Consultant Security Forces Hospital Riaydh. KSA
PANCREAS v Only 2% of the pancreas weight is beta cell. v Those cell produce insulin in the rate of one unit per each kilogram of body weight
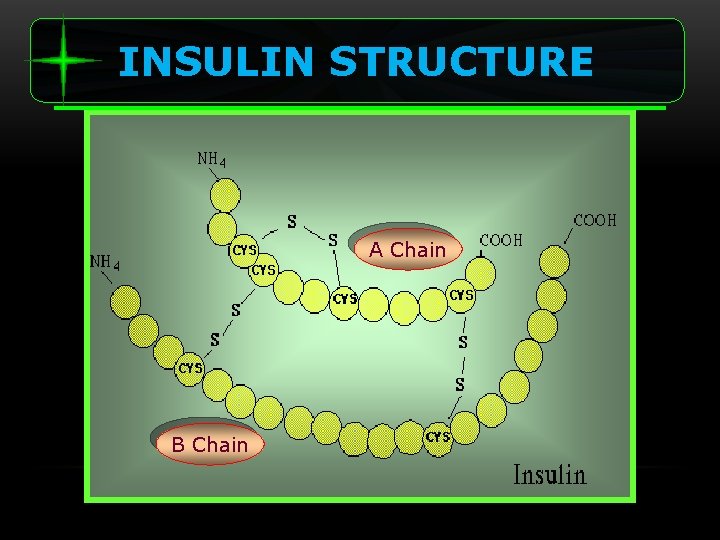
INSULIN STRUCTURE A Chain B Chain

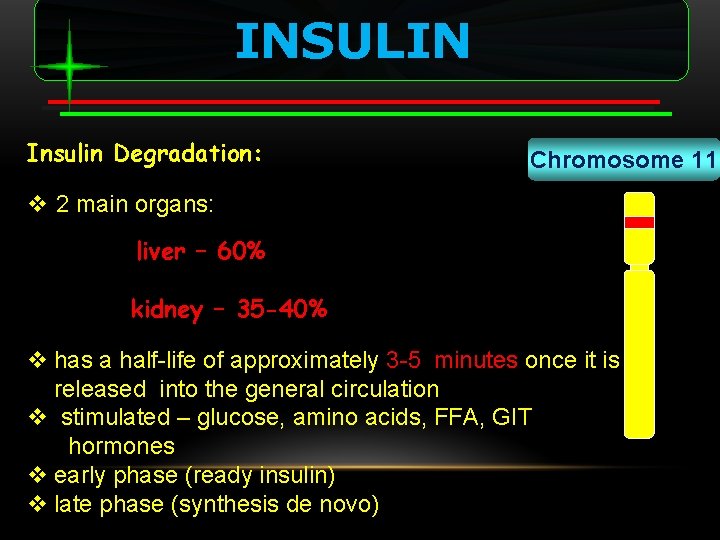
INSULIN Insulin Degradation: Chromosome 11 v 2 main organs: liver – 60% kidney – 35 -40% v has a half-life of approximately 3 -5 minutes once it is released into the general circulation v stimulated – glucose, amino acids, FFA, GIT hormones v early phase (ready insulin) v late phase (synthesis de novo)

HOW INSULIN IS RELEASED? Insulin secretion is continuous BASAL INSULIN SECTRETION Increases after carbohydrate consumption BIPHASIC
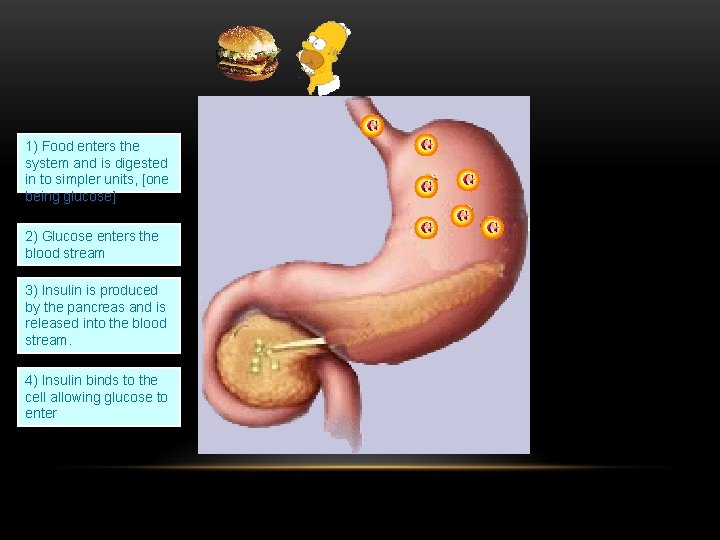
1) Food enters the system and is digested in to simpler units, [one being glucose] 2) Glucose enters the blood stream 3) Insulin is produced by the pancreas and is released into the blood stream. 4) Insulin binds to the cell allowing glucose to enter
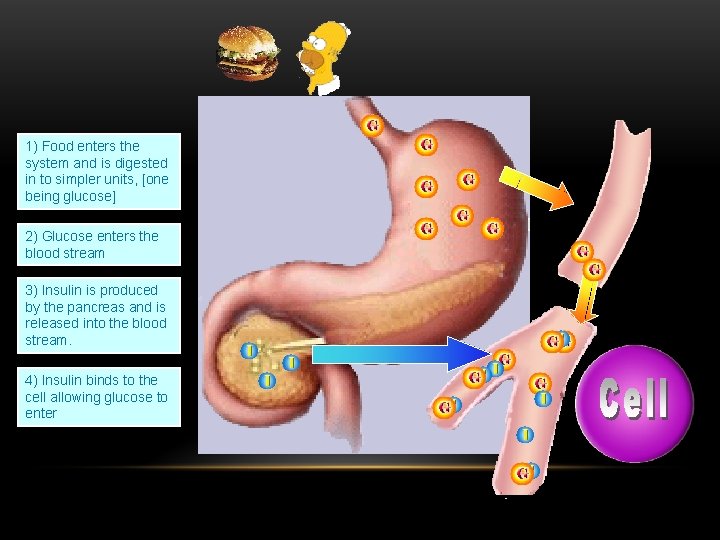
1) Food enters the system and is digested in to simpler units, [one being glucose] 2) Glucose enters the blood stream 3) Insulin is produced by the pancreas and is released into the blood stream. 4) Insulin binds to the cell allowing glucose to enter

