inorganic organic The original meanings of the terms







- Slides: 69

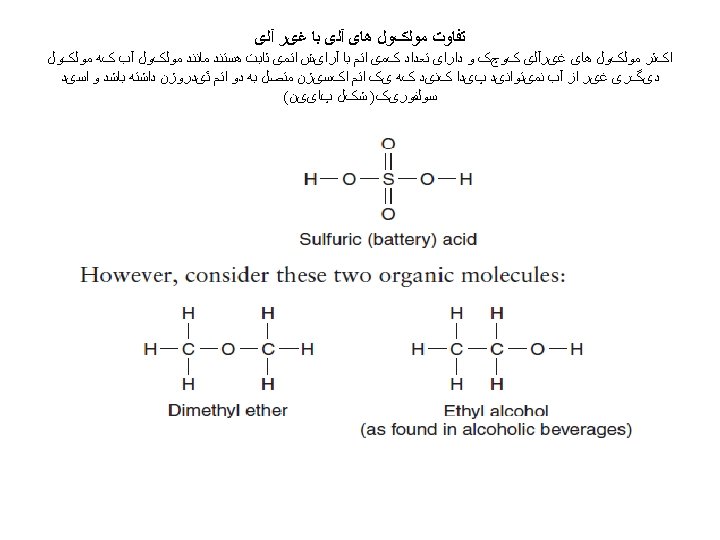
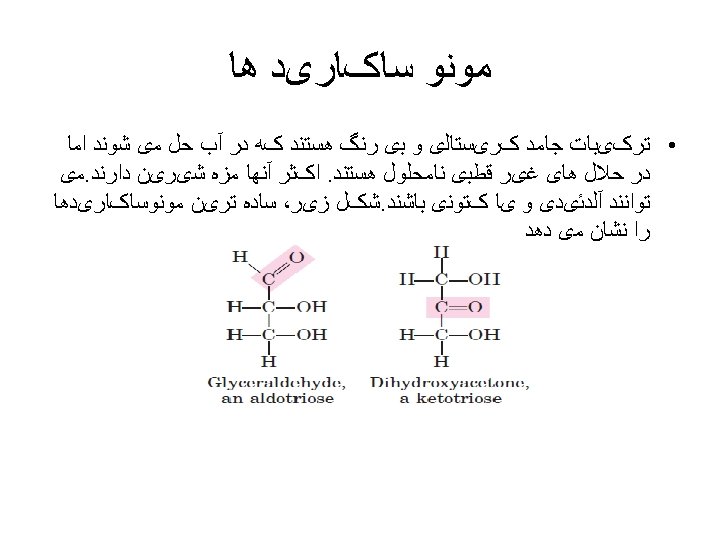
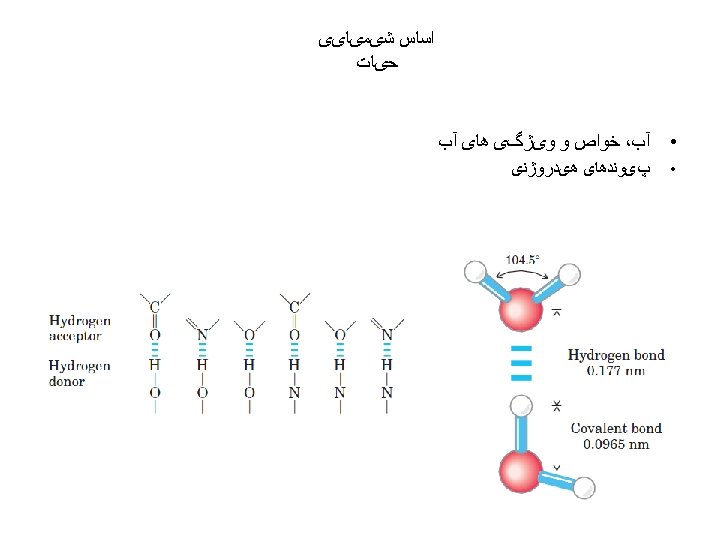
ﺑیﻮﺷیﻤی ﺗﺮکیﺒﺎﺕ آﻠی ﻣﻮﺟﻮﺩﺍﺕ ﺯﻧﺪﻩ (inorganic) ( ﻭ ﻏیﺮ آﻠی organic ) آﻠی : ﻣﻮﺍﺩ The original meanings of the terms inorganic and organic came from the fact that organic materials were thought to be either alive or produced only by living things. The words organism, organize, and organic are all related.

