Instructor Eng M Alzira Dinis Materials Science Atomic


![[3] Available at: http: //www. ill. fr/dif/3 D-crystals/images/buckeyb. gif All Atoms are made up [3] Available at: http: //www. ill. fr/dif/3 D-crystals/images/buckeyb. gif All Atoms are made up](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-4.jpg)
![The Structure of Some Important Atoms. . . [8] Available at: http: //www. terrificscientific. The Structure of Some Important Atoms. . . [8] Available at: http: //www. terrificscientific.](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-6.jpg)
![[12] Atoms. . . Atoms of different elements are distinguished from each other by [12] Atoms. . . Atoms of different elements are distinguished from each other by](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-7.jpg)
![Modern Atom Models [16] Available at: http: //astronomy. nmsu. edu/nicole/teaching/ASTR 110/lectures/lecture 18/pics/atoms 4. gif Modern Atom Models [16] Available at: http: //astronomy. nmsu. edu/nicole/teaching/ASTR 110/lectures/lecture 18/pics/atoms 4. gif](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-9.jpg)
![Modern Atom Models 1) Thomson Model of the Atom [17] Available at: http: //www. Modern Atom Models 1) Thomson Model of the Atom [17] Available at: http: //www.](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-10.jpg)
![[21] [23] It is considerable that the scattering angle of the alpha particle by [21] [23] It is considerable that the scattering angle of the alpha particle by](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-12.jpg)
![[25] [26] [27] [26]Available at: http: //dbhs. wvusd. k 12. ca. us/webdocs/Atomic. Structure/Thomson. Model. [25] [26] [27] [26]Available at: http: //dbhs. wvusd. k 12. ca. us/webdocs/Atomic. Structure/Thomson. Model.](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-13.jpg)
![Modern Atom Models 2) The Rutherford Model of the Nuclear Atom [28] Available at: Modern Atom Models 2) The Rutherford Model of the Nuclear Atom [28] Available at:](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-14.jpg)
![Modern Atom Models 3) The Bohr Model of the Atom [34] Available at: http: Modern Atom Models 3) The Bohr Model of the Atom [34] Available at: http:](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-17.jpg)
![Modern Atom Models 4) Schroedinger Picture of the Atom [40] Available at: http: //www. Modern Atom Models 4) Schroedinger Picture of the Atom [40] Available at: http: //www.](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-20.jpg)
![Erwin Schroedinger (1887 -1961) [44] Available at: http: //physics. bgsu. edu/~stoner/P 202/atoms/img 016. JPG Erwin Schroedinger (1887 -1961) [44] Available at: http: //physics. bgsu. edu/~stoner/P 202/atoms/img 016. JPG](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-22.jpg)

- Slides: 24

Instructor: Engª M. Alzira Dinis Materials Science Atomic Structure and Bonding Aras Keropyan 15386 Computer Engineering Universidade Fernando Pessoa

Atomic Structure and Modern Atom Models Index Atomic Structure The Bases and Particles of Atom Structure of Nucleus and Atom Modern Atom Models Formulas Summary

? What are atoms ? Atoms are the basic building blocks of matter that make up everyday objects. A desk, the air, even we are made up of atoms [1]. There are 90 naturally occurring kinds of atoms. Scientists in labs have been able to make about 25 more [2]. [1] Frumar M. (1997), Polak K. , Cernosek Z. , Frumarova B. , Wagner T. , Chem. pp. 51 [2] Golberg D. (2003), Xu F. -F. , Bando Y. , Appl. Phys. A 76 479.
![3 Available at http www ill frdif3 Dcrystalsimagesbuckeyb gif All Atoms are made up [3] Available at: http: //www. ill. fr/dif/3 D-crystals/images/buckeyb. gif All Atoms are made up](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-4.jpg)
[3] Available at: http: //www. ill. fr/dif/3 D-crystals/images/buckeyb. gif All Atoms are made up of 3 basic things. These are: electrons , protons and neutrons . These particles have different charasteristics. Electrons are petty, very light that those have a negative electrical charge (-). Protons are much larger and heavier than electrons and carry positive (+) charge. Neutrons are large and heavy like protons, but neutrons have no electrical charge [4]. Each atom is made up of a combination of p+’s n 0’s ana e-’s [5]. [4] Tanaka K. (1990), Rev. Solid State Sci. 4 [5] Mickelson W. (2003), Aloni S. , Han W. , Cumings J. , Science 300 We can see a one type of atom next -> [6] Available at: http: //www. fdu. edu/images/atom. gif [6]

