Inside the Atom Lets Investigate the Atom Atoms






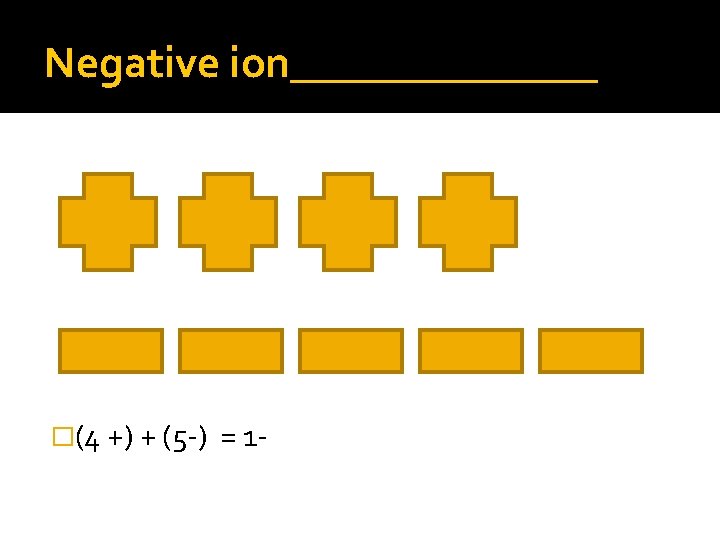



- Slides: 18

Inside the Atom

Let’s Investigate the Atom

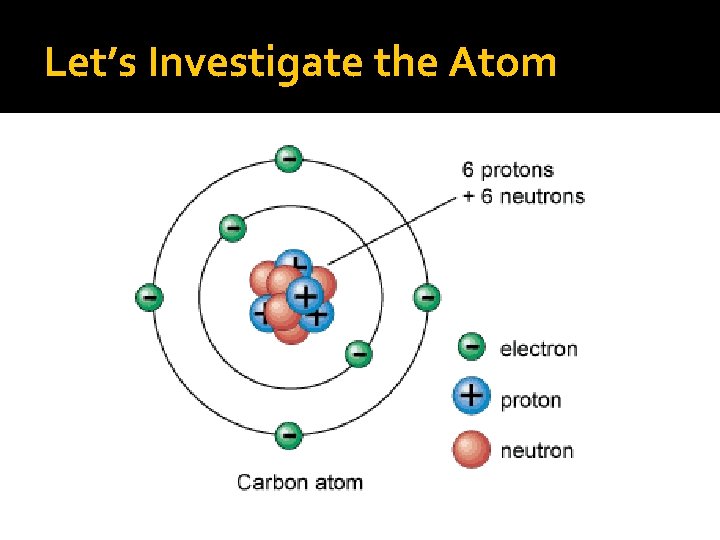
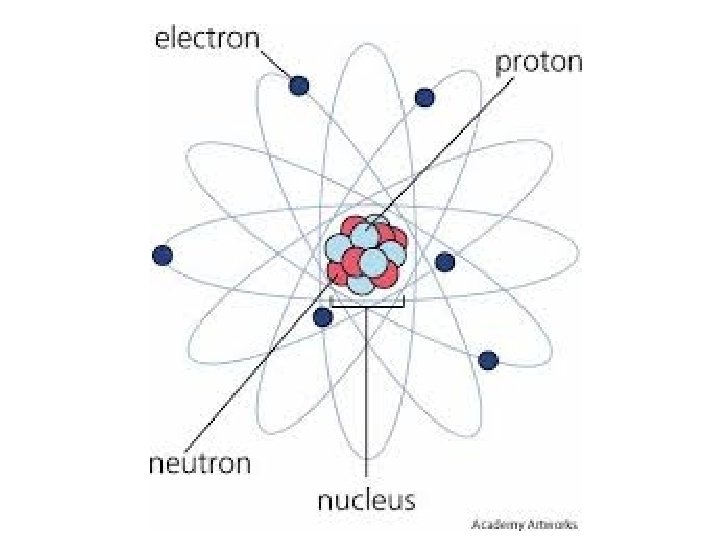
Atoms are composed of three type of particles: SUBATOMIC PARTICLES: protons, neutrons, and electron. Protons and neutrons are responsible for most of the atomic mass of an atom, while electrons contribute a very small amount of mass(9. 108 X 10 -28 grams).

