Inorganic structure prediction too much and not enough














![Aluminoborates Example : [Al. B 4 O 9]-2, cubic, SG : Pn-3, a = Aluminoborates Example : [Al. B 4 O 9]-2, cubic, SG : Pn-3, a =](https://slidetodoc.com/presentation_image_h2/dcfc21bc4dd84834d096bc126870f7b5/image-40.jpg)
![Unknown : PCOD 1010005 - [Ca 3 Al 4 F 21]3 - Unknown : PCOD 1010005 - [Ca 3 Al 4 F 21]3 -](https://slidetodoc.com/presentation_image_h2/dcfc21bc4dd84834d096bc126870f7b5/image-42.jpg)




- Slides: 59

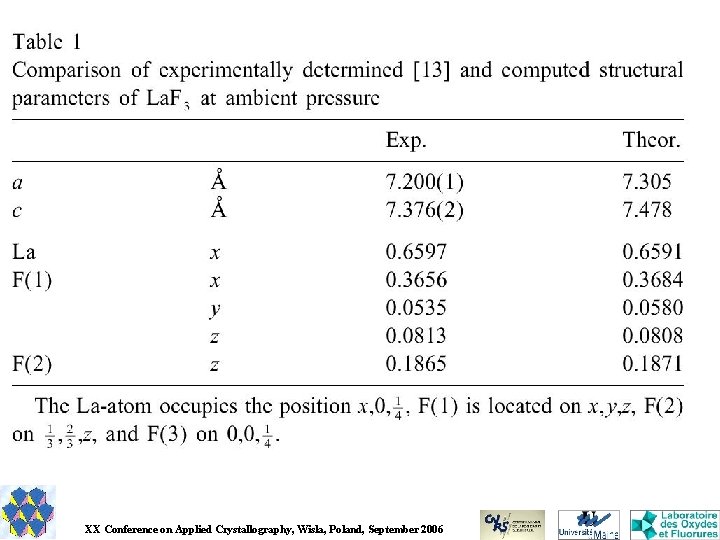
Inorganic structure prediction : too much and not enough Armel Le Bail Université du Maine, Laboratoire des oxydes et Fluorures, CNRS UMR 6010, Avenue O. Messiaen, 72085 Le Mans Cedex 9, France. Email : alb@cristal. org XX Conference on Applied Crystallography, Wisla, Poland, September 2006

CONTENTS - Introduction - Prediction software and examples - More examples from the GRINSP software (especially Al. F 3 polymorphs and titanosilicates) - Opened doors, limitations, problems - Conclusion XX Conference on Applied Crystallography, Wisla, Poland, September 2006

INTRODUCTION Personnal views about crystal structure prediction : “Exact” description before synthesis or discovery in nature. These “exact” descriptions should be used for the calculation of powder patterns included in a database for automatic identification of real compounds not yet characterized crystallographycally. XX Conference on Applied Crystallography, Wisla, Poland, September 2006

Where are we with inorganic crystal structure prediction? If the state of the art had dramatically evolved in the past ten years, we should have huge databases of predicted compounds, and not any new crystal structure would surprise us since it would corespond already to an entry in that database. Moreover, we would have obtained in advance the physical properties and we would have preferably synthesized those interesting compounds. Of course, this is absolutely not the case. XX Conference on Applied Crystallography, Wisla, Poland, September 2006

But things are changing, maybe : Two databases of hypothetical compounds were built in 2004. One is exclusively devoted to zeolites : M. D. Foster & M. M. J. Treacy - Hypothetical Zeolites – http: //www. hypotheticalzeolites. net/ The other includes zeolites as well as other predicted oxides (phosphates, borosilicates, etc) and fluorides : the PCOD (Predicted Crystallography Open Database) http: //www. crystallography. net/pcod/ XX Conference on Applied Crystallography, Wisla, Poland, September 2006

Prediction software Especially recommended lectures (review papers) : 1 - S. M. Woodley, in: Application of Evolutionary Computation in Chemistry, R. L. Johnston (ed), Structure and bonding series, Springer. Verlag 110 (2004) 95 -132. 2 - J. C. Schön & M. Jansen, Z. Krist. 216 (2001) 307 -325; 361 -383. Software : CASTEP, program for Zeolites, GULP, G 42, Spuds, AASBU, GRINSP XX Conference on Applied Crystallography, Wisla, Poland, September 2006

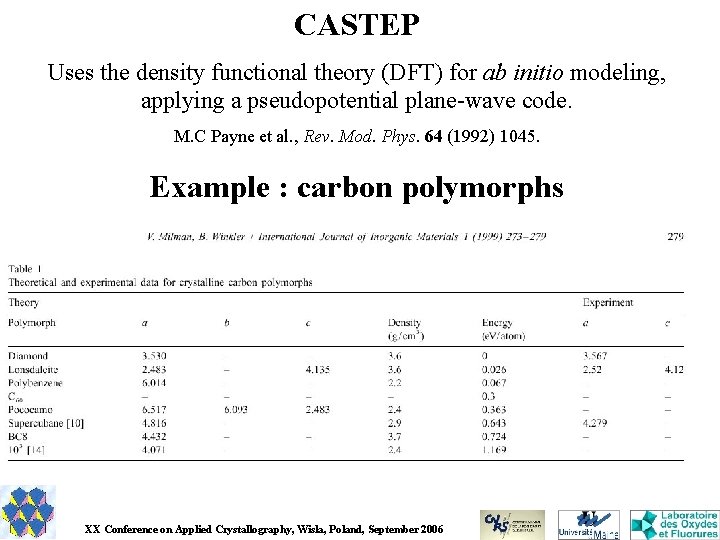
CASTEP Uses the density functional theory (DFT) for ab initio modeling, applying a pseudopotential plane-wave code. M. C Payne et al. , Rev. Mod. Phys. 64 (1992) 1045. Example : carbon polymorphs XX Conference on Applied Crystallography, Wisla, Poland, September 2006

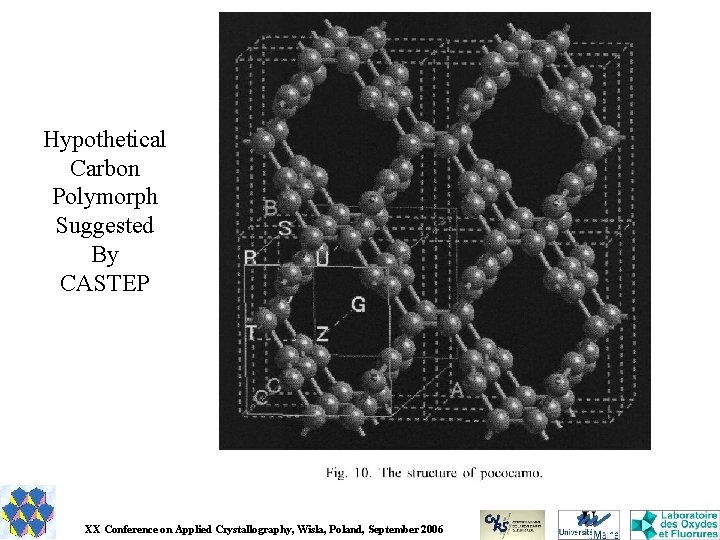
Hypothetical Carbon Polymorph Suggested By CASTEP XX Conference on Applied Crystallography, Wisla, Poland, September 2006