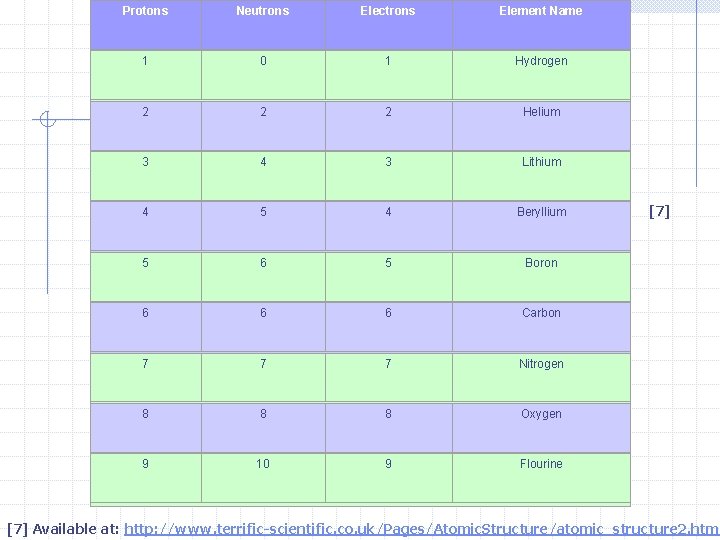
Protons Neutrons Electrons Element Name 1 0 1 Hydrogen 2 2 2 Helium 3 4 3 Lithium 4 5 4 Beryllium 5 6 5 Boron 6 6 6 Carbon 7 7 7 Nitrogen 8 8 8 Oxygen 9 10 9 Flourine [7] Available at: http: //www. terrific-scientific. co. uk/Pages/Atomic. Structure/atomic_structure 2. htm
![The Structure of Some Important Atoms 8 Available at http www terrificscientific The Structure of Some Important Atoms. . . [8] Available at: http: //www. terrificscientific.](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-6.jpg)
The Structure of Some Important Atoms. . . [8] Available at: http: //www. terrificscientific. co. uk/Pages/Atomic. S tructure/topic%20 graphics/ato mic_2_helium. gif [9] Available at: http: //www. terrificscientific. co. uk/Pages/Atomic. Structure/topic%20 graphics/atomic_8_oxygen. gif
![12 Atoms Atoms of different elements are distinguished from each other by [12] Atoms. . . Atoms of different elements are distinguished from each other by](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-7.jpg)
[12] Atoms. . . Atoms of different elements are distinguished from each other by their number of protons (the number of protons is constant for all atoms of a single element, the number of neutrons and electrons can vary under some circumstances). To Atoms are extremely identify this important small. One hydrogen characteristic of atoms, the term atomic number (z) is used to atom (the smallest atom describe the number of protons known) is approximately 5 x 10 -8 mm in in an atom [10]. diameter [11]. [10] Kysar W. (1998), Models of Engineering and Applied Sciences, Harvard University [11] Walko D. A. (1999), Robinson I. K. , Phys. Rev. B 15446. [12] Available at: http: //static. howstuffworks. com/gif/laser 1. jpg

