Institute of Organic Chemistry National Academy of Sciences






- Slides: 20

Institute of Organic Chemistry National Academy of Sciences of Ukraine Innovative Opportunities and Prospects Murmanska str. , 5, 02660, Кyiv-94 www. ioch. kiev. ua 1

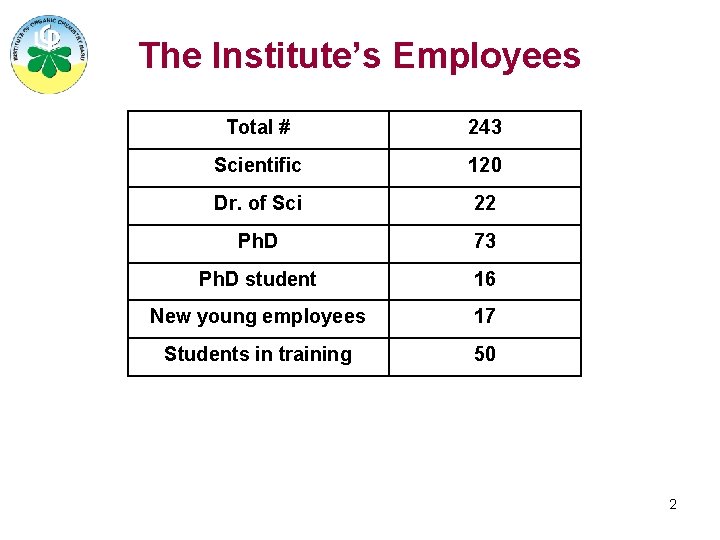
The Institute’s Employees Total # 243 Scientific 120 Dr. of Sci 22 Ph. D 73 Ph. D student 16 New young employees 17 Students in training 50 2

The Institute’s Scientific Department 1. Organophosphorus Compounds 2. Heteroatom Chemistry 3. Colour and Structure of Organic Compounds 4. Mechanisms of Organic Reactions 5. Chemistry of Phosphoranes 6. Chemistry of Bio-active Compounds 7. Physico-chemical Investigations 8. Chemistry of Organo-fluorine Compounds 9. Chemistry of Organo-sulfur Compounds 10. Bio-medical Investigations 3

The Institute’s Basic Research • fine organic synthesis • organoelement chemistry (P, S, Si) • heterocyclic chemistry • fluoroorganic chemistry • polymethyne dyes, colour theory • mechanisms of organic reactions • chemistry of biologically active compounds • supramolecular chemistry • calixarene chemistry 4

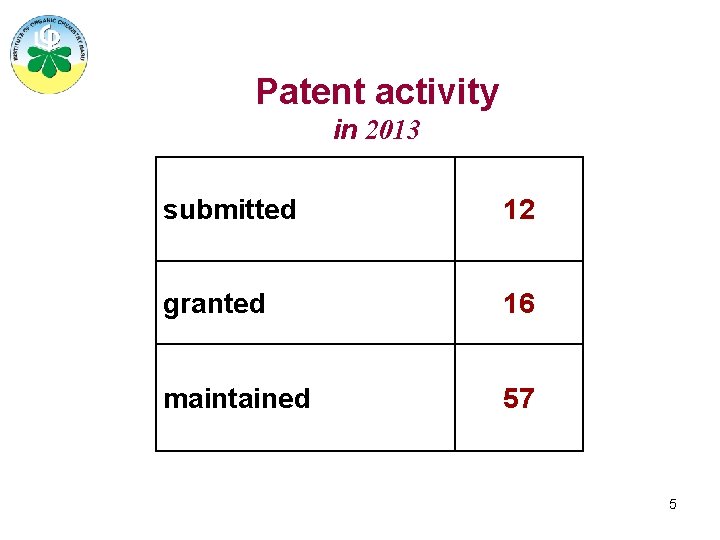
Patent activity in 2013 submitted 12 granted 16 maintained 57 5