REGULATION OF INSULIN SECRETION v Glucose rapidly increase the translation of the insulin m. RNA and slowly increases transcription of the insulin gene v No insulin is produced when plasma glucose below 50 mg/d. I v Threshold of glucose-stimulated insulin secretion is 100 mg/dl. v Half-maximal insulin response occurs at 150 mg/dl v A maximum insulin response occurs at 300 mg/dl v above 300 mg/dl platue
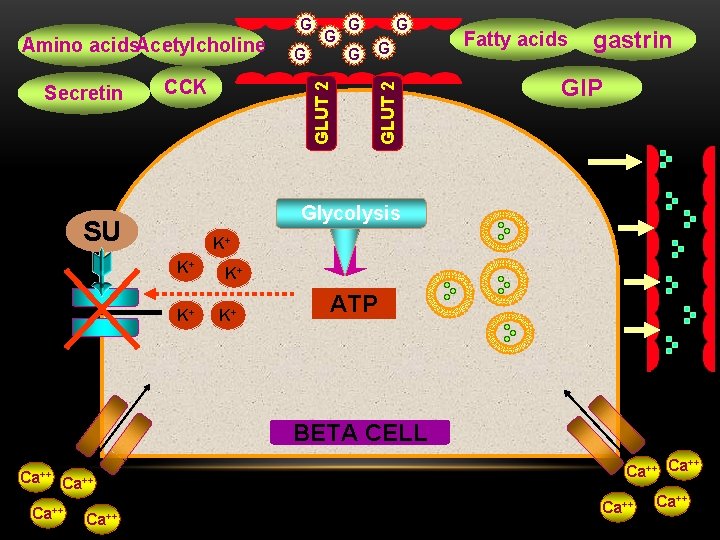
Amino acids. Acetylcholine CCK G GLUT 2 Secretin G G Fatty acids G gastrin GIP GLUT 2 G Glycolysis SU K+ K+ K+ ATP BETA CELL Ca++ ++ Ca Ca++
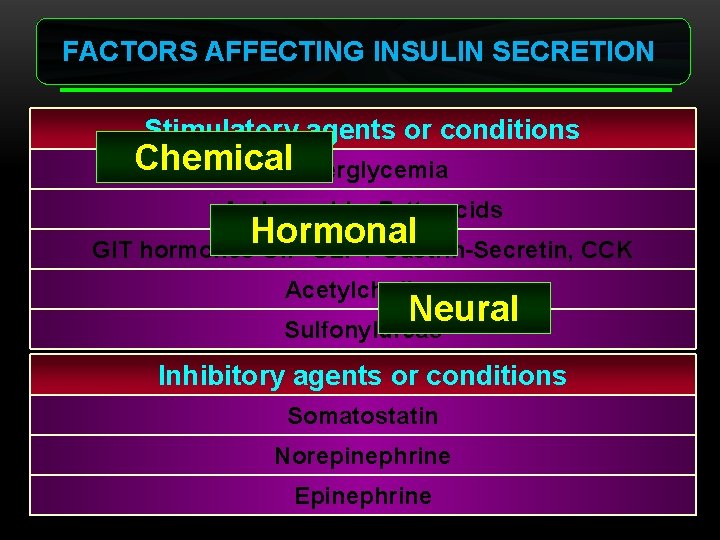
FACTORS AFFECTING INSULIN SECRETION Stimulatory agents or conditions Chemical. Hyperglycemia Amino acids, Fatty acids Hormonal GIT hormones GIP-GLP 1 -Gastrin-Secretin, CCK Acetylcholine Neural Sulfonylureas Inhibitory agents or conditions Somatostatin Norepinephrine Epinephrine
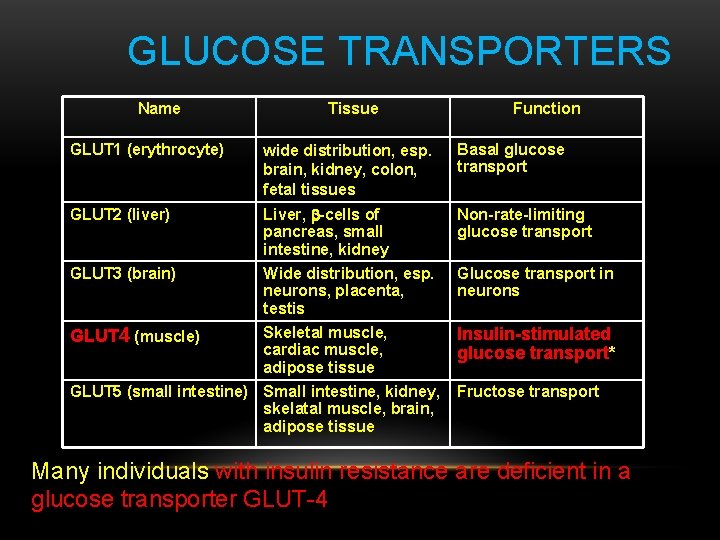
GLUCOSE TRANSPORTERS Name Tissue Function GLUT 1 (erythrocyte) wide distribution, esp. brain, kidney, colon, fetal tissues Basal glucose transport GLUT 2 (liver) Liver, b-cells of pancreas, small intestine, kidney Non-rate-limiting glucose transport GLUT 3 (brain) Wide distribution, esp. neurons, placenta, testis Glucose transport in neurons GLUT 4 (muscle) Skeletal muscle, cardiac muscle, adipose tissue Insulin-stimulated glucose transport* GLUT 5 (small intestine) Small intestine, kidney, skelatal muscle, brain, adipose tissue Fructose transport Many individuals with insulin resistance are deficient in a glucose transporter GLUT-4
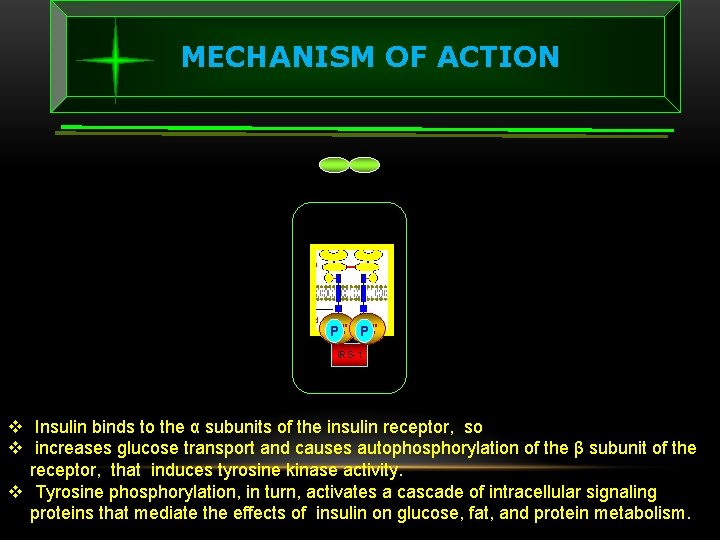
MECHANISM OF ACTION Tyrosine Kinase P IRS-1 v Insulin binds to the α subunits of the insulin receptor, so v increases glucose transport and causes autophosphorylation of the β subunit of the receptor, that induces tyrosine kinase activity. v Tyrosine phosphorylation, in turn, activates a cascade of intracellular signaling proteins that mediate the effects of insulin on glucose, fat, and protein metabolism.
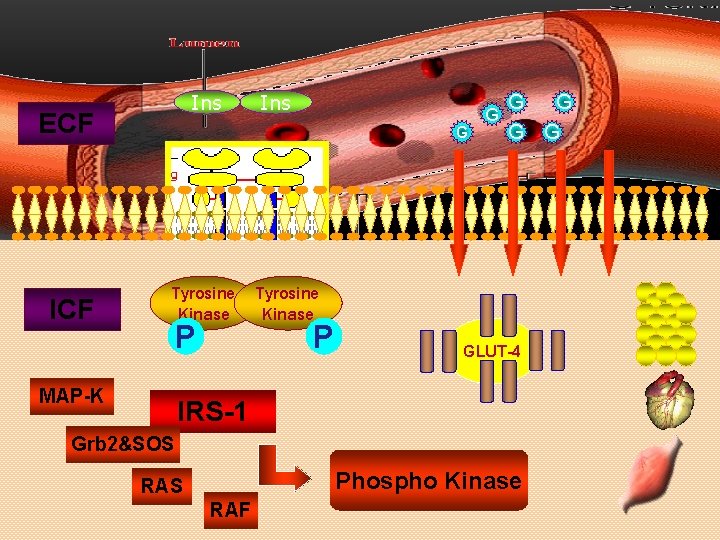
Ins ECF Ins G Tyrosine Kinase P MAP-K G G G Tyrosine Kinase P GLUT-4 IRS-1 Grb 2&SOS Phospho Kinase RAS RAF G G
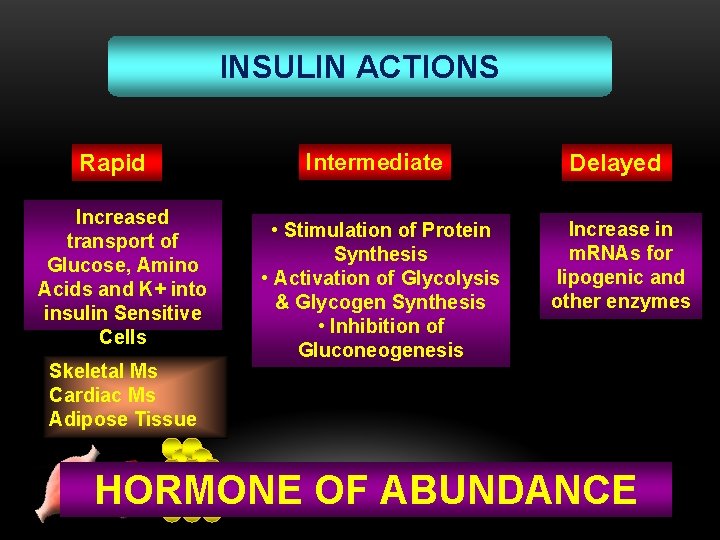
INSULIN ACTIONS Rapid Increased transport of Glucose, Amino Acids and K+ into insulin Sensitive Cells Skeletal Ms Cardiac Ms Adipose Tissue Intermediate • Stimulation of Protein Synthesis • Activation of Glycolysis & Glycogen Synthesis • Inhibition of Gluconeogenesis Delayed Increase in m. RNAs for lipogenic and other enzymes HORMONE OF ABUNDANCE