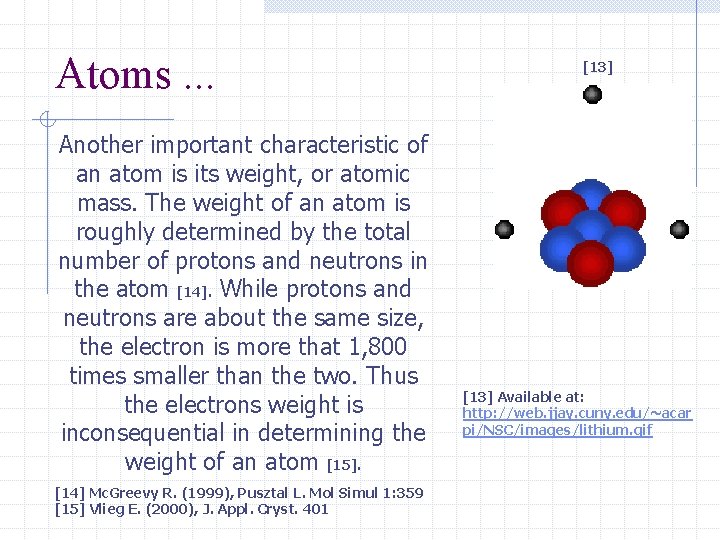
Atoms. . . Another important characteristic of an atom is its weight, or atomic mass. The weight of an atom is roughly determined by the total number of protons and neutrons in the atom [14]. While protons and neutrons are about the same size, the electron is more that 1, 800 times smaller than the two. Thus the electrons weight is inconsequential in determining the weight of an atom [15]. [14] Mc. Greevy R. (1999), Pusztal L. Mol Simul 1: 359 [15] Vlieg E. (2000), J. Appl. Cryst. 401 [13] Available at: http: //web. jjay. cuny. edu/~acar pi/NSC/images/lithium. gif
![Modern Atom Models 16 Available at http astronomy nmsu edunicoleteachingASTR 110lectureslecture 18picsatoms 4 gif Modern Atom Models [16] Available at: http: //astronomy. nmsu. edu/nicole/teaching/ASTR 110/lectures/lecture 18/pics/atoms 4. gif](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-9.jpg)
Modern Atom Models [16] Available at: http: //astronomy. nmsu. edu/nicole/teaching/ASTR 110/lectures/lecture 18/pics/atoms 4. gif
![Modern Atom Models 1 Thomson Model of the Atom 17 Available at http www Modern Atom Models 1) Thomson Model of the Atom [17] Available at: http: //www.](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-10.jpg)
Modern Atom Models 1) Thomson Model of the Atom [17] Available at: http: //www. sdmiramar. edu/faculty/fgarces/z. Course/Fall 05/Ch 100_MM/a. My_File. Lec/04_Lec. Note s_Ch 100/04_Model. Atom/401_Atomic. Evolution/401_pic/thomson. gif