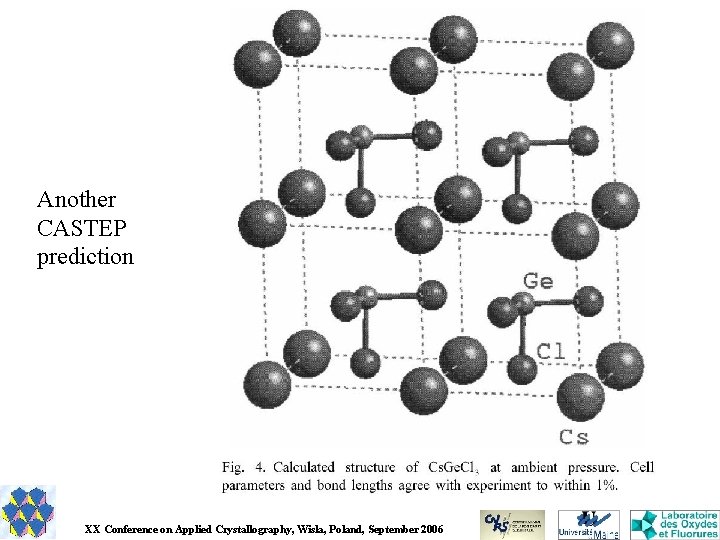
Another CASTEP prediction XX Conference on Applied Crystallography, Wisla, Poland, September 2006

XX Conference on Applied Crystallography, Wisla, Poland, September 2006

XX Conference on Applied Crystallography, Wisla, Poland, September 2006

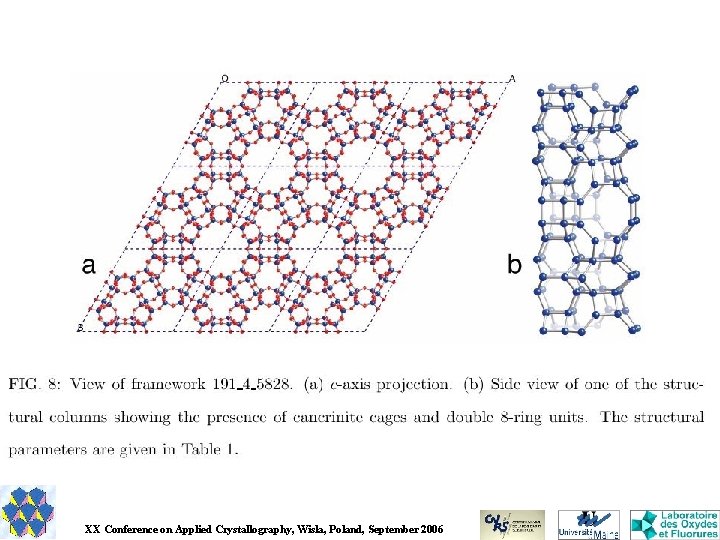
ZEOLITES The structures gathered in the database of hypothetical zeolites are produced from a 64 -processor computer cluster grinding away non-stop, generating graphs and annealing them, the selected frameworks being then re-optimized using the General Utility Lattice Program (GULP, written by Julian Gale) using atomic potentials. M. D. Foster & M. M. J. Treacy - Hypothetical Zeolites – http: //www. hypotheticalzeolites. net/ XX Conference on Applied Crystallography, Wisla, Poland, September 2006


XX Conference on Applied Crystallography, Wisla, Poland, September 2006

XX Conference on Applied Crystallography, Wisla, Poland, September 2006

XX Conference on Applied Crystallography, Wisla, Poland, September 2006

Zeolite predictions are probably too much… Less than 200 zeotypes are known Less than 10 new zeotypes are discovered every year Less than half of them are listed in that >1. 000 database So that zeolite predictions will continue up to attain several millions more… Quantum chemistry validation of these prediction is required, not only empirical energy calculations, for elimination of a large number of models that will certainly never be confirmed.

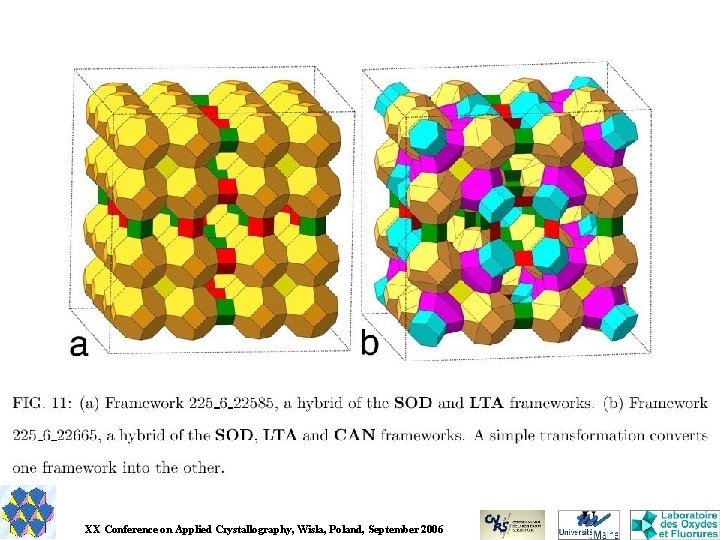
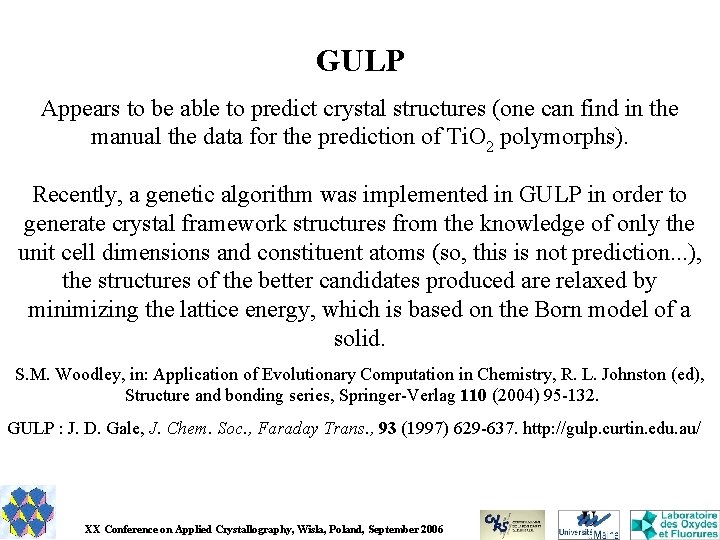
GULP Appears to be able to predict crystal structures (one can find in the manual the data for the prediction of Ti. O 2 polymorphs). Recently, a genetic algorithm was implemented in GULP in order to generate crystal framework structures from the knowledge of only the unit cell dimensions and constituent atoms (so, this is not prediction. . . ), the structures of the better candidates produced are relaxed by minimizing the lattice energy, which is based on the Born model of a solid. S. M. Woodley, in: Application of Evolutionary Computation in Chemistry, R. L. Johnston (ed), Structure and bonding series, Springer-Verlag 110 (2004) 95 -132. GULP : J. D. Gale, J. Chem. Soc. , Faraday Trans. , 93 (1997) 629 -637. http: //gulp. curtin. edu. au/ XX Conference on Applied Crystallography, Wisla, Poland, September 2006