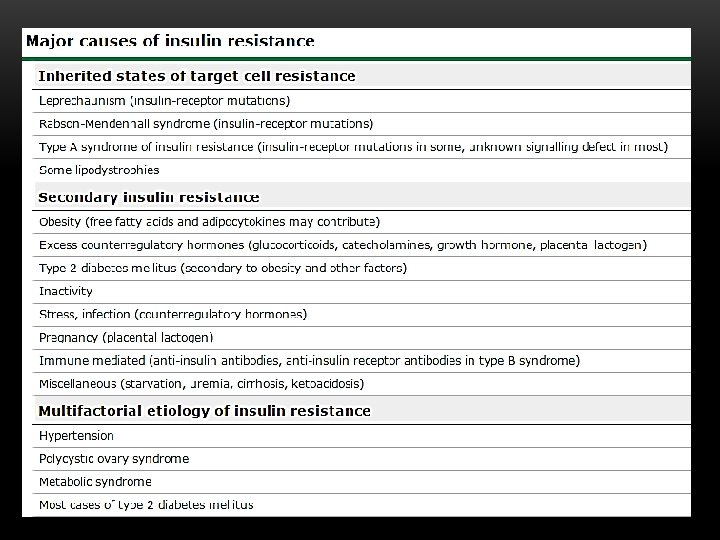
INSULIN RESISTANCE v Diminishe the ability of the cells to respond to the action of insulin in transporting glucose from the bloodstream into muscle and other tissues v. Cells become less responsive to insulin high plasma insulin Compensatory hyperinsulinemia causes down regulation of insulin receptor Defects in insulin receptor Reduction of glucose uptake/use by cells hyperglycemia