Fluorination reagents and processes 6

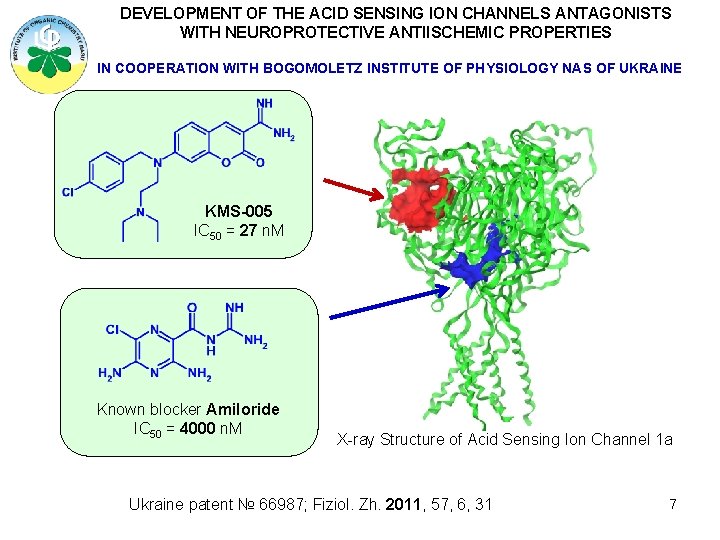
DEVELOPMENT OF THE ACID SENSING ION CHANNELS ANTAGONISTS WITH NEUROPROTECTIVE ANTIISCHEMIC PROPERTIES IN COOPERATION WITH BOGOMOLETZ INSTITUTE OF PHYSIOLOGY NAS OF UKRAINE KMS-005 IC 50 = 27 n. M Known blocker Amiloride IC 50 = 4000 n. M X-ray Structure of Acid Sensing Ion Channel 1 a Ukraine patent № 66987; Fiziol. Zh. 2011, 57, 6, 31 7

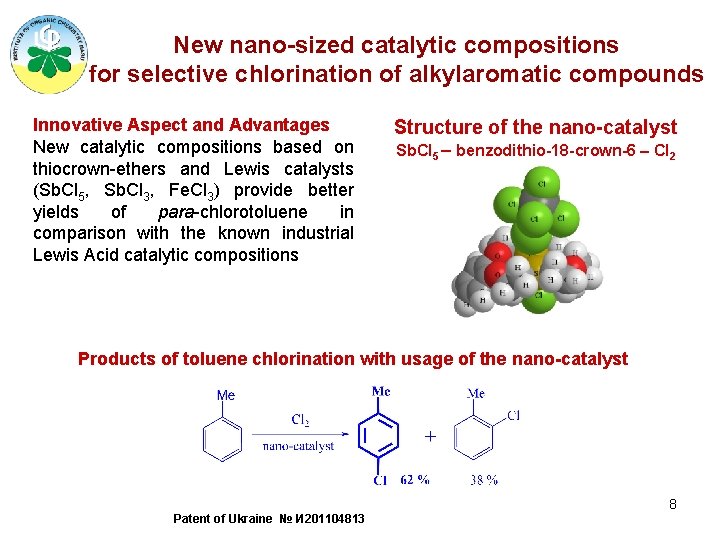
New nano-sized catalytic compositions for selective chlorination of alkylaromatic compounds Innovative Aspect and Advantages New catalytic compositions based on thiocrown-ethers and Lewis catalysts (Sb. Cl 5, Sb. Cl 3, Fe. Cl 3) provide better yields of para-chlorotoluene in comparison with the known industrial Lewis Acid catalytic compositions Structure of the nano-catalyst Sb. Cl 5 – benzodithio-18 -crown-6 – Cl 2 Products of toluene chlorination with usage of the nano-catalyst 8 Patent of Ukraine № И 201104813