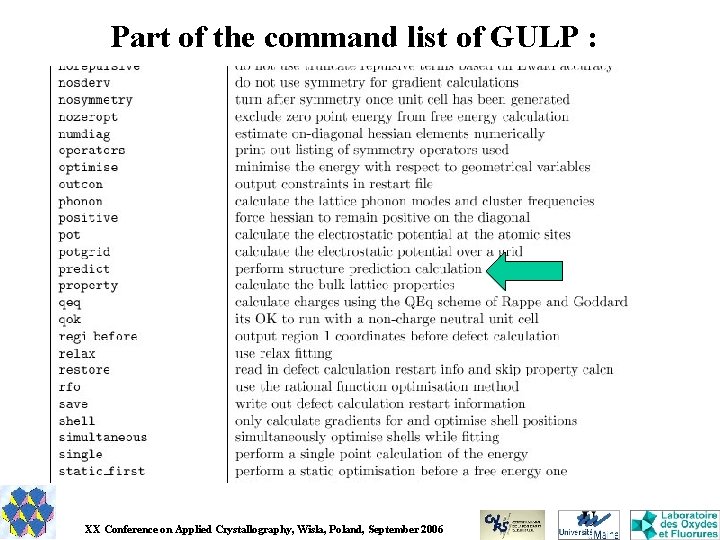
Part of the command list of GULP : XX Conference on Applied Crystallography, Wisla, Poland, September 2006

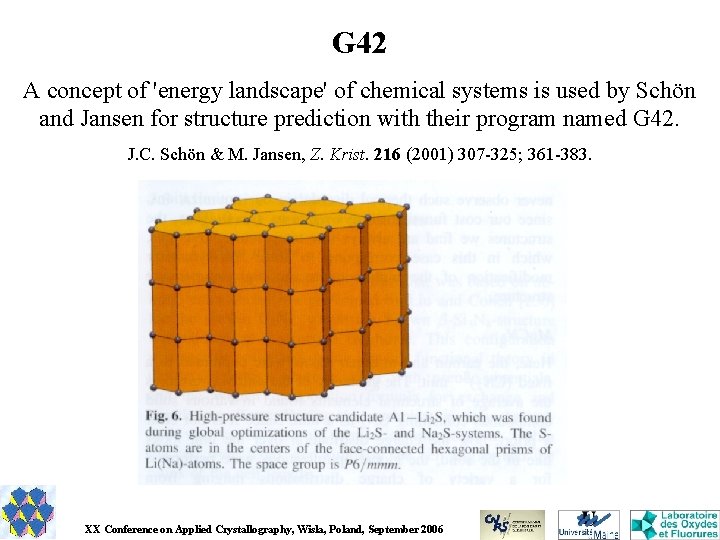
G 42 A concept of 'energy landscape' of chemical systems is used by Schön and Jansen for structure prediction with their program named G 42. J. C. Schön & M. Jansen, Z. Krist. 216 (2001) 307 -325; 361 -383. XX Conference on Applied Crystallography, Wisla, Poland, September 2006

XX Conference on Applied Crystallography, Wisla, Poland, September 2006

XX Conference on Applied Crystallography, Wisla, Poland, September 2006

XX Conference on Applied Crystallography, Wisla, Poland, September 2006

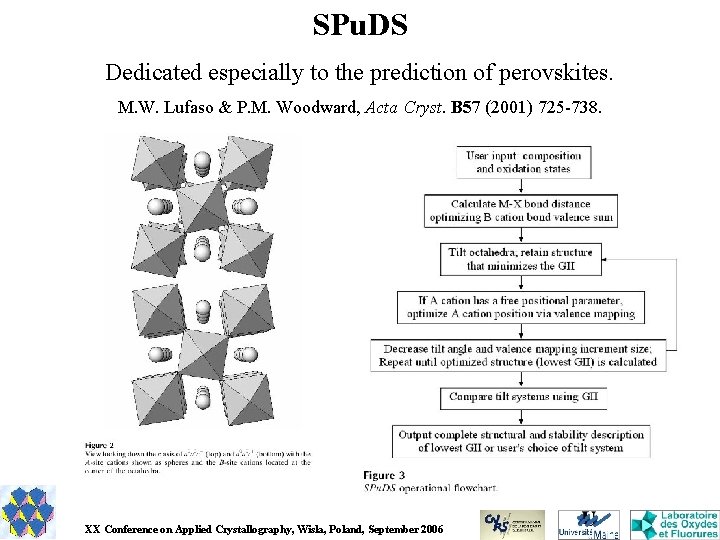
SPu. DS Dedicated especially to the prediction of perovskites. M. W. Lufaso & P. M. Woodward, Acta Cryst. B 57 (2001) 725 -738. XX Conference on Applied Crystallography, Wisla, Poland, September 2006

AASBU method (Automated Assembly of Secondary Building Units) Developed by Mellot-Draznieks et al. , C. Mellot-Drazniek, J. M. Newsam, A. M. Gorman, C. M. Freeman & G. Férey, Angew. Chem. Int. Ed. 39 (2000) 2270 -2275; C. Mellot-Drazniek, S. Girard, G. Férey, C. Schön, Z. Cancarevic, M. Jansen, Chem. Eur. J. 8 (2002) 4103 -4113. Using Cerius 2 and GULP in a sequence of simulated annealing plus minimization steps for the aggregation of large structural motifs. Cerius 2, Version 4. 2, Molecular Simulations Inc. , Cambridge, UK, 2000. XX Conference on Applied Crystallography, Wisla, Poland, September 2006

XX Conference on Applied Crystallography, Wisla, Poland, September 2006

XX Conference on Applied Crystallography, Wisla, Poland, September 2006

XX Conference on Applied Crystallography, Wisla, Poland, September 2006

Not enough If zeolites are excluded, the productions of these prediction software a few dozen… not enough, not available in any database. A recent (2005) prediction program is able to extend the investigations to larger series of inorganic compounds characterized by corner-sharing polyhedra.