What makes up the atom? Protons and neutrons reside in the nucleus. Protons have a positive (+) charge, neutrons have no charge. They are neutral. Electrons are in orbitals, or electron clouds, around the nucleus. They have a negative charge (-).


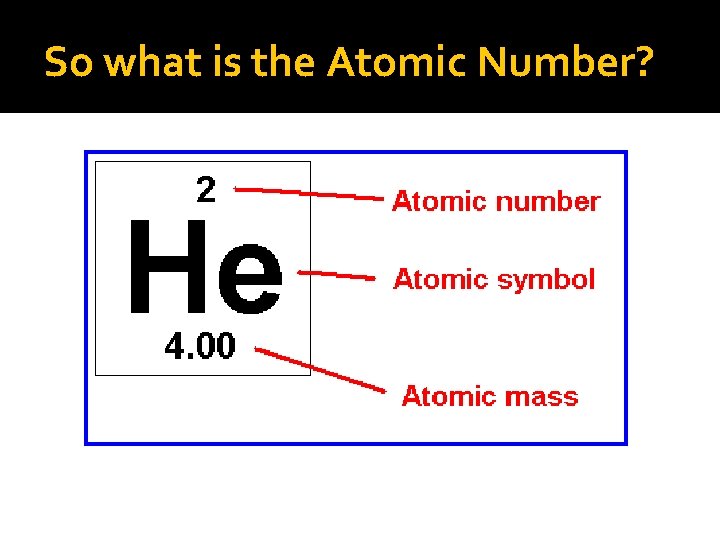
Atomic Number The number of _____ in the nucleus of an atom determines an element's atomic number.

So what is the Atomic Number?

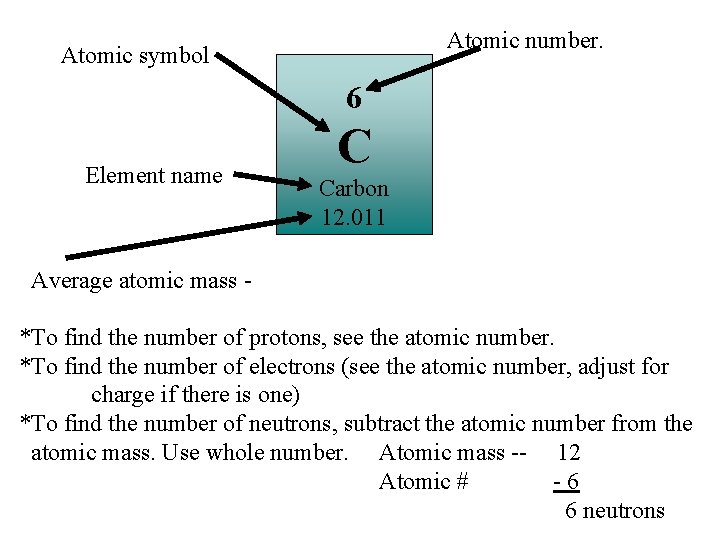
Atomic number. Atomic symbol 6 Element name C Carbon 12. 011 Average atomic mass *To find the number of protons, see the atomic number. *To find the number of electrons (see the atomic number, adjust for charge if there is one) *To find the number of neutrons, subtract the atomic number from the atomic mass. Use whole number. Atomic mass -- 12 Atomic # -6 6 neutrons

The number of electrons, protons, and neutrons can be changed in an atom and changes the atom!

I. Changing Electrons �An atom that changes the number of electrons is called an _____. �The number of ____ and ____ stays the same. No change in atomic number or ______________.
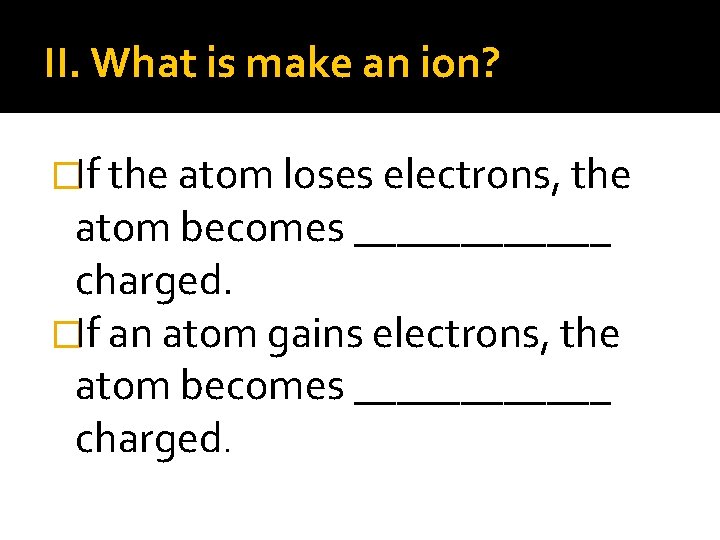
II. What is make an ion? �If the atom loses electrons, the atom becomes ______ charged. �If an atom gains electrons, the atom becomes ______ charged.