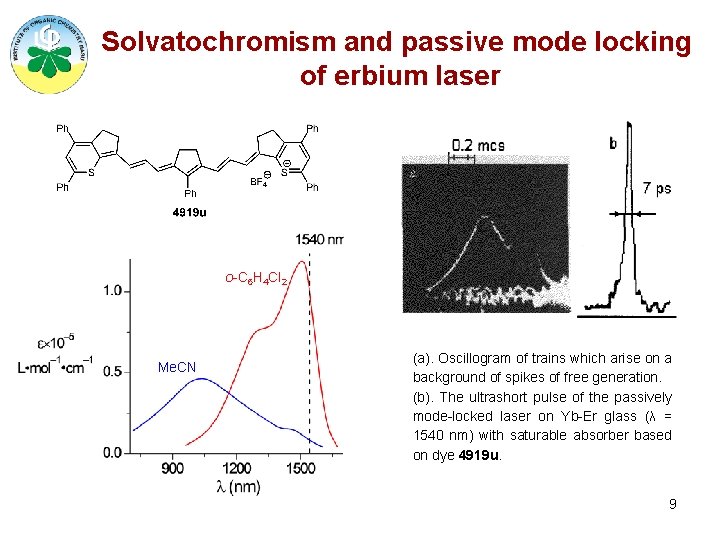
Solvatochromism and passive mode locking of erbium laser o-C 6 H 4 Cl 2 Me. CN (a). Oscillogram of trains which arise on a background of spikes of free generation. (b). The ultrashort pulse of the passively mode-locked laser on Yb-Er glass (λ = 1540 nm) with saturable absorber based on dye 4919 u. 9

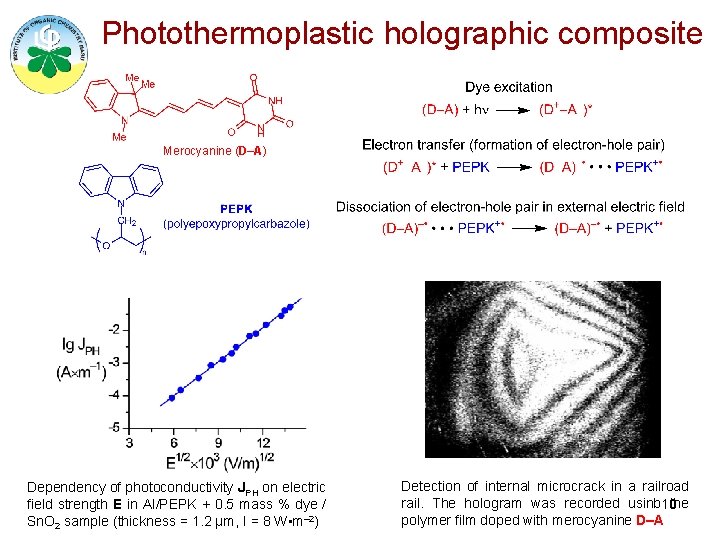
Photothermoplastic holographic composite Merocyanine (D–A) Dependency of photoconductivity JPH on electric field strength E in Al/PEPK + 0. 5 mass % dye / Sn. O 2 sample (thickness = 1. 2 μm, I = 8 W • m– 2) Detection of internal microcrack in a railroad rail. The hologram was recorded usinb 10 the polymer film doped with merocyanine D–A

CALIXARENE RECEPTORS Calixarenes selectively recognize, bind and separate similar in properties species 11

Institute of Organic Chemistry NAS Ukraine Calixarene R&D Research Design of selective (chiral) receptors of cations, anions, molecules and bio-molecules Development Radionuclide Extractants Chemosensors Drug Design J. Am. Chem. Soc. 2007. 129. 1123 -1131. J. Org. Chem. 2007. 72. 3223 -3231. Pure Appl. Chem. 2008. 80. 1449 -1458 J. Phys. Chem. C. 2009. 1137 -1142 Curr. Med. Chem. 2009. 1630 -1655 Bioorg. Med. Chem. Lett. 2010, 20, 483– 487 J. of Catalysis. 2011. 284. 42 -49 FEBS Journal. 2011. 278. 1244 -1251 12