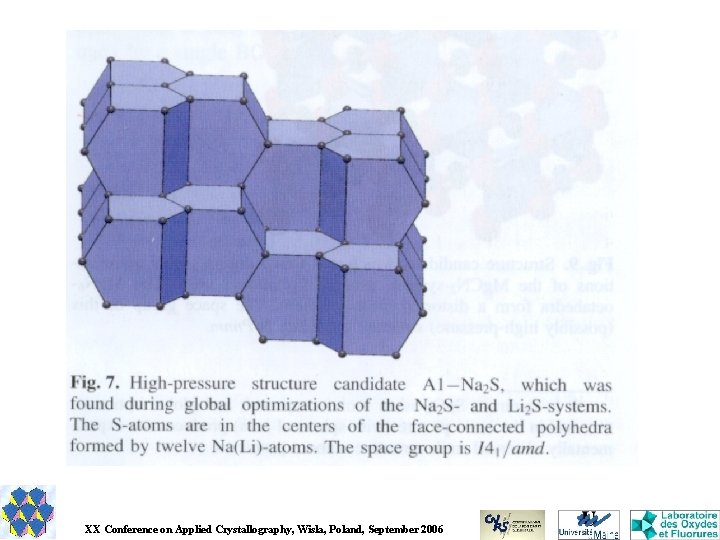
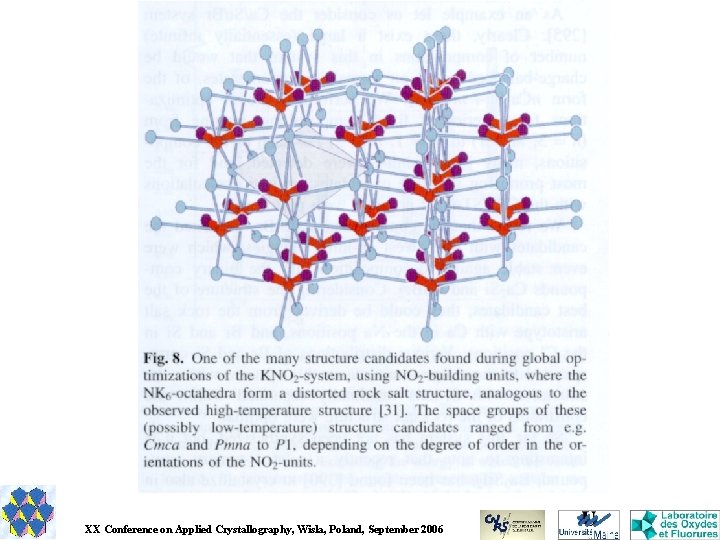
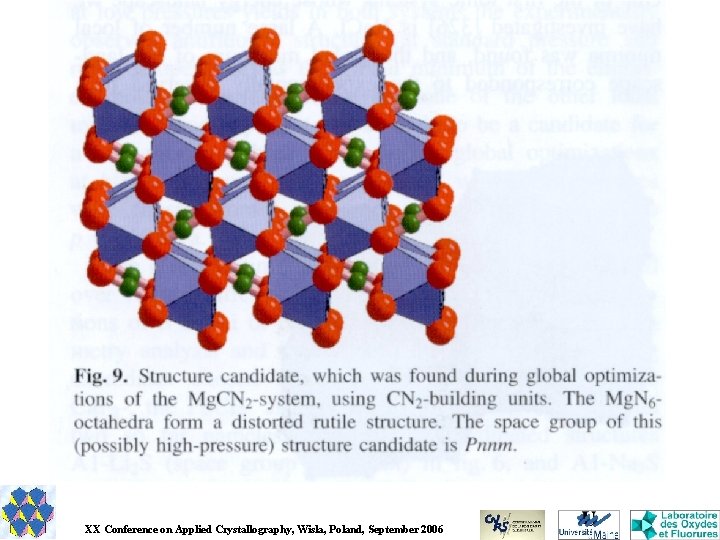
GRINSP Geometrically Restrained INorganic Structure Prediction Applies the knowledge about the geometrical characteristics of a particular group of inorganic crystal structures (N-connected 3 D networks with N = 3, 4, 5, 6, for one or two N values). Explores that limited and special space (exclusive corner-sharing polyhedra) by a Monte Carlo approach. The cost function is very basic, depending on weighted differences between ideal and calculated interatomic distances for first neighbours M-X, X-X and M-M for binary Ma. Xb or ternary Ma. M'b. Xc compounds. J. Appl. Cryst. 38, 2005, 389 -395. J. Solid State Chem. 179, 2006, 3159 -3166.

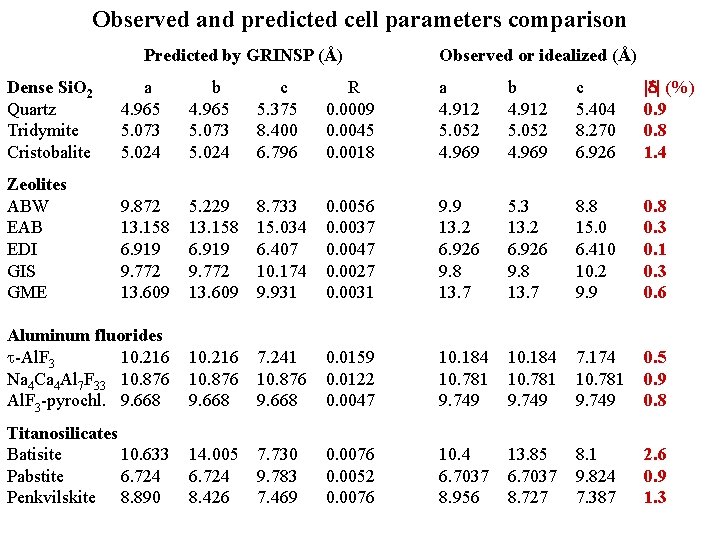
Observed and predicted cell parameters comparison Predicted by GRINSP (Å) Observed or idealized (Å) Dense Si. O 2 Quartz Tridymite Cristobalite a 4. 965 5. 073 5. 024 b 4. 965 5. 073 5. 024 c 5. 375 8. 400 6. 796 R 0. 0009 0. 0045 0. 0018 a 4. 912 5. 052 4. 969 b 4. 912 5. 052 4. 969 c 5. 404 8. 270 6. 926 (%) 0. 9 0. 8 1. 4 Zeolites ABW EAB EDI GIS GME 9. 872 13. 158 6. 919 9. 772 13. 609 5. 229 13. 158 6. 919 9. 772 13. 609 8. 733 15. 034 6. 407 10. 174 9. 931 0. 0056 0. 0037 0. 0047 0. 0027 0. 0031 9. 9 13. 2 6. 926 9. 8 13. 7 5. 3 13. 2 6. 926 9. 8 13. 7 8. 8 15. 0 6. 410 10. 2 9. 9 0. 8 0. 3 0. 1 0. 3 0. 6 Aluminum fluorides -Al. F 3 10. 216 Na 4 Ca 4 Al 7 F 33 10. 876 Al. F 3 -pyrochl. 9. 668 10. 216 10. 876 9. 668 7. 241 10. 876 9. 668 0. 0159 0. 0122 0. 0047 10. 184 10. 781 9. 749 7. 174 10. 781 9. 749 0. 5 0. 9 0. 8 Titanosilicates Batisite 10. 633 Pabstite 6. 724 Penkvilskite 8. 890 14. 005 6. 724 8. 426 7. 730 9. 783 7. 469 0. 0076 0. 0052 0. 0076 10. 4 6. 7037 8. 956 13. 85 6. 7037 8. 727 8. 1 9. 824 7. 387 2. 6 0. 9 1. 3

Predictions produced by GRINSP Binary compounds Formulations M 2 X 3, MX 2, M 2 X 5 et MX 3 were examined. Zeolites MX 2 (= 4 -connected 3 D nets) More than 1000 zeolites (not 1. 000) are proposed with cell parameters < 16 Å, placed into the PCOD database : http: //www. crystallography. net/pcod/ GRINSP recognizes a zeotype by comparing the coordination sequences (CS) of a model with a previously established list of CS and with the CS of the models already proposed during the current calculation).