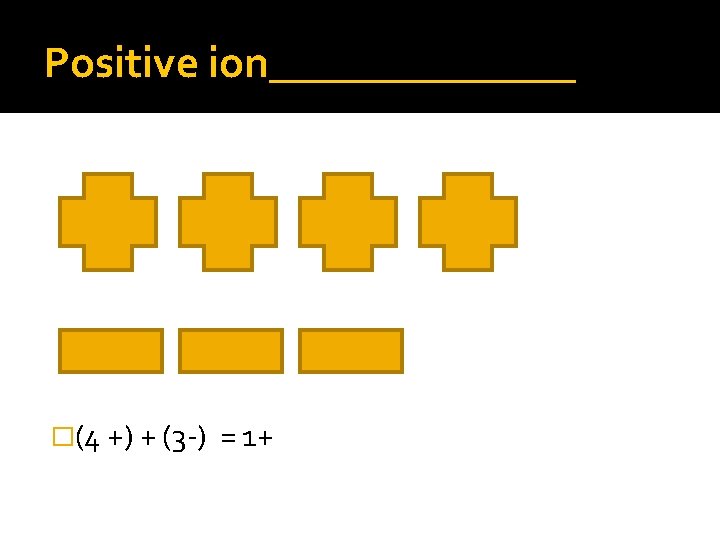
Positive ion_______ �(4 +) + (3 -) = 1+
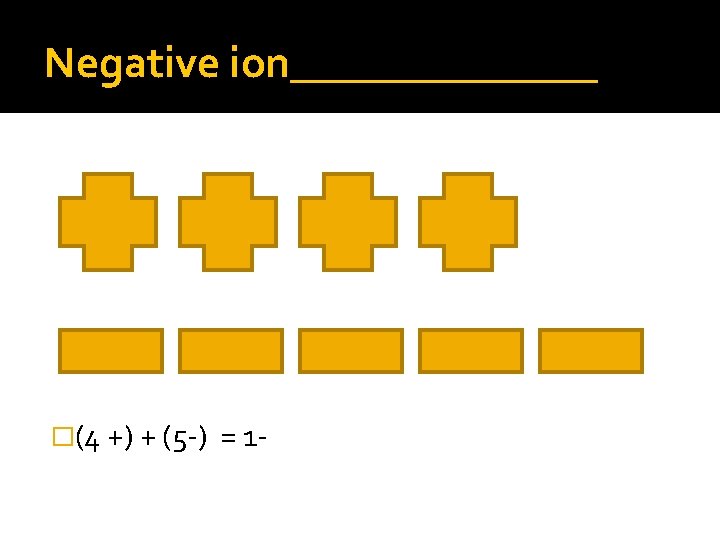
Negative ion_______ �(4 +) + (5 -) = 1 -

III. Changing Neutrons �Changing the number of neutrons in an atom makes an ________. �Have the same _____________ �Have different mass numbers (number of _____ + ______)

IV. Changing Protons �Changing the number of protons causes ________- changing from one element to another. �The _________and _________ changes

Sample Problem 1: For an atom with 15 protons, 16 neutrons, and 18 electrons… � What is the atom’s net charge? � What is the atomic number of the atom? What is the mass number? � This is an atom of what element?

Sample Problem 2: For an atom with 28 protons, 31 neutrons, and 26 electrons… � What is the atom’s net charge? � What is the atomic number of the atom? What is the mass number? � This is an atom of what element?

Vocabulary 79. Democritus 80. John Dalton 81. Atomic theory 82. J. J. Thomason 83. Ernest Rutherford 84. Niels Bohr 85. James Chadwick 86. Electron clouds 87. Nucleus 88. Protons 89. Neutrons 90. Electrons 91. Atomic mass 92. Atomic number 93. Ion 94. Isotope 95. Transmutation