INSTANTGMP ELECTRONIC BATCH RECORDS FOR GMP MANUFACTURING SOFTWARE

INSTANTGMP™ ELECTRONIC BATCH RECORDS FOR GMP MANUFACTURING SOFTWARE DEMONSTRATION Instant. GMP: Electronic Batch Records System for GMP Manufacturing

c. GMP: Challenges of Traditional Systems GMP manufacturing is a complex process. Wide array of regulatory requirements Long time and high cost to set up a quality system Long cycle times for QA reviews and corrections Training on SOPs is lengthy Keeping track of Master Production Records and Batch Production Records is laborious GMP compliance can be resource intensive Instant. GMP™ is the GMP manufacturing system that has been built from the ground up to meet these challenges Instant. GMP: Electronic Batch Records System for GMP Manufacturing

Instant. GMP™ - A new approach to GMP manufacturing Electronic Batch Records System for GMP Manufacturing Seamlessly incorporates everything necessary for GMP manufacturing in one place Cloud-based application makes all data visible to everyone at all times Uses built-in quality procedures to make GMP compliance easy Provides more flexibility, visibility and productivity than paper based manufacturing Instant. GMP: Electronic Batch Records System for GMP Manufacturing

Instant. GMP starts with the Quality System Structure First step was design of the quality system structure Next was writing SOPs and Policies that link all GMP manufacturing processes together Finally writing comprehensive manufacturing software with GMP compliance built-in Instant. GMP: Electronic Batch Records System for GMP Manufacturing 4

Automated Compliance c. GMP compliance controls are built into the system Part numbers automatically assigned to all materials Materials quarantine/restricted until QA approval of vendors and specifications Specifications system has automated version control Purchasing restricted to approved materials and vendors Master Batch Records have version control Digital signatures at specified batch record steps record operator and date/time Deviations must be approved before batch is released Limited access based on user/role security settings Complete automated audit trail Instant. GMP: Electronic Batch Records System for GMP Manufacturing

Materials Only QA approved materials from approved vendors can be used in GMP manufacturing Containers/Closures Excipients Components Instant. GMP: Electronic Batch Records System for GMP Manufacturing Work in Process 6

Getting ready for manufacturing – Control of Materials Instant. GMP: Electronic Batch Records System for GMP Manufacturing 7

Automatic Control Automated rules and entries, that are based on the SOPs, are integrated in the system. Some of the many features: System assigns lot numbers upon receipt QA enters and controls raw material status based on QC Lab input System issues purchase requisitions Production can only use approved raw materials Drop downs for entries in batch records ensure accuracy Electronic signatures allow remote, easy approvals Security settings limit access to only authorized personnel Instant. GMP: Electronic Batch Records System for GMP Manufacturing

Rooms and Equipment Room List – names of manufacturing rooms Room Log – record of activities in rooms, e. g. Cleaning Maintenance Batch use Equipment List – names of production equipment Shows up in drop down lists in batch records Equipment Log – similar to Room Log Instant. GMP: Electronic Batch Records System for GMP Manufacturing 9

Specifications QA shall approve specifications of materials and components Test - A measurement of a quality attribute such as Method - The procedure by which the quality Limit - The acceptable range for the attribute potency or water content attribute is measured Other Requirements Sampling Instructions Safety and Handling Instant. GMP: Electronic Batch Records System for GMP Manufacturing 10

Process Flow – Material Management Instant. GMP: Electronic Batch Records System for GMP Manufacturing 11

Process Flow - Production Instant. GMP: Electronic Batch Records System for GMP Manufacturing 12

Tracking of Inventory Distribution Instant. GMP: Electronic Batch Records System for GMP Manufacturing 13
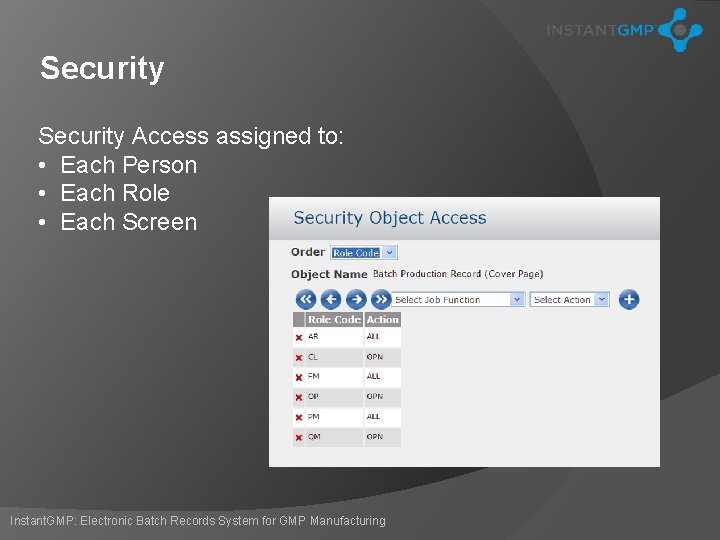
Security Access assigned to: • Each Person • Each Role • Each Screen Instant. GMP: Electronic Batch Records System for GMP Manufacturing

Quick Facts Developed by the manufacturing and quality experts at Pharma. Directions Integrated internet based application developed through “Quality by Design” approach Standard Operating Procedures and Policies come with the application 21 CFR Part 11 Compliant System has been in use since 2004 Instant. GMP: Electronic Batch Records System for GMP Manufacturing

Instant. GMP™ Summary Electronic Batch Records for GMP manufacturing Takes full advantage of cloud-based application 21 CFR Part 11 compliant database with SOP requirements hard coded into the software Streamlines entire process of producing GMP materials Simplifies the documentation and approval procedures to reduce production time lines Ideally suited for any GMP manufacturing such as APIs, drugs, OTCs, generics and dietary supplements For more information, contact: JPittman@Instant. GMP. com Instant. GMP: Electronic Batch Records System for GMP Manufacturing

Instant. GMP™ www. instantgmp. com Electronic Batch Records for GMP Manufacturing Instant. GMP: Electronic Batch Records System for GMP Manufacturing
- Slides: 17