Modern chemistry has considerably altered the original meanings of the terms organic and inorganic, because it is now possible to manufacture unique organic molecules that cannot be produced by living things. Many of the materials we use daily are the result of the organic chemist’s art. Nylon, aspirin, polyurethane varnish, silicones, food wrap, Teflon, and insecticides are just a few of the unique molecules that have been invented by organic chemists (figure 1) . •

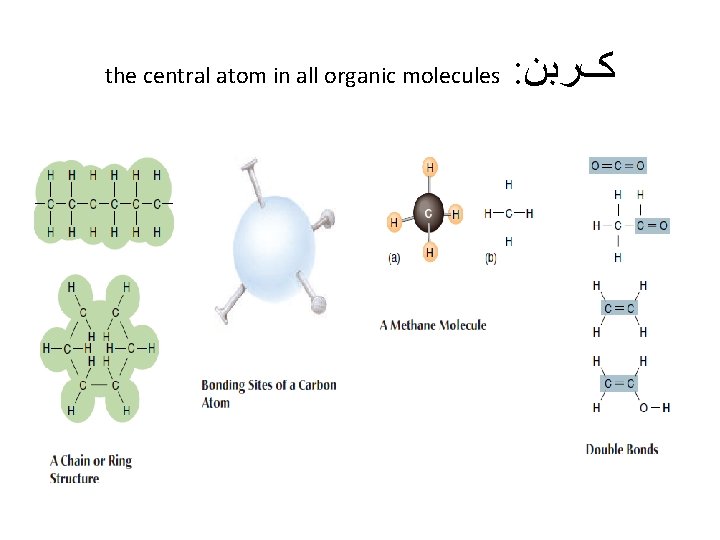
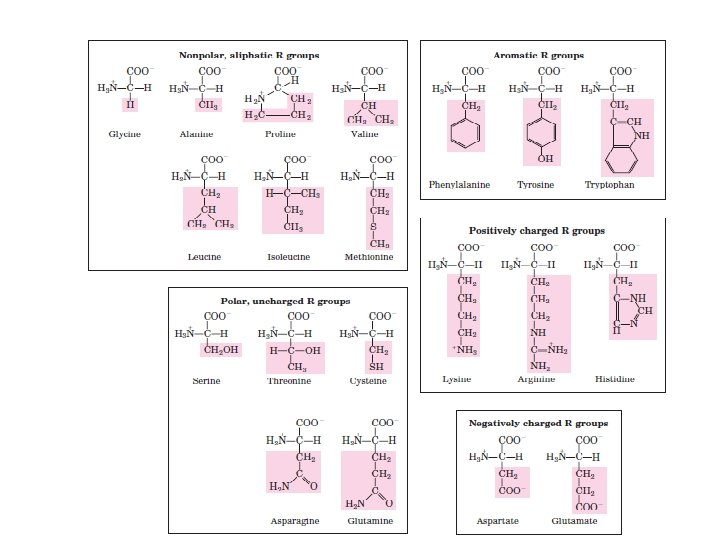