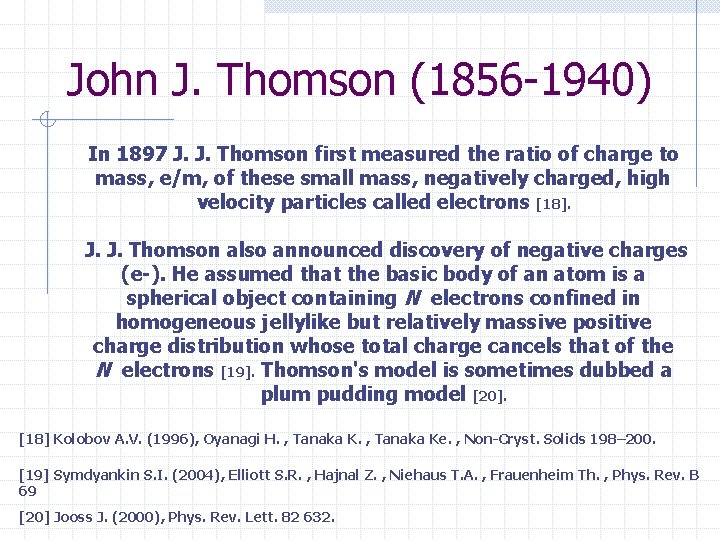
John J. Thomson (1856 -1940) In 1897 J. J. Thomson first measured the ratio of charge to mass, e/m, of these small mass, negatively charged, high velocity particles called electrons [18]. J. J. Thomson also announced discovery of negative charges (e-). He assumed that the basic body of an atom is a spherical object containing N electrons confined in homogeneous jellylike but relatively massive positive charge distribution whose total charge cancels that of the N electrons [19]. Thomson's model is sometimes dubbed a plum pudding model [20]. [18] Kolobov A. V. (1996), Oyanagi H. , Tanaka Ke. , Non-Cryst. Solids 198– 200. [19] Symdyankin S. I. (2004), Elliott S. R. , Hajnal Z. , Niehaus T. A. , Frauenheim Th. , Phys. Rev. B 69 [20] Jooss J. (2000), Phys. Rev. Lett. 82 632.
![21 23 It is considerable that the scattering angle of the alpha particle by [21] [23] It is considerable that the scattering angle of the alpha particle by](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-12.jpg)
[21] [23] It is considerable that the scattering angle of the alpha particle by Thomson's model is at most 0. 01 degrees [22]. The thickness of the metal foil in the scattering experiment of the alpha rays is about 10 -6 m. When assuming that the atoms are tightly packed in the metal, there about 10000 atoms lining up in the direction of thickness, because the size of an atom is approximately 10 -10 m. [24]. [21] Available at: http: //www 2. kutl. kyushu-u. ac. jp/seminar/Micro. World 1_E/Part 2_E/P 24_E/Th [22] Bérardi G. (1996), Jaeger M. , Martin R. and Carpentier C. [23] Available at: http: //www. sc. ehu. es/sbweb/fisica/cuantica/rutherford_5. gif [24] Kato H. , Matsubara E. , Inoue A. , Nishiyama N. , Mater Sci Eng 2004; 375– 377: 444.
![25 26 27 26Available at http dbhs wvusd k 12 ca uswebdocsAtomic StructureThomson Model [25] [26] [27] [26]Available at: http: //dbhs. wvusd. k 12. ca. us/webdocs/Atomic. Structure/Thomson. Model.](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-13.jpg)
[25] [26] [27] [26]Available at: http: //dbhs. wvusd. k 12. ca. us/webdocs/Atomic. Structure/Thomson. Model. GIF [25], [27]Available at: http: //w 3. balikesir. edu. tr/~taner/dersler/genel_kimya/atomik_yapi/rutherford_atom_modeli/r utherford_atom_modeli. htm
![Modern Atom Models 2 The Rutherford Model of the Nuclear Atom 28 Available at Modern Atom Models 2) The Rutherford Model of the Nuclear Atom [28] Available at:](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-14.jpg)
Modern Atom Models 2) The Rutherford Model of the Nuclear Atom [28] Available at: http: //www. newgenevacenter. org/portrait/rutherford. jpg

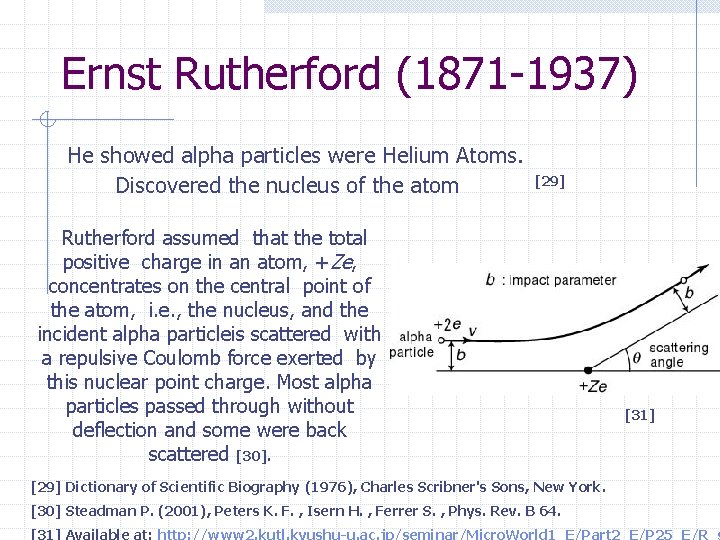
Ernst Rutherford (1871 -1937) He showed alpha particles were Helium Atoms. Discovered the nucleus of the atom [29] Rutherford assumed that the total positive charge in an atom, +Ze, concentrates on the central point of the atom, i. e. , the nucleus, and the incident alpha particleis scattered with a repulsive Coulomb force exerted by this nuclear point charge. Most alpha particles passed through without deflection and some were back scattered [30]. [31] [29] Dictionary of Scientific Biography (1976), Charles Scribner's Sons, New York. [30] Steadman P. (2001), Peters K. F. , Isern H. , Ferrer S. , Phys. Rev. B 64. [31] Available at: http: //www 2. kutl. kyushu-u. ac. jp/seminar/Micro. World 1_E/Part 2_E/P 25_E/R_s