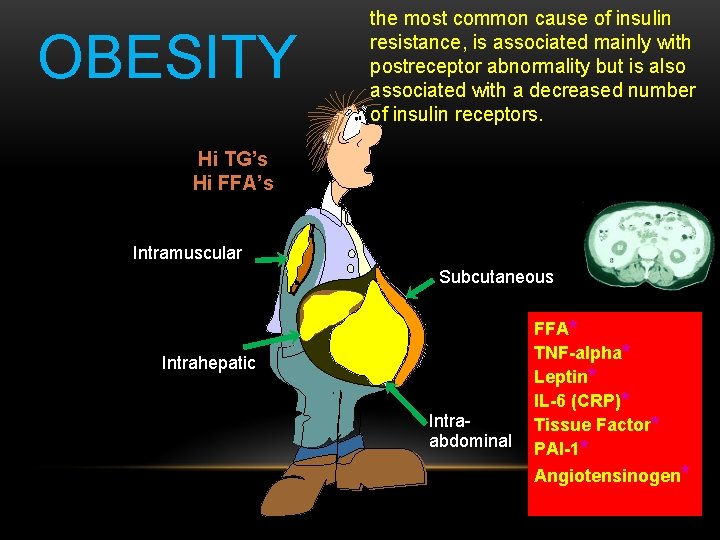
OBESITY the most common cause of insulin resistance, is associated mainly with postreceptor abnormality but is also associated with a decreased number of insulin receptors. Hi TG’s Hi FFA’s Intramuscular Subcutaneous Intrahepatic Intraabdominal FFA* TNF-alpha* Leptin* IL-6 (CRP)* Tissue Factor* PAI-1* Angiotensinogen*
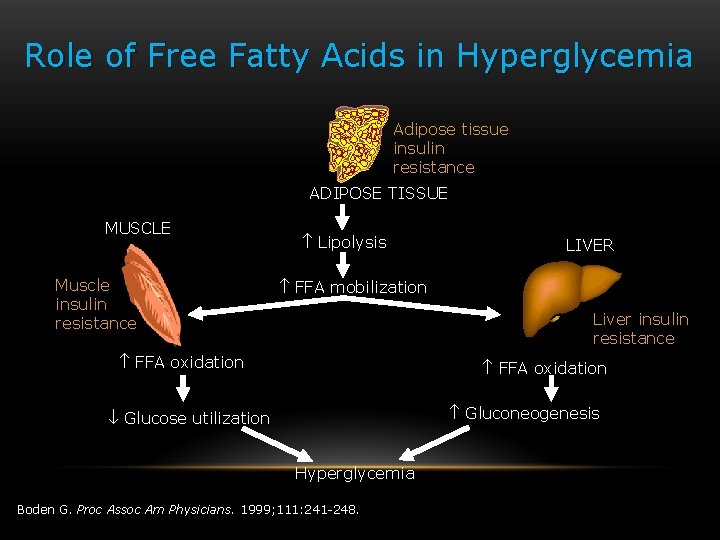
Role of Free Fatty Acids in Hyperglycemia Adipose tissue insulin resistance ADIPOSE TISSUE MUSCLE Muscle insulin resistance Lipolysis LIVER FFA mobilization Liver insulin resistance FFA oxidation Gluconeogenesis Glucose utilization Hyperglycemia Boden G. Proc Assoc Am Physicians. 1999; 111: 241 -248.
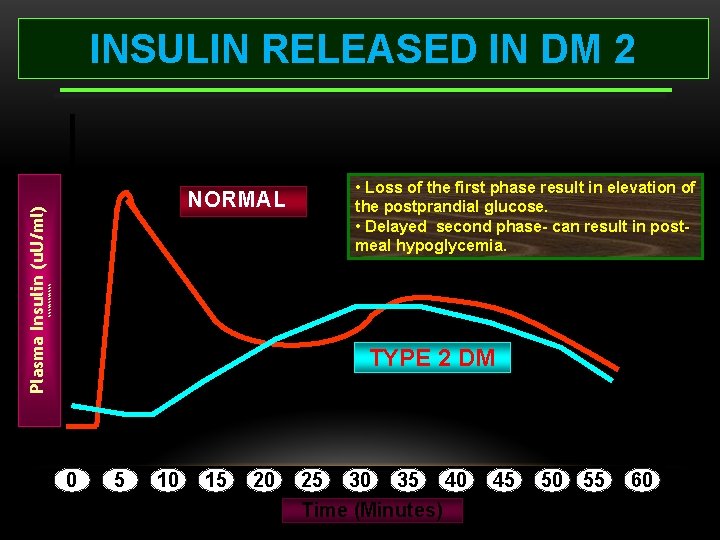
NORMAL • Loss of the first phase result in elevation of the postprandial glucose. • Delayed second phase- can result in postmeal hypoglycemia. %%%%%% Plasma Insulin (u. U/ml) INSULIN RELEASED IN DM 2 TYPE 2 DM 0 5 10 15 20 25 30 35 40 Time (Minutes) 45 50 55 60