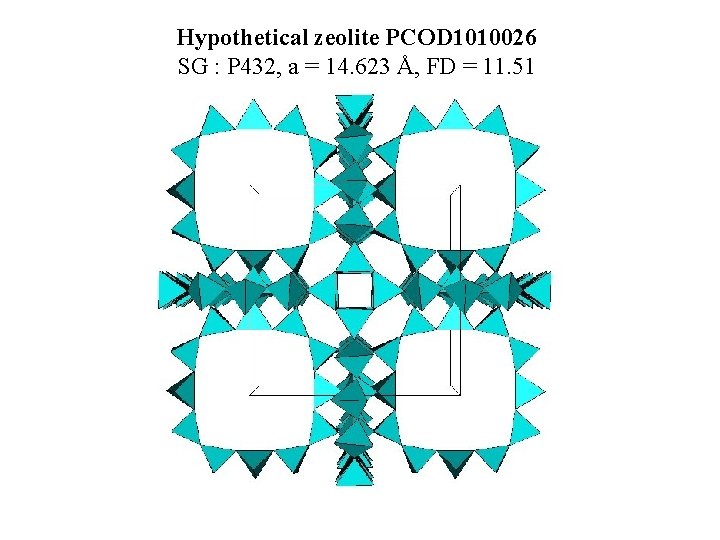
Hypothetical zeolite PCOD 1010026 SG : P 432, a = 14. 623 Å, FD = 11. 51

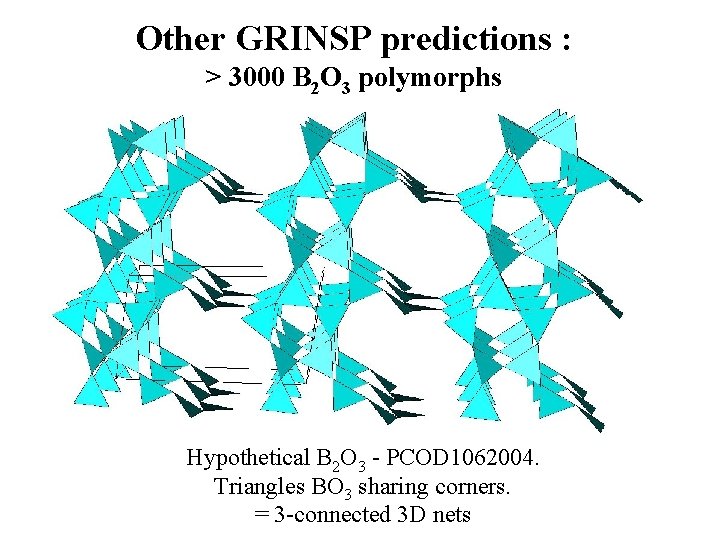
Other GRINSP predictions : > 3000 B 2 O 3 polymorphs Hypothetical B 2 O 3 - PCOD 1062004. Triangles BO 3 sharing corners. = 3 -connected 3 D nets

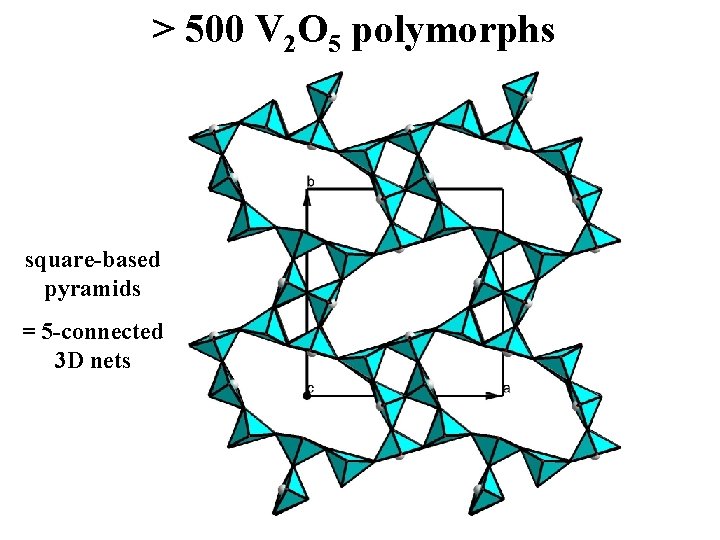
> 500 V 2 O 5 polymorphs square-based pyramids = 5 -connected 3 D nets

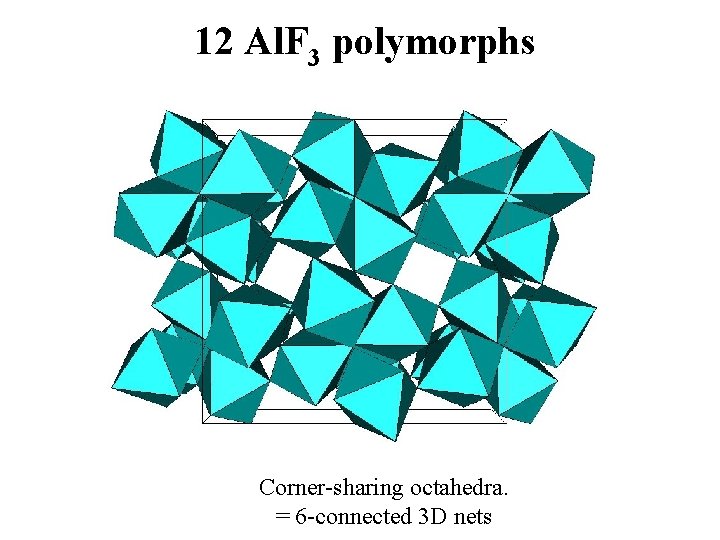
12 Al. F 3 polymorphs Corner-sharing octahedra. = 6 -connected 3 D nets

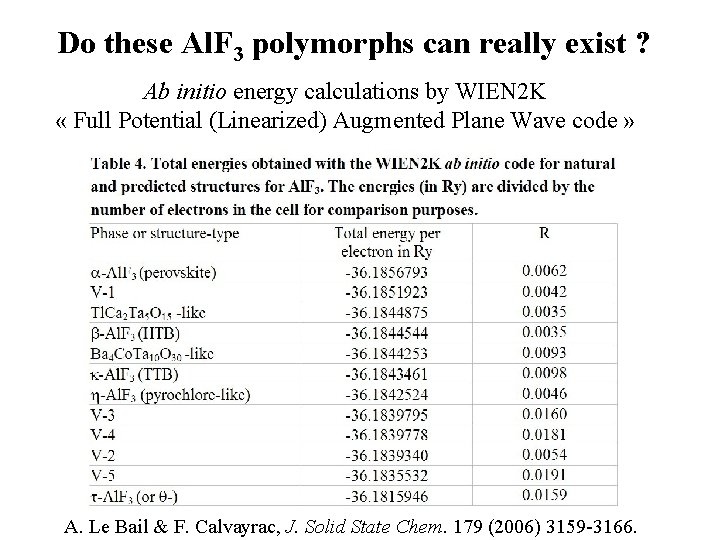
Do these Al. F 3 polymorphs can really exist ? Ab initio energy calculations by WIEN 2 K « Full Potential (Linearized) Augmented Plane Wave code » A. Le Bail & F. Calvayrac, J. Solid State Chem. 179 (2006) 3159 -3166.

Ternary compounds Ma. M’b. Xc in 3 D networks of polyhedra connected by corners Either M/M’ with same coordination but different ionic radii or with different coordinations (mixed N-N’-connected 3 D frameworks) These ternary compounds are not always electrically neutral.