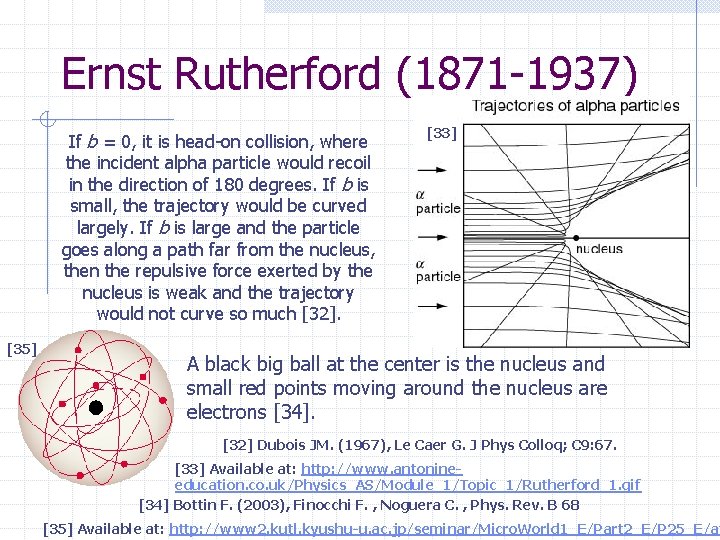
Ernst Rutherford (1871 -1937) If b = 0, it is head-on collision, where the incident alpha particle would recoil in the direction of 180 degrees. If b is small, the trajectory would be curved largely. If b is large and the particle goes along a path far from the nucleus, then the repulsive force exerted by the nucleus is weak and the trajectory would not curve so much [32]. [35] [33] A black big ball at the center is the nucleus and small red points moving around the nucleus are electrons [34]. [32] Dubois JM. (1967), Le Caer G. J Phys Colloq; C 9: 67. [33] Available at: http: //www. antonineeducation. co. uk/Physics_AS/Module_1/Topic_1/Rutherford_1. gif [34] Bottin F. (2003), Finocchi F. , Noguera C. , Phys. Rev. B 68 [35] Available at: http: //www 2. kutl. kyushu-u. ac. jp/seminar/Micro. World 1_E/Part 2_E/P 25_E/at
![Modern Atom Models 3 The Bohr Model of the Atom 34 Available at http Modern Atom Models 3) The Bohr Model of the Atom [34] Available at: http:](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-17.jpg)
Modern Atom Models 3) The Bohr Model of the Atom [34] Available at: http: //nobelprize. org/physics/laureates/1922/bohr. gif

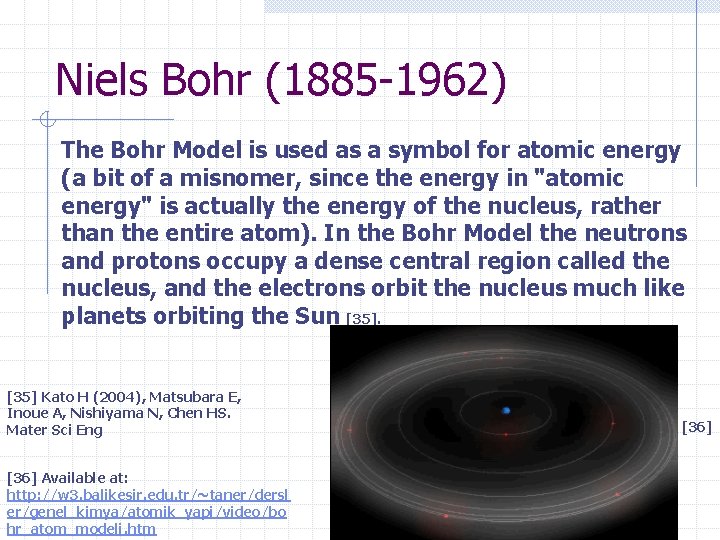
Niels Bohr (1885 -1962) The Bohr Model is used as a symbol for atomic energy (a bit of a misnomer, since the energy in "atomic energy" is actually the energy of the nucleus, rather than the entire atom). In the Bohr Model the neutrons and protons occupy a dense central region called the nucleus, and the electrons orbit the nucleus much like planets orbiting the Sun [35] Kato H (2004), Matsubara E, Inoue A, Nishiyama N, Chen HS. Mater Sci Eng [36] Available at: http: //w 3. balikesir. edu. tr/~taner/dersl er/genel_kimya/atomik_yapi/video/bo hr_atom_modeli. htm [36]