the central atom in all organic molecules : کﺮﺑﻦ

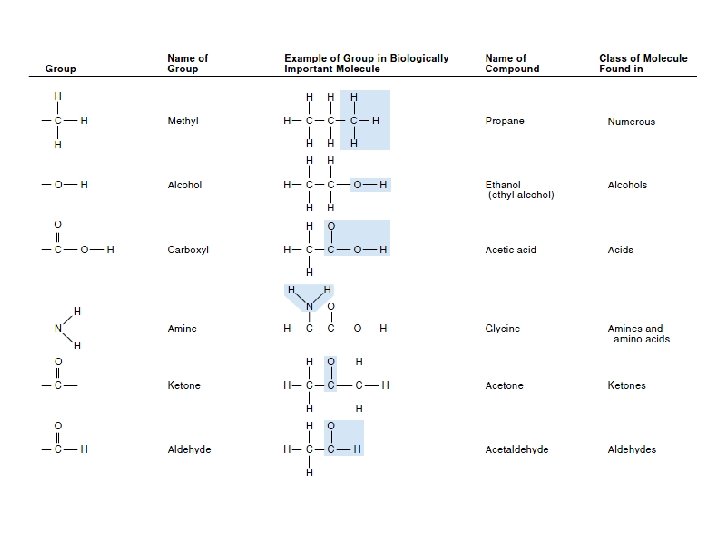
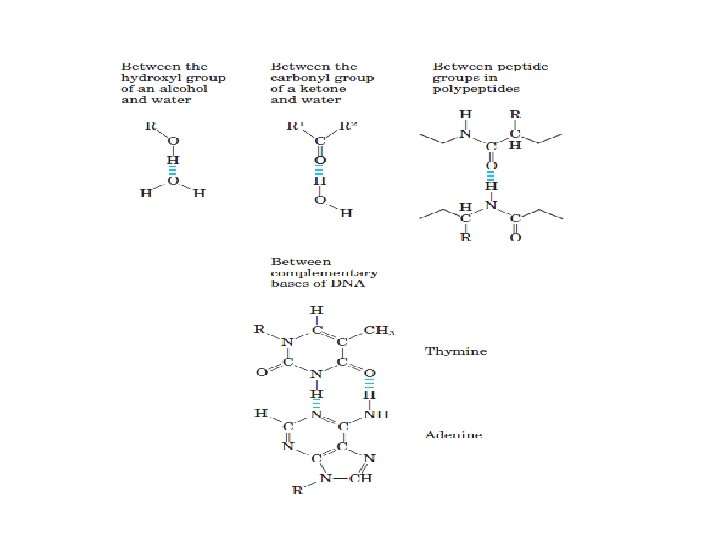
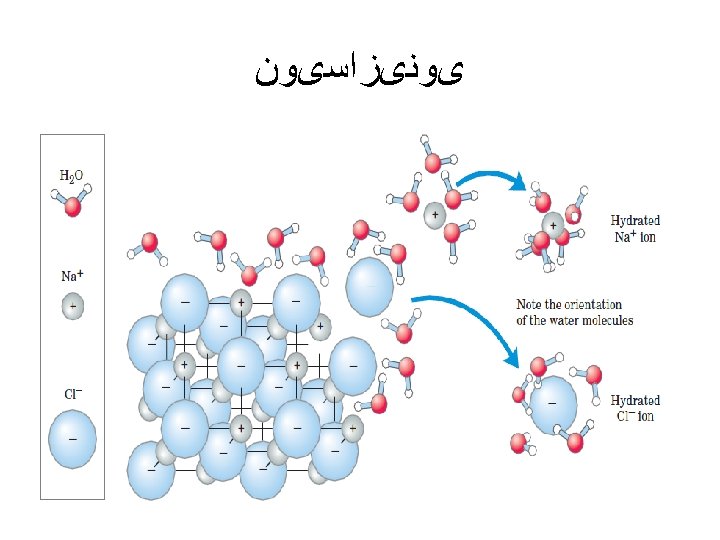
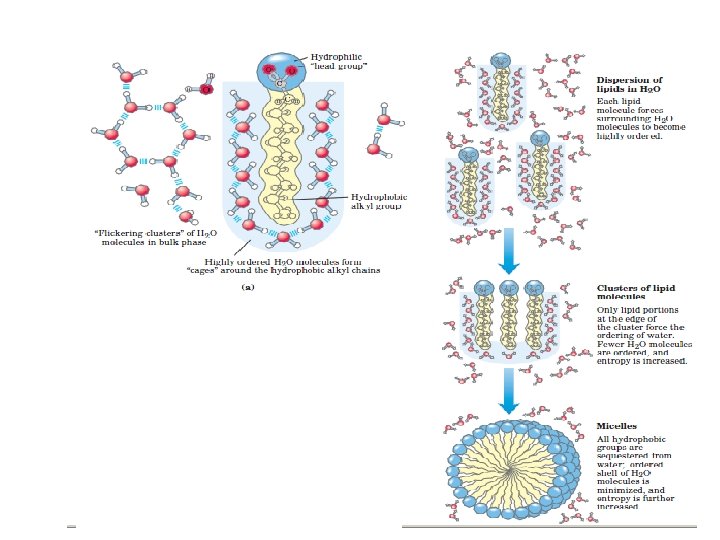
ﺳﺎیﺮ ﺍﺗﻢ ﻫﺎی ﻣﻮﺟﻮﺩ ﺩﺭ ﻣﻮﺍﺩ آﻠی Although most atoms can be involved in the structure of an organic • molecule, only a few are commonly found. Hydrogen (H) and oxygen (O) are almost always present. Nitrogen (N), sulfur (S), and phosphorus (P) are also very important in specific types of organic molecules.