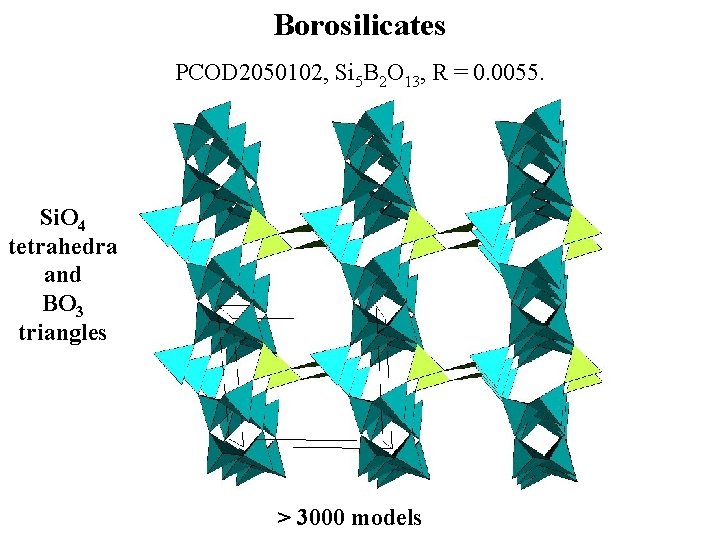
Borosilicates PCOD 2050102, Si 5 B 2 O 13, R = 0. 0055. Si. O 4 tetrahedra and BO 3 triangles > 3000 models
![Aluminoborates Example Al B 4 O 92 cubic SG Pn3 a Aluminoborates Example : [Al. B 4 O 9]-2, cubic, SG : Pn-3, a =](https://slidetodoc.com/presentation_image_h2/dcfc21bc4dd84834d096bc126870f7b5/image-40.jpg)
Aluminoborates Example : [Al. B 4 O 9]-2, cubic, SG : Pn-3, a = 15. 31 Å, R = 0. 0051: Al. O 6 octahedra and BO 3 triangles > 2000 models

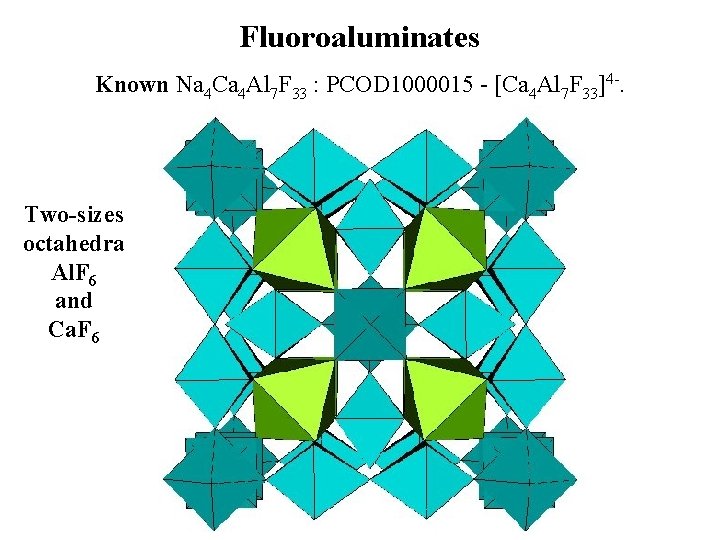
Fluoroaluminates Known Na 4 Ca 4 Al 7 F 33 : PCOD 1000015 - [Ca 4 Al 7 F 33]4 -. Two-sizes octahedra Al. F 6 and Ca. F 6
![Unknown PCOD 1010005 Ca 3 Al 4 F 213 Unknown : PCOD 1010005 - [Ca 3 Al 4 F 21]3 -](https://slidetodoc.com/presentation_image_h2/dcfc21bc4dd84834d096bc126870f7b5/image-42.jpg)
Unknown : PCOD 1010005 - [Ca 3 Al 4 F 21]3 -

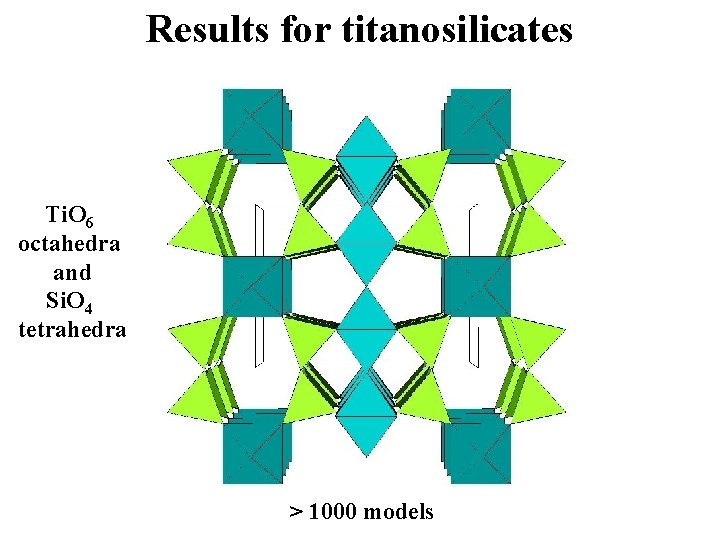
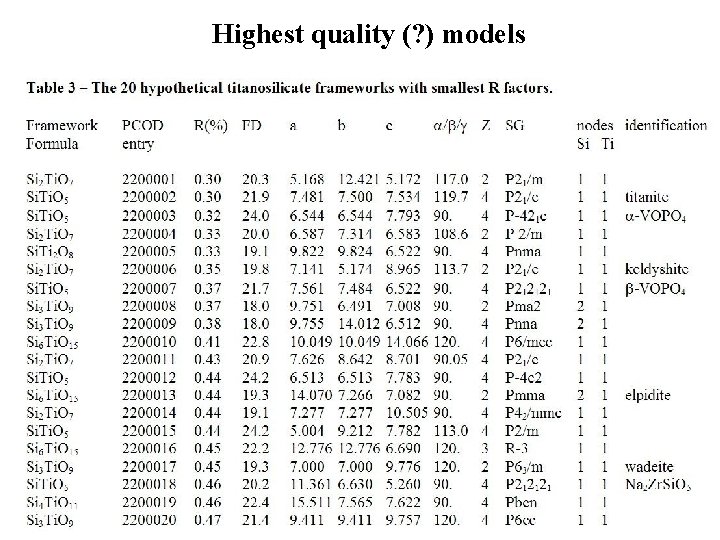
Results for titanosilicates Ti. O 6 octahedra and Si. O 4 tetrahedra > 1000 models