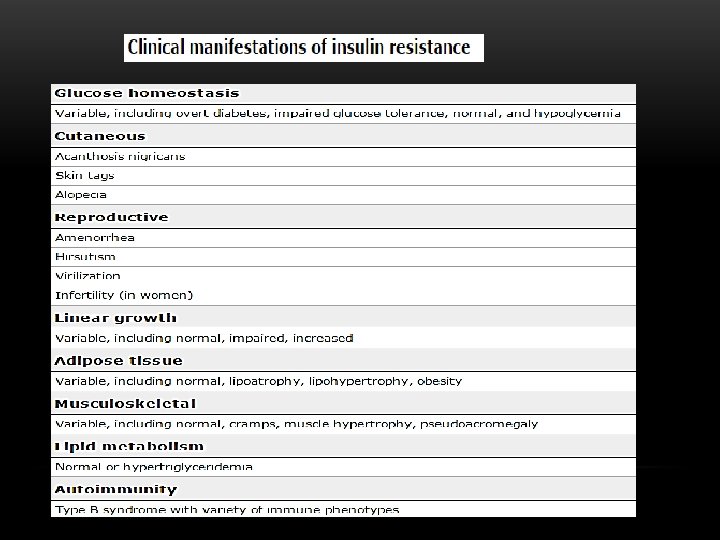
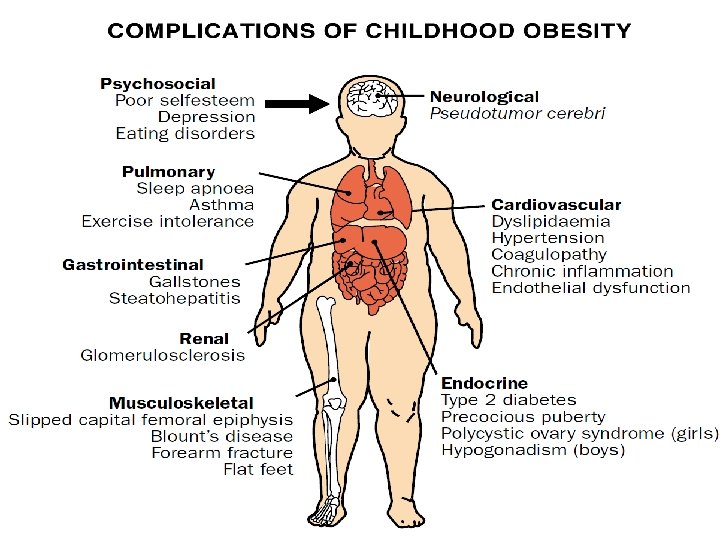
CLINICAL SPECTRUM
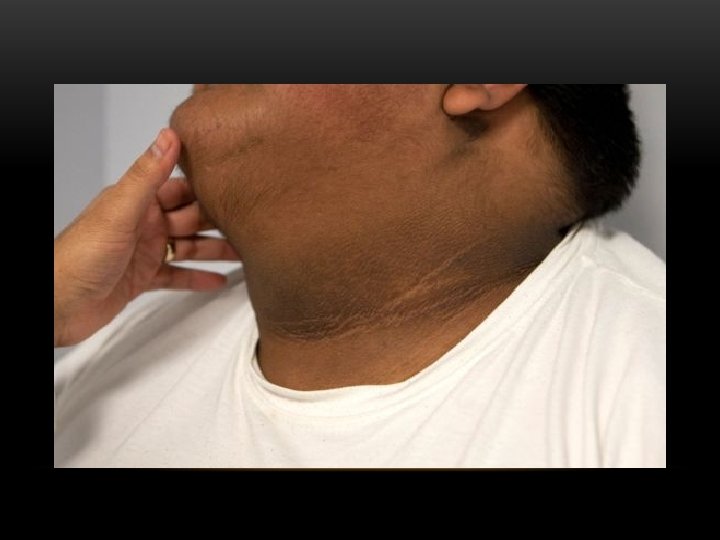
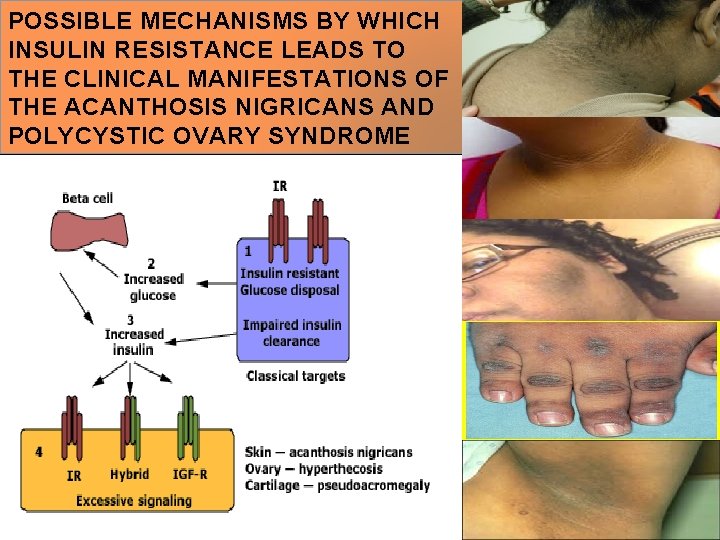
POSSIBLE MECHANISMS BY WHICH INSULIN RESISTANCE LEADS TO THE CLINICAL MANIFESTATIONS OF THE ACANTHOSIS NIGRICANS AND POLYCYSTIC OVARY SYNDROME
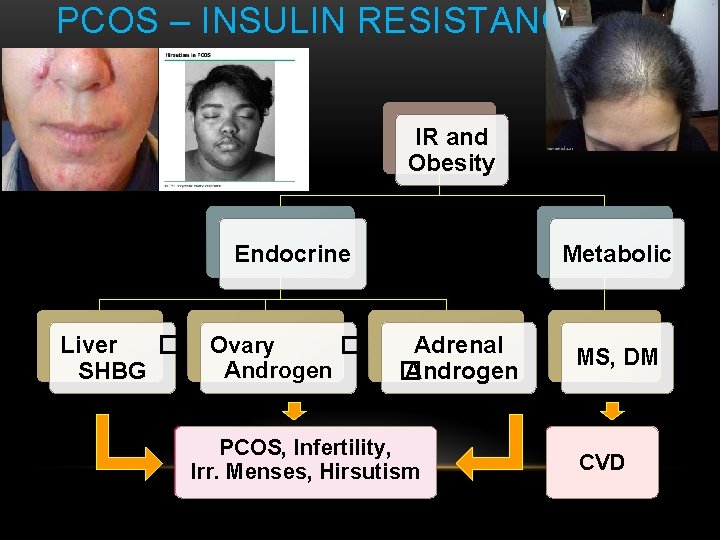
PCOS – INSULIN RESISTANCE IR and Obesity Endocrine Liver � SHBG Ovary � Androgen Metabolic Adrenal � Androgen PCOS, Infertility, Irr. Menses, Hirsutism MS, DM CVD

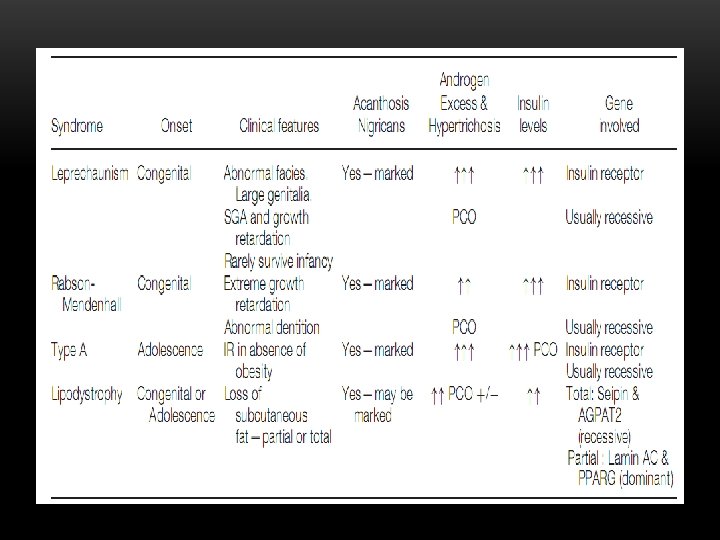
GENETIC CAUSES OF INSULIN RESISTANCE v. Leprechaunism v. Rabson-Mendenhall syndrome v. Lipoatropic diabetes (Lorens syndrome) v. Type A Ins resistance The key feature of all insulin resistance syndromes are acanthosis nigricans, androgen excess and massively raised insulin concentrations in the absence of obesity

LEPRECHAUNISM –DONAHUE SYNDROME v Congenital v Abnormal faces v Large genitalia v SGA and growth retardation vfasting hypoglycemia v Rarely survive infancy Death within the first 1 to 2 years of life v. Acanthosis Nigricans v. Gene involved Insulin receptor & GHresistence v. Recessive
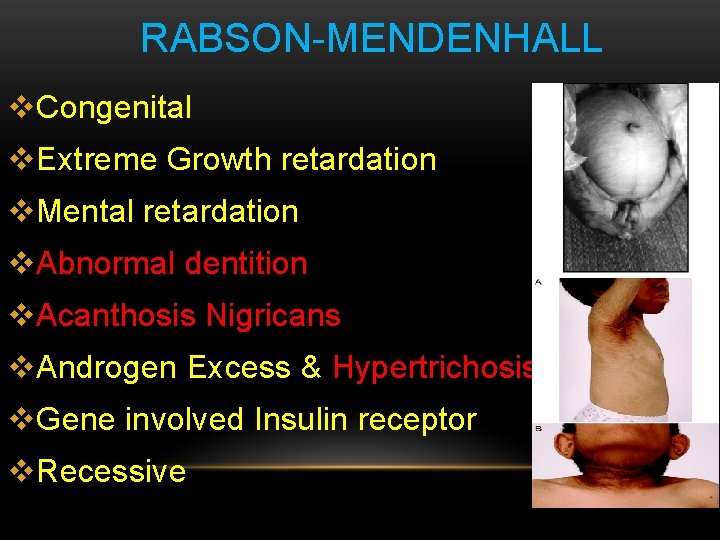
RABSON-MENDENHALL v. Congenital v. Extreme Growth retardation v. Mental retardation v. Abnormal dentition v. Acanthosis Nigricans v. Androgen Excess & Hypertrichosis v. Gene involved Insulin receptor v. Recessive

LIPODYSTROPHY LORENS SYNDROME v Congenital or Adolescence v Loss of subcutaneous fat – partial or total v Acanthosis Nigricans v Androgen Excess & Hypertrichosis v Insulin resistance, high TG, large fatty liver v Gene involved Total: Seipin & AGPAT 2 (A. recessive) Partial : Lamin AC & PPARG (dominant)

TYPE A INS RESISTANCE v. Adolescence v. Ins-resistance in absence of obesity v. Acanthosis Nigricans v. Androgen Excess & Hypertrichosis v. Gene involved Insulin receptor v. Recessive

Metabolic Syndrome

CHILDREN AND ADOLESCENTS v The International Diabetes Federation (IDF) definition of metabolic syndrome in children 10 to 16 years old is similar to that used by the IDF for adults v For children 16 years and older, the adult criteria can be used v For children younger than 10 years of age, metabolic syndrome cannot be diagnosed, but vigilance is recommended if the waist circumference is ≥ 90 percentile.

CRITERIA FOR DIAGNOSIS v World Health Organization (WHO) v International Diabetes Federation (IDF) - European Association for the Study of Diabetes (EASD) v National Cholesterol Education Project, Adult Treatment Panel (NCEP-ATP III) v Others
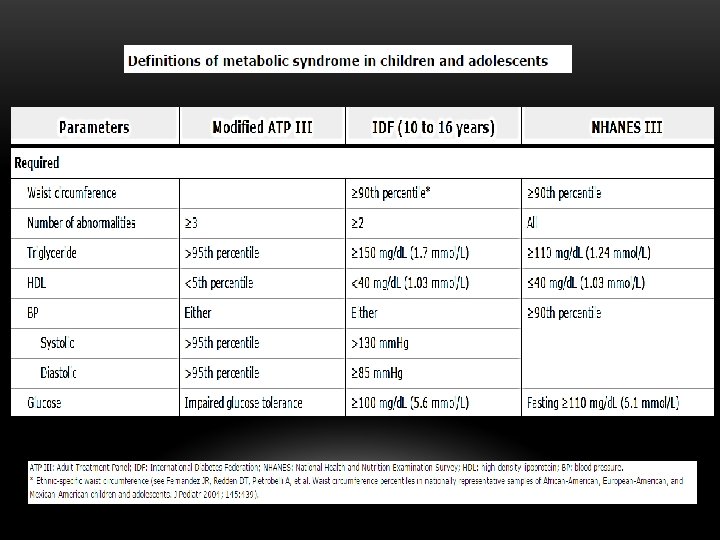

NECESSARY CRITERIA TO MAKE DIAGNOSIS v IDF: Require central obesity plus two of the other abnormalities v WHO: Also requires microalbuminuria - Albumen/ creatinine ratio >30 mg/gm creatinine v ATP III: Require three or more of the five criteria

OBESITY v IDF: Central obesity - waist circumference >94 cm for men, >80 cm women with ethnicity specific values for other groups v WHO: Waist-hip ratio >0. 9 - men or >0. 85 – women v ATP III: Waist circumference >101. 6 cm in men and 88. 9 cm in women

GLUCOSE ABNORMALITIES v IDF: FPG >100 mg/d. L (5. 6 mmol. L) or previously diagnosed type 2 diabetes v WHO: Presence of diabetes, IGT, IFG, insulin resistance v ATP III: FBS >110 mg%, <126 mg (ADA: FBS >100)
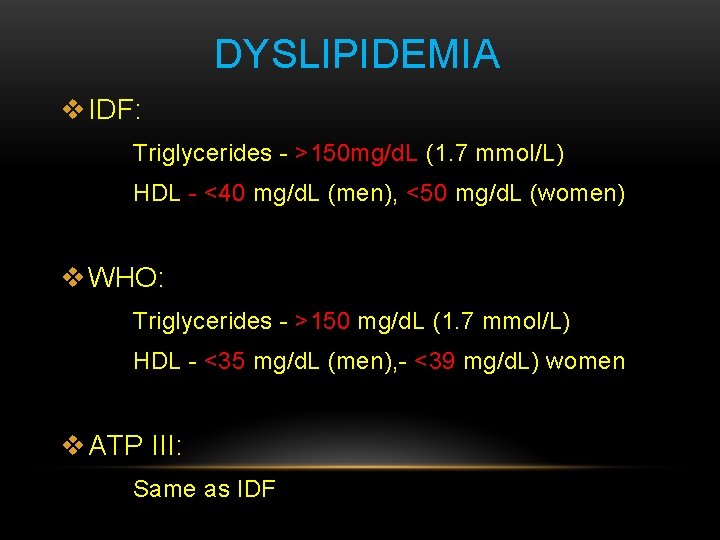
DYSLIPIDEMIA v IDF: Triglycerides - >150 mg/d. L (1. 7 mmol/L) HDL - <40 mg/d. L (men), <50 mg/d. L (women) v WHO: Triglycerides - >150 mg/d. L (1. 7 mmol/L) HDL - <35 mg/d. L (men), - <39 mg/d. L) women v ATP III: Same as IDF

HYPERTENSION v. IDF: BP >130/85 or on Rx for previously Dxed hypertension v. WHO: BP >140/90 v. NCEP ATP III: BP >130/80
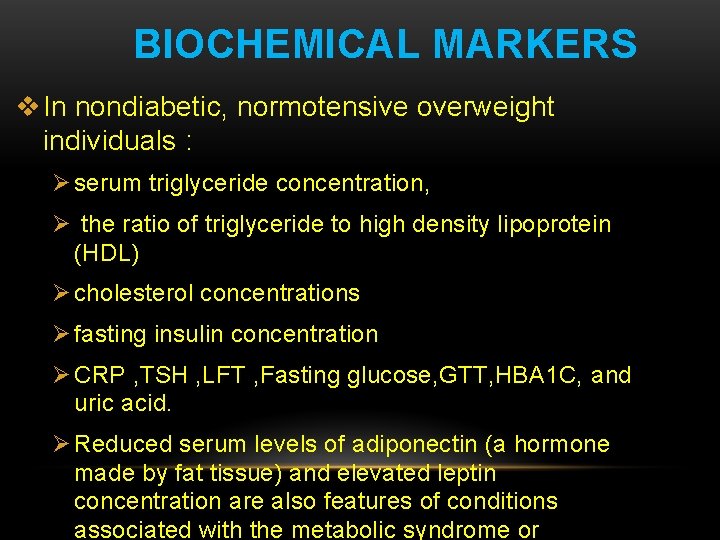
BIOCHEMICAL MARKERS v In nondiabetic, normotensive overweight individuals : Ø serum triglyceride concentration, Ø the ratio of triglyceride to high density lipoprotein (HDL) Ø cholesterol concentrations Ø fasting insulin concentration Ø CRP , TSH , LFT , Fasting glucose, GTT, HBA 1 C, and uric acid. Ø Reduced serum levels of adiponectin (a hormone made by fat tissue) and elevated leptin concentration are also features of conditions associated with the metabolic syndrome or

THERAPY

MULTIPLE RISK FACTOR MANAGEMENT v. Obesity v. Glucose Intolerance v. Insulin Resistance v. Lipid Disorders v. Hypertension v. Goals: Minimize Risk of Type 2 Diabetes and Cardiovascular Disease

v. Lifestyle modification v. Diet v. Exercise v. Prevention of type 2 diabetes v. Oral hypoglycemic agents v. Cardiovascular risk reduction v. Prevention