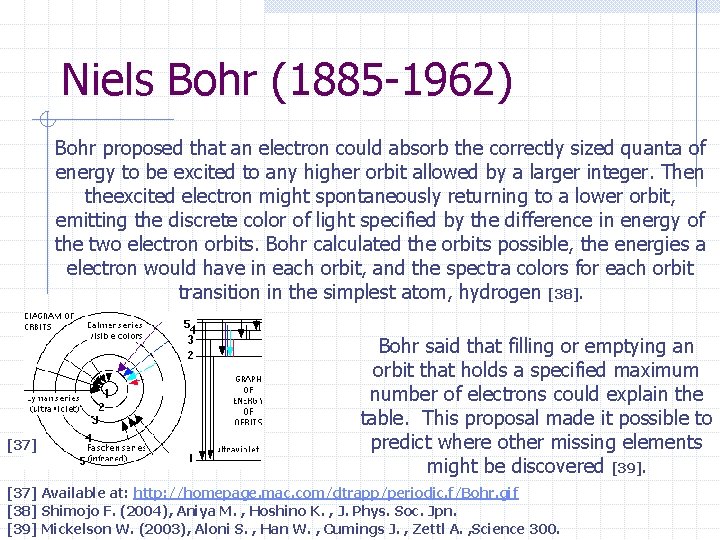
Niels Bohr (1885 -1962) Bohr proposed that an electron could absorb the correctly sized quanta of energy to be excited to any higher orbit allowed by a larger integer. Then theexcited electron might spontaneously returning to a lower orbit, emitting the discrete color of light specified by the difference in energy of the two electron orbits. Bohr calculated the orbits possible, the energies a electron would have in each orbit, and the spectra colors for each orbit transition in the simplest atom, hydrogen [38]. [37] Bohr said that filling or emptying an orbit that holds a specified maximum number of electrons could explain the table. This proposal made it possible to predict where other missing elements might be discovered [39]. [37] Available at: http: //homepage. mac. com/dtrapp/periodic. f/Bohr. gif [38] Shimojo F. (2004), Aniya M. , Hoshino K. , J. Phys. Soc. Jpn. [39] Mickelson W. (2003), Aloni S. , Han W. , Cumings J. , Zettl A. , Science 300.
![Modern Atom Models 4 Schroedinger Picture of the Atom 40 Available at http www Modern Atom Models 4) Schroedinger Picture of the Atom [40] Available at: http: //www.](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-20.jpg)
Modern Atom Models 4) Schroedinger Picture of the Atom [40] Available at: http: //www. pbs. org/wgbh/nova/photo 51/images/befo-schroedinger. jpg

Erwin Schroedinger (1887 -1961) • In 1926 he published the “Schroedinger Wave Equation” applied to the H atom [41]. • Bohr Orbits became resonances – stading electron waves [42]. • The electron’s mass and charge spread over space in a “cloud” of probability [43]. • Orbitals are like standing wave patterns with definite frequency (energy). [41] Bird R. B. (2002), Stewart W. E. , Lightfoot E. N. , Transport Phenomena, Wiley, New York [42] Jepps O. G. (2004), Bhatia S. K. , Searles D. J. , J. Chem. Phys. 120 [43] Ferrer S. (1995), Comin F. , Rev. Sci. Instrum. 66 1674.
![Erwin Schroedinger 1887 1961 44 Available at http physics bgsu edustonerP 202atomsimg 016 JPG Erwin Schroedinger (1887 -1961) [44] Available at: http: //physics. bgsu. edu/~stoner/P 202/atoms/img 016. JPG](https://slidetodoc.com/presentation_image_h/373ec7151ed7f98b90f041df7f6cfc36/image-22.jpg)
Erwin Schroedinger (1887 -1961) [44] Available at: http: //physics. bgsu. edu/~stoner/P 202/atoms/img 016. JPG

Any Questions?

Thank you for Listening to me. .