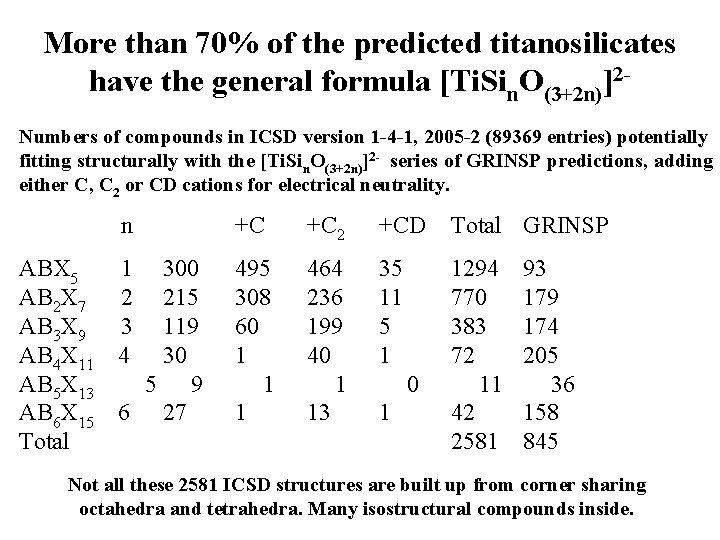
More than 70% of the predicted titanosilicates have the general formula [Ti. Sin. O(3+2 n)]2 Numbers of compounds in ICSD version 1 -4 -1, 2005 -2 (89369 entries) potentially fitting structurally with the [Ti. Sin. O(3+2 n)]2 - series of GRINSP predictions, adding either C, C 2 or CD cations for electrical neutrality. n ABX 5 1 300 AB 2 X 7 2 215 AB 3 X 9 3 119 AB 4 X 11 4 30 AB 5 X 13 5 9 AB 6 X 15 6 27 Total +C +C 2 +CD Total GRINSP 495 308 60 1 1 1 464 236 199 40 1 13 35 11 5 1 1294 770 383 72 11 42 2581 0 1 93 179 174 205 36 158 845 Not all these 2581 ICSD structures are built up from corner sharing octahedra and tetrahedra. Many isostructural compounds inside.

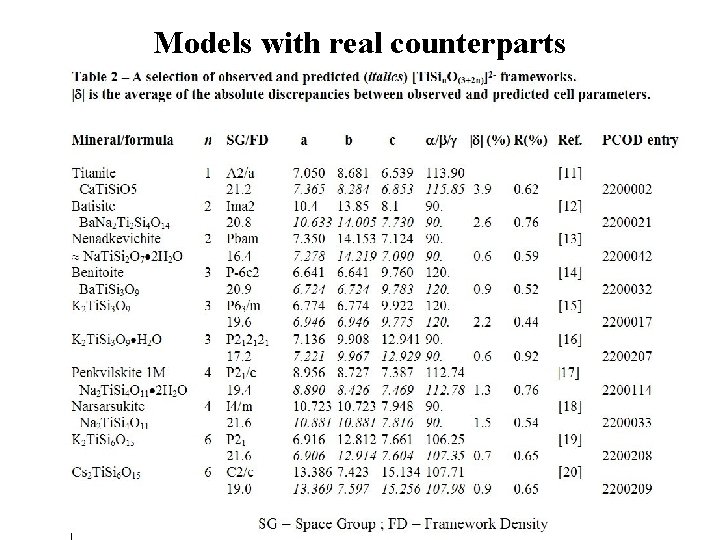
Models with real counterparts

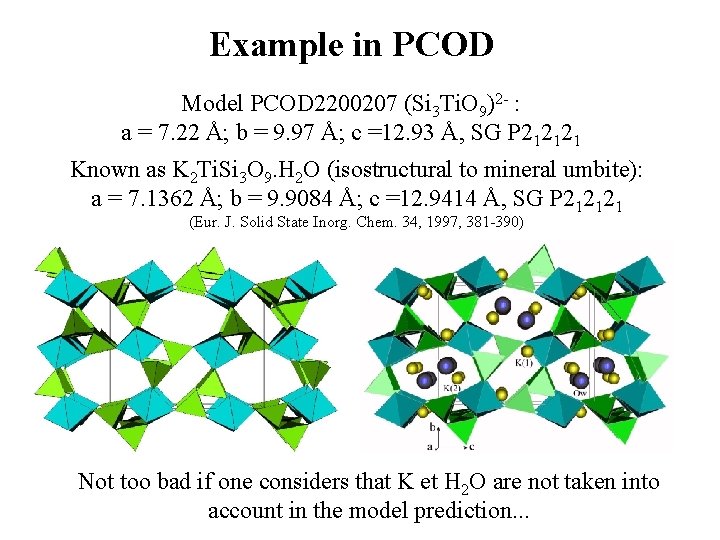
Example in PCOD Model PCOD 2200207 (Si 3 Ti. O 9)2 - : a = 7. 22 Å; b = 9. 97 Å; c =12. 93 Å, SG P 212121 Known as K 2 Ti. Si 3 O 9. H 2 O (isostructural to mineral umbite): a = 7. 1362 Å; b = 9. 9084 Å; c =12. 9414 Å, SG P 212121 (Eur. J. Solid State Inorg. Chem. 34, 1997, 381 -390) Not too bad if one considers that K et H 2 O are not taken into account in the model prediction. . .