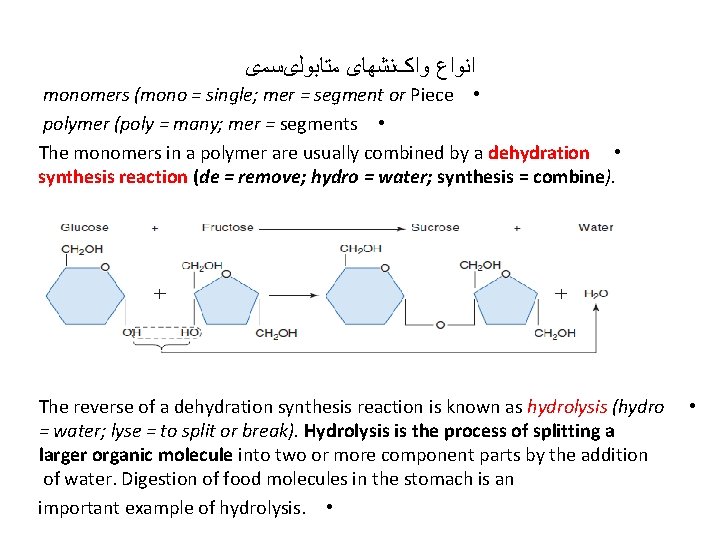
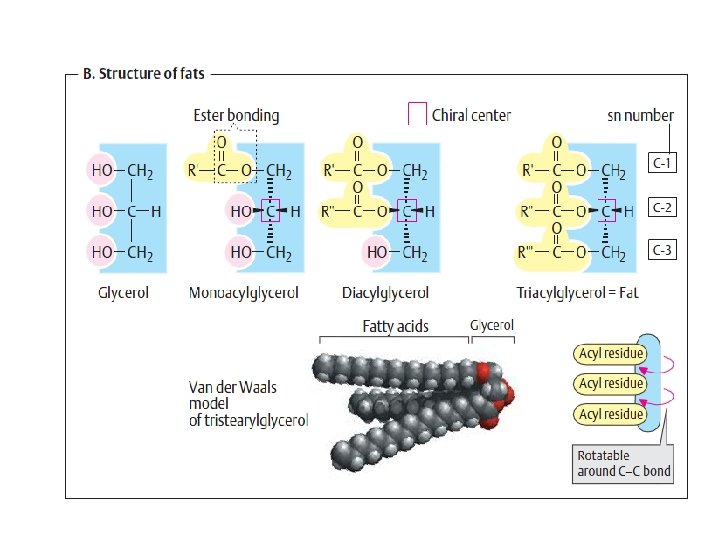
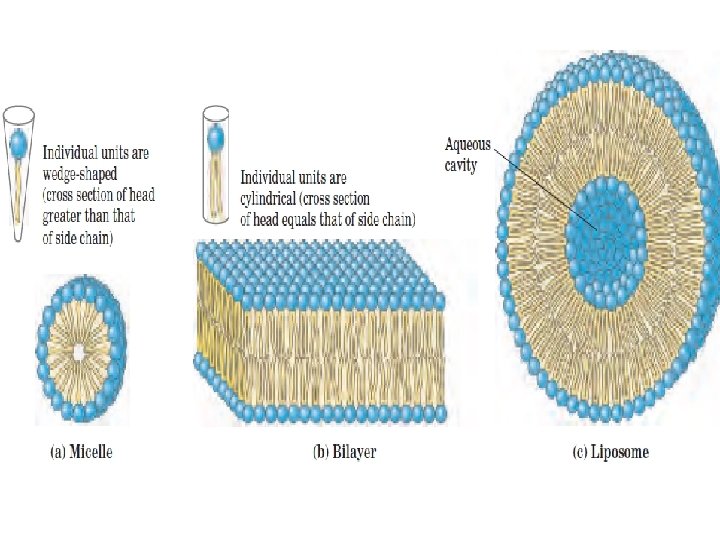