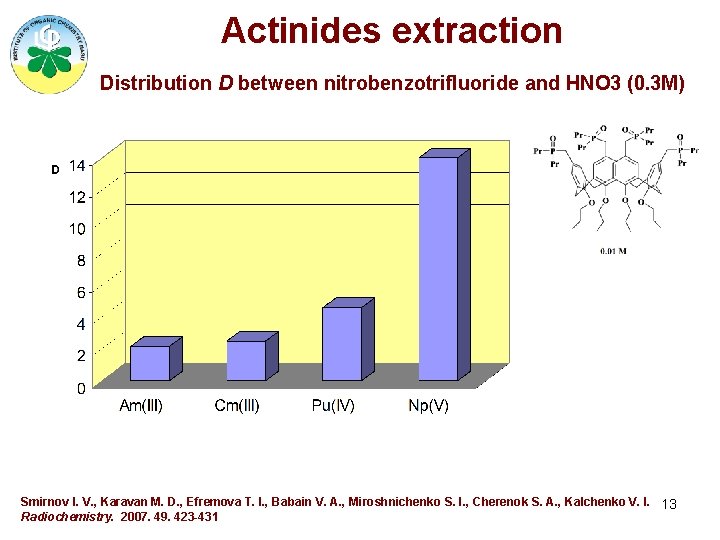
Actinides extraction Distribution D between nitrobenzotrifluoride and HNO 3 (0. 3 M) Smirnov I. V. , Karavan M. D. , Efremova T. I. , Babain V. A. , Miroshnichenko S. I. , Cherenok S. A. , Kalchenko V. I. 13 Radiochemistry. 2007. 49. 423 -431

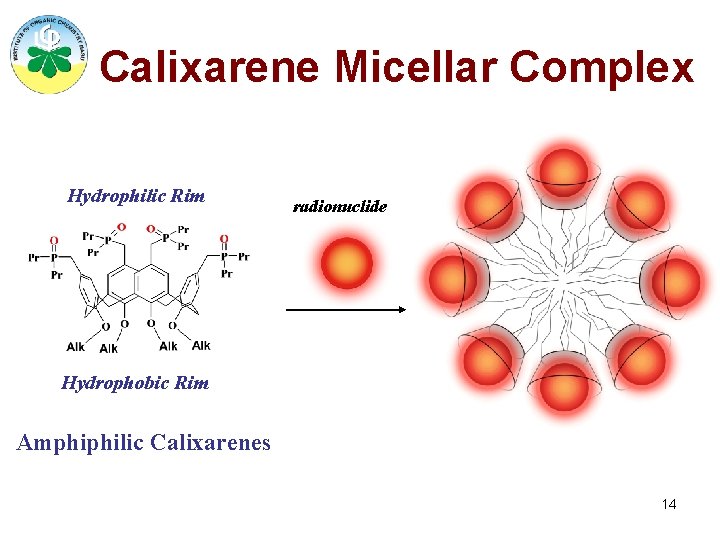
Calixarene Micellar Complex Hydrophilic Rim radionuclide Hydrophobic Rim Amphiphilic Calixarenes 14