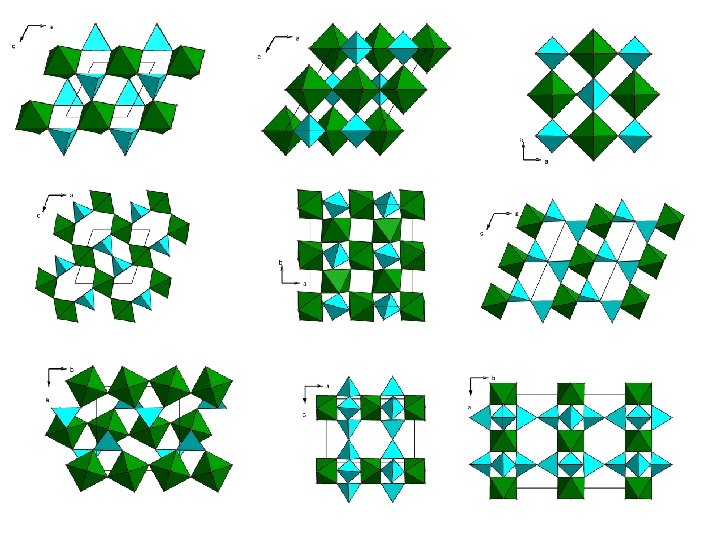
Highest quality (? ) models



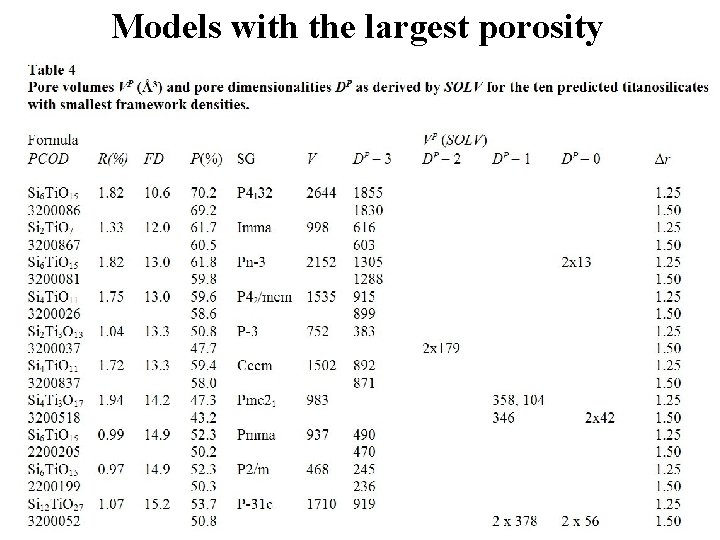
Models with the largest porosity

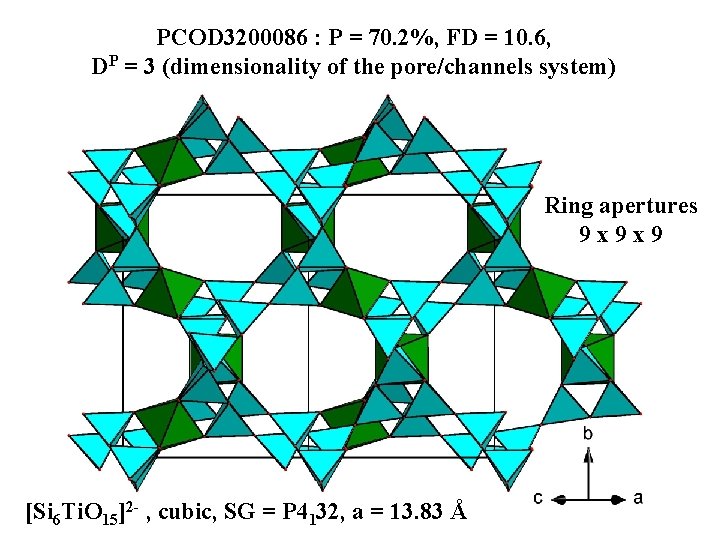
PCOD 3200086 : P = 70. 2%, FD = 10. 6, DP = 3 (dimensionality of the pore/channels system) Ring apertures 9 x 9 x 9 [Si 6 Ti. O 15]2 - , cubic, SG = P 4132, a = 13. 83 Å

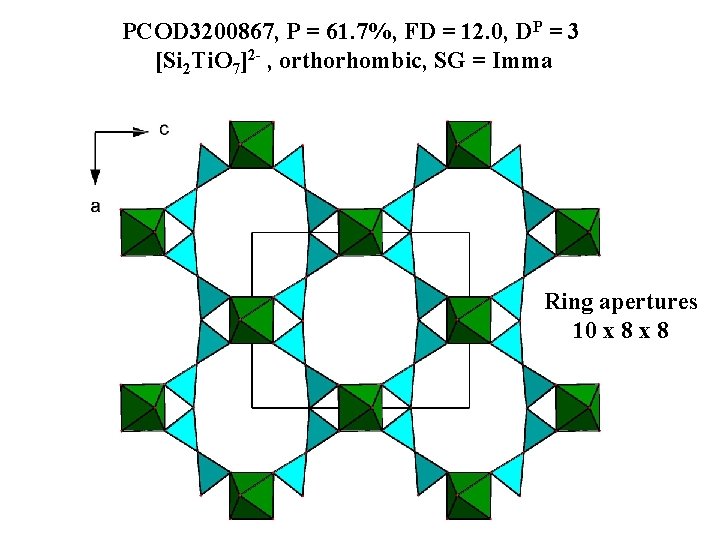
PCOD 3200867, P = 61. 7%, FD = 12. 0, DP = 3 [Si 2 Ti. O 7]2 - , orthorhombic, SG = Imma Ring apertures 10 x 8

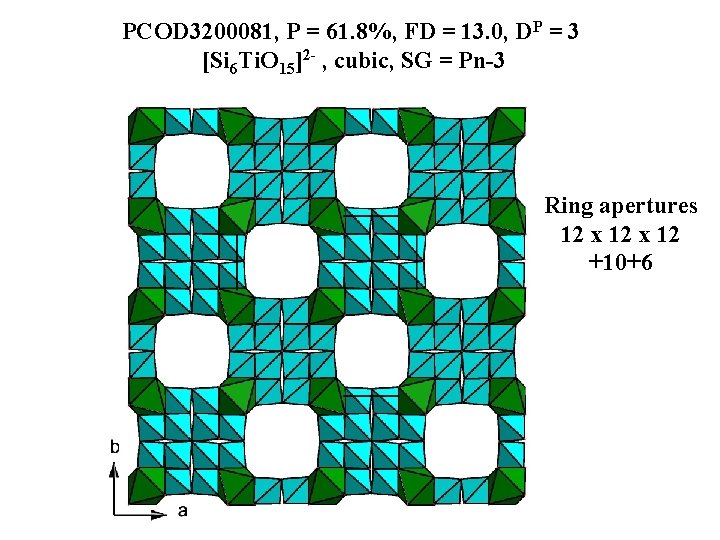
PCOD 3200081, P = 61. 8%, FD = 13. 0, DP = 3 [Si 6 Ti. O 15]2 - , cubic, SG = Pn-3 Ring apertures 12 x 12 +10+6

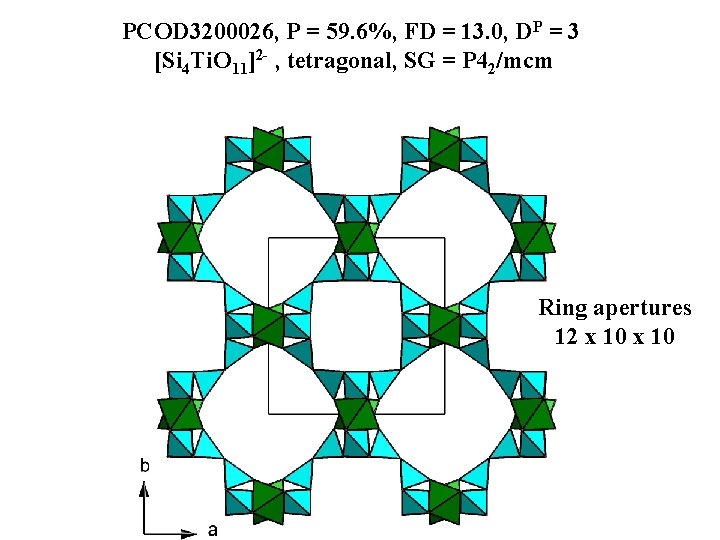
PCOD 3200026, P = 59. 6%, FD = 13. 0, DP = 3 [Si 4 Ti. O 11]2 - , tetragonal, SG = P 42/mcm Ring apertures 12 x 10

Opened doors, Limitations, Problems GRINSP limitation : exclusively corner-sharing polyhedra. Opening the door potentially to > 50. 000 hypothetical compounds. The predicted titanosilicates can be extrapolated to phosphates, sulfates, and/or replacing Ti by Nb, V, Zr, Ga, etc. More than 10. 000 should be included into PCOD before the end of 2006. Then, their powder patterns will be calculated and possibly used for search-match identification.

Expected improvements : Edge, face, corner-sharing, mixed. Hole detection, filling them automatically, appropriately, for electrical neutrality. Using bond valence rules or/and energy calculations to define a new cost function. Extension to quaternary compounds, combining more than two different polyhedra. Etc, etc. Do it yourself, the GRINSP software is open source…

Two things that don’t work well enough up to now… Validation - Ab initio calculations (WIEN 2 K, etc) : not fast enough for the validation of > 10000 structure candidates (was 2 months for 12 Al. F 3 models) Identification - There is no efficient tool for the identification of the known structures (from the ICSD) among >10000 hypothetical compounds

One advice, if you become a structure predictor Send your data (CIFs) to the PCOD, thanks… (no proteins, no nucleic acid, not 1. 000 zeolites)

CONCLUSIONS Structure and properties prediction is THE challenge of this XXIth century in crystallography. Advantages are obvious (less serendipity and fishing-type syntheses). We have to establish databases of predicted compounds, preferably open access on the Internet, finding some equilibrium between too much and not enough. If we are unable to do that, we have to stop pretending to understand master the crystallography laws.