Instrumental Analysis Discuss theory and background for 1
Instrumental Analysis Discuss theory and background for 1. 2. 3. 4. Spectrophotometry Chromatography Electrochemistry signal processing and relationship between readout to property measured

Introduction (Chapter 1) n Classification of Analytical Methods n n n Qualitative instrumental analysis is that measured property indicates presence of analyte in matrix Quantitative instrumental analysis is that magnitude of measured property is proportional to concentration of analyte (species of interest) in matrix (all constituents including analyte. n Matrix-analyte = concomitants(相隨共存物) Often need pretreatment - chemical extraction, distillation, separation, precipitation……
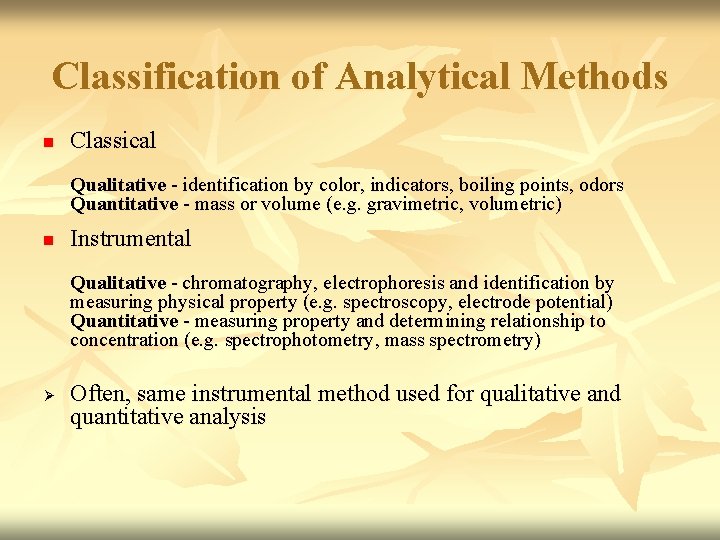
Classification of Analytical Methods n Classical Qualitative - identification by color, indicators, boiling points, odors Quantitative - mass or volume (e. g. gravimetric, volumetric) n Instrumental Qualitative - chromatography, electrophoresis and identification by measuring physical property (e. g. spectroscopy, electrode potential) Quantitative - measuring property and determining relationship to concentration (e. g. spectrophotometry, mass spectrometry) Ø Often, same instrumental method used for qualitative and quantitative analysis
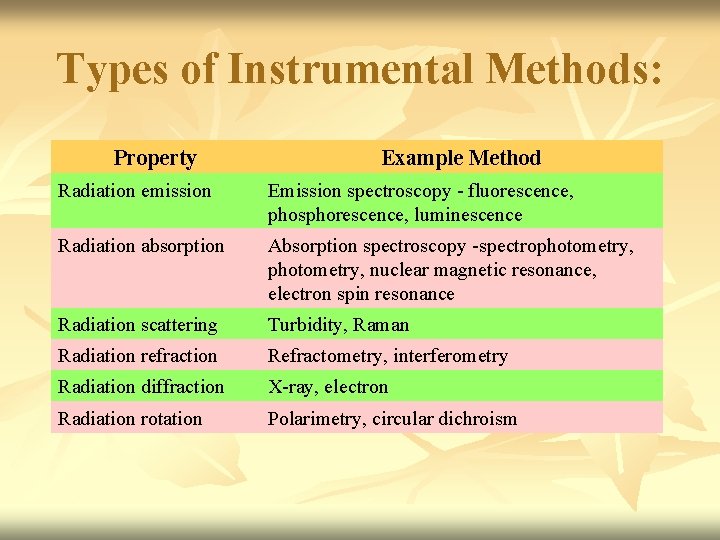
Types of Instrumental Methods: Property Example Method Radiation emission Emission spectroscopy - fluorescence, phosphorescence, luminescence Radiation absorption Absorption spectroscopy -spectrophotometry, nuclear magnetic resonance, electron spin resonance Radiation scattering Turbidity, Raman Radiation refraction Refractometry, interferometry Radiation diffraction X-ray, electron Radiation rotation Polarimetry, circular dichroism