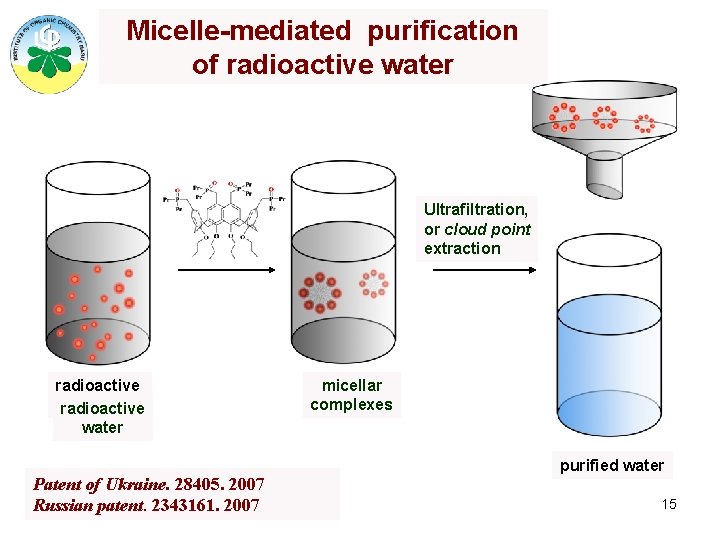
Micelle-mediated purification of radioactive water Ultrafiltration, or cloud point extraction radioactive water Patent of Ukraine. 28405. 2007 Russian patent. 2343161. 2007 micellar complexes purified water 15

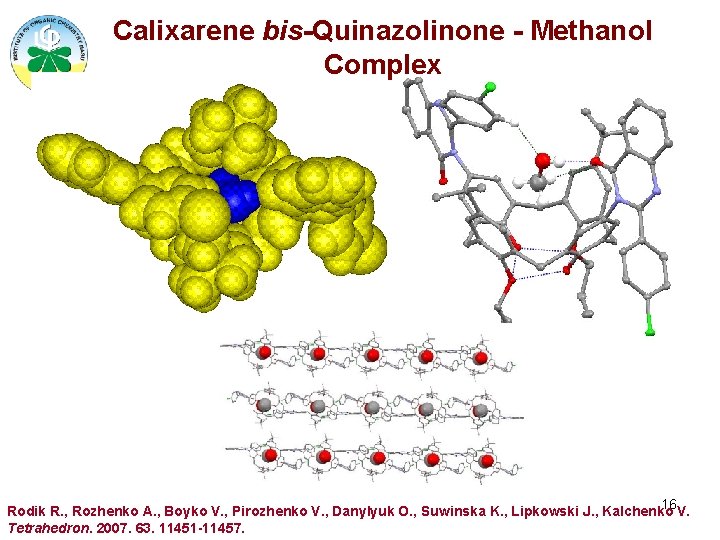
Calixarene bis-Quinazolinone - Methanol Complex 16 Rodik R. , Rozhenko A. , Boyko V. , Pirozhenko V. , Danylyuk O. , Suwinska K. , Lipkowski J. , Kalchenko V. Tetrahedron. 2007. 63. 11451 -11457.

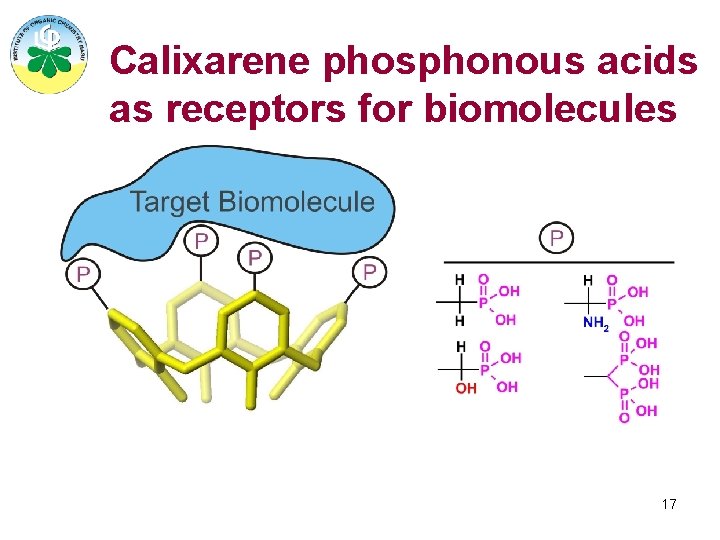
Calixarene phosphonous acids as receptors for biomolecules 17