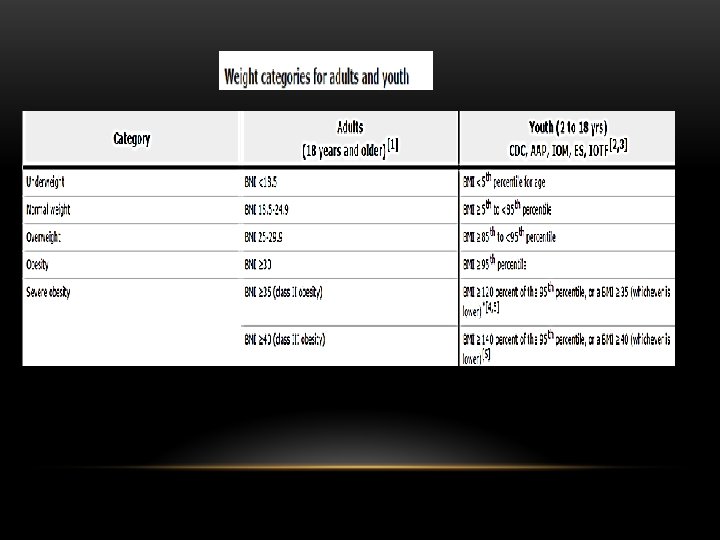
LIFE-STYLE MODIFICATION: IS IT IMPORTANT? v Abdominal obesity Year 1: reduce body weight 7 to 10 percent Continue weight loss thereafter with ultimate goal BMI <25 kg/m 2 v Exercise Improves CV fitness, weight control, sensitivity to insulin, reduces incidence of diabetes v Atherogenic diet Reduced intake saturate fat, trans fat, cholesterol v Weight loss Improves lipids, insulin sensitivity, BP levels, reduces incidence of diabetes v Goals: Brisk walking - 30 min. /day

DIABETES CONTROL - HOW IMPORTANT? v For every 1% rise in Hb A 1 c there is an 18% rise in risk of cardiovascular events & a 28% increase in peripheral arterial disease v Evidence is accumulating to show that tight blood sugar control in both Type 1 and Type 2 diabetes reduces risk of CVD v
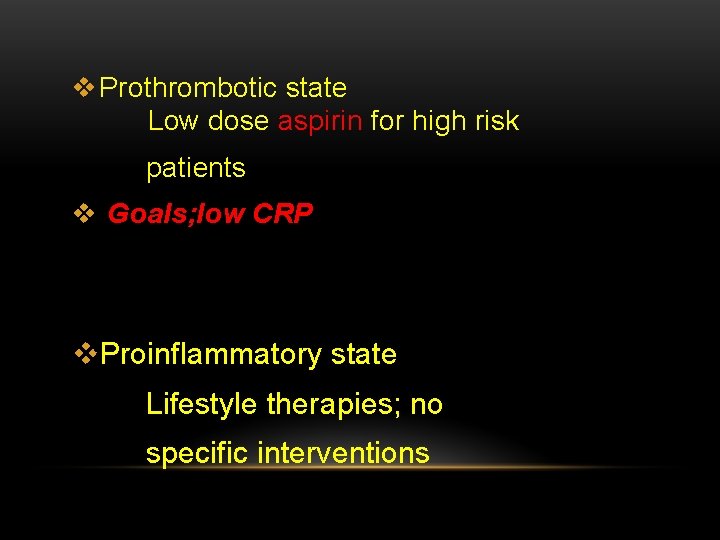
v Prothrombotic state Low dose aspirin for high risk patients v Goals; low CRP v. Proinflammatory state Lifestyle therapies; no specific interventions
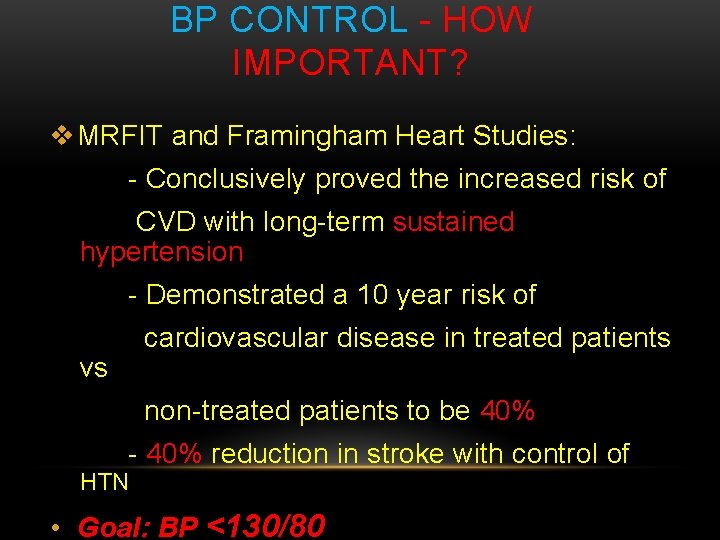
BP CONTROL - HOW IMPORTANT? v MRFIT and Framingham Heart Studies: - Conclusively proved the increased risk of CVD with long-term sustained hypertension - Demonstrated a 10 year risk of cardiovascular disease in treated patients vs non-treated patients to be 40% - 40% reduction in stroke with control of HTN • Goal: BP <130/80

LIPID CONTROL - HOW IMPORTANT? v. Multiple major studies show 24 - 37% reductions in cardiovascular disease risk with use of statins and fibrates in the control of hyperlipidemia v. Goals: LDL <100 mg/d. L (<3. 0 mmol /l) (high risk <70 mg/d. L- <2. 6 mmol/L) TG <150 mg% (<1. 7 mmol /l) HDL >40 mg% (>1. 1 mmol /l)

Medications

v Hypertension: ACE inhibitors, ARBs Others - thiazides, calcium channel blockers, beta blockers, alpha blockers v Hyperlipidemia: Statins, Fibrates, Niacin v Platelet inhibitors: ASA v Insulin Resistance/Diabetes Insulin Sensitizers: - Biguanides - metformin - PPAR α, γ & δ agonists - Glitazones, Glitazars Can be used in combination Insulin Secretagogues: - Sulfonylureas - glipizide, glyburide, glimeparide, glibenclamide - Meglitinides - repaglanide, netiglamide

prevention


- Slides: 57