Types of Instrumental Methods: Property Example Method Electrical potential Potentiometry Electrical charge Coulometry Electrical current Voltammetry - amperometry, polarography Electrical resistance Conductometry Mass Gravimetry Mass-to-charge ratio Mass spectrometry Rate of reaction Stopped flow, flow injection analysis Thermal gravimetry, calorimetry Radioactivity Activation, isotope dilution Often combined with chromatographic or electrophoretic methods
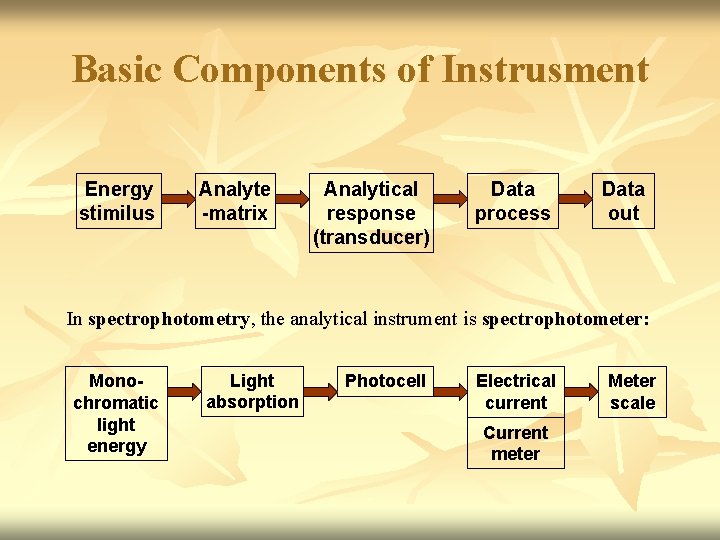
Basic Components of Instrusment Energy stimilus Analyte -matrix Analytical response (transducer) Data process Data out In spectrophotometry, the analytical instrument is spectrophotometer: Monochromatic light energy Light absorption Photocell Electrical current Current meter Meter scale
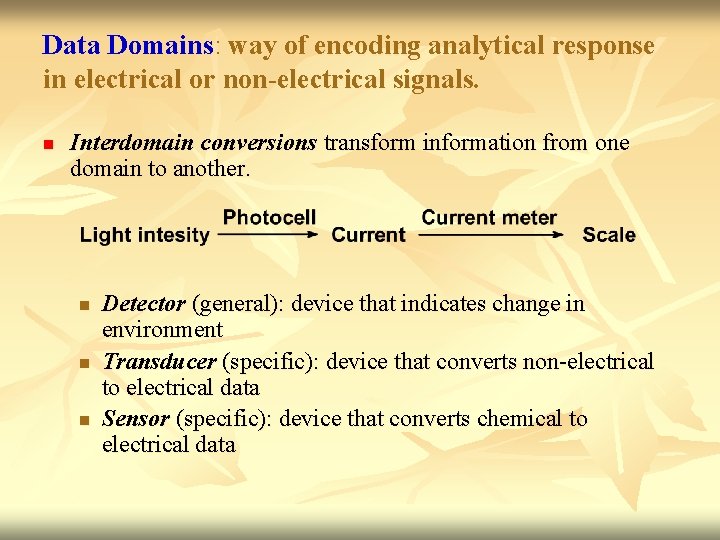
Data Domains: way of encoding analytical response in electrical or non-electrical signals. n Interdomain conversions transform information from one domain to another. n n n Detector (general): device that indicates change in environment Transducer (specific): device that converts non-electrical to electrical data Sensor (specific): device that converts chemical to electrical data
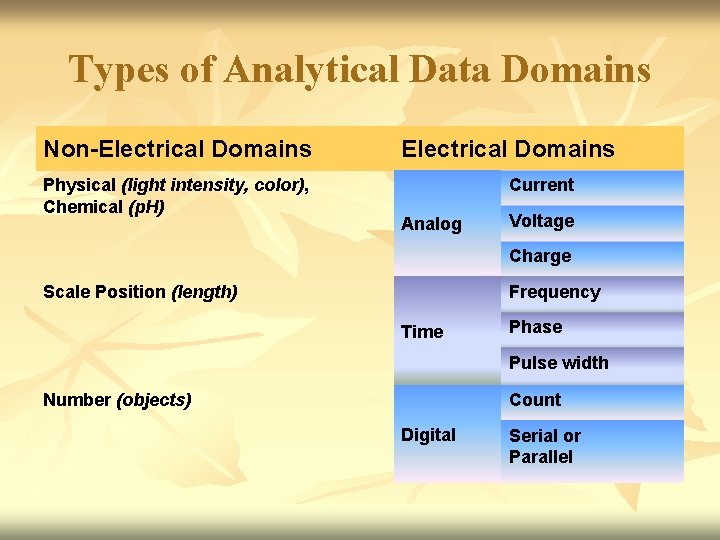
Types of Analytical Data Domains Non-Electrical Domains Physical (light intensity, color), Chemical (p. H) Electrical Domains Current Analog Voltage Charge Scale Position (length) Frequency Time Phase Pulse width Number (objects) Count Digital Serial or Parallel
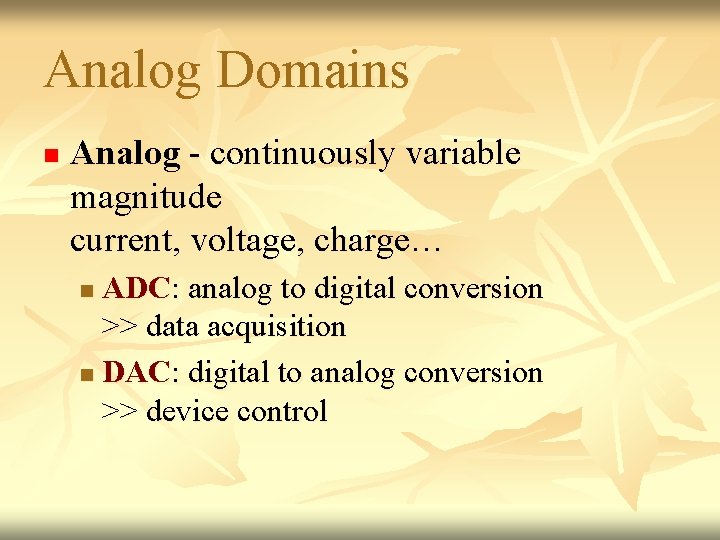
Analog Domains n Analog - continuously variable magnitude current, voltage, charge… ADC: analog to digital conversion >> data acquisition n DAC: digital to analog conversion >> device control n

Digital Domains n n Discrete values count, serial, parallel, number Advantages: (1) easy to store (2) not susceptible to noise

Time Domains n Time - vary with time frequency, phase, pulse width…

Selecting an analytical method n n n How reproducible? - Precision How close to true value? - Accuracy/Bias How small a difference can be measured? Sensitivity What range of amounts? - Dynamic Range How much interference? - Selectivity How many samples? – Efficience (time, money cost)
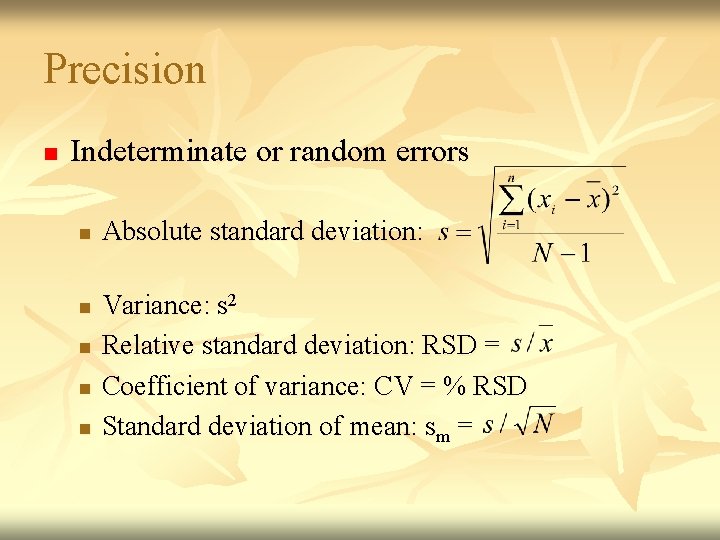
Precision n Indeterminate or random errors n n n Absolute standard deviation: Variance: s 2 Relative standard deviation: RSD = Coefficient of variance: CV = % RSD Standard deviation of mean: sm =

Accuracy n n Determinate errors from operator, method, instrumental… Bias(偏差 ): μ – x true Bias(

Sensitivity n Calibration sensitivity: larger slope of calibration curve m, more sensitive measurement

Detection Limit n n n Signal must be bigger than random noise of blank Minimum signal: Signal min = Av. Signal blank + k.Signal blank From statistics (at 95% confidence level) k = signal/noise >= 3

Dynamic Range n n At detection limit we can say confidently analyte is present butcannot perform reliable quantitation Level of quantitation (LOQ): k = 10 Limit of linearity (LOL): when signal is no longer proportional to conc. Dynamic range: LOL / LOQ = 102 to > 106
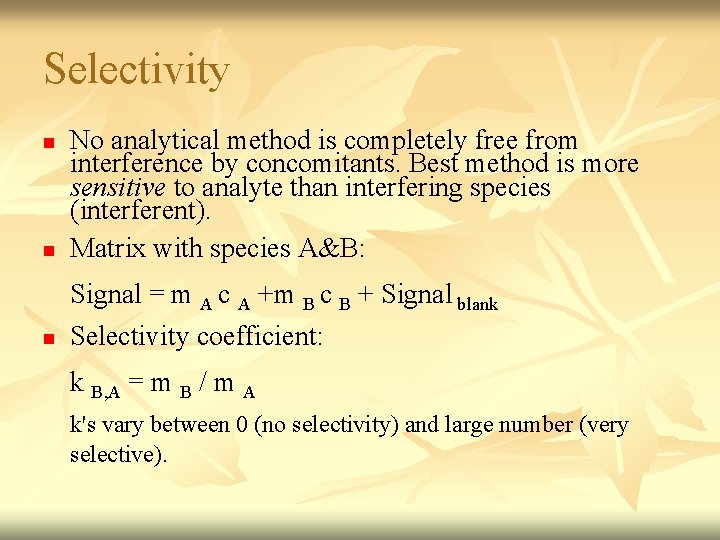
Selectivity n No analytical method is completely free from interference by concomitants. Best method is more sensitive to analyte than interfering species (interferent). Matrix with species A&B: n Signal = m A c A +m B c B + Signal blank Selectivity coefficient: n k B, A = m B / m A k's vary between 0 (no selectivity) and large number (very selective).
- Slides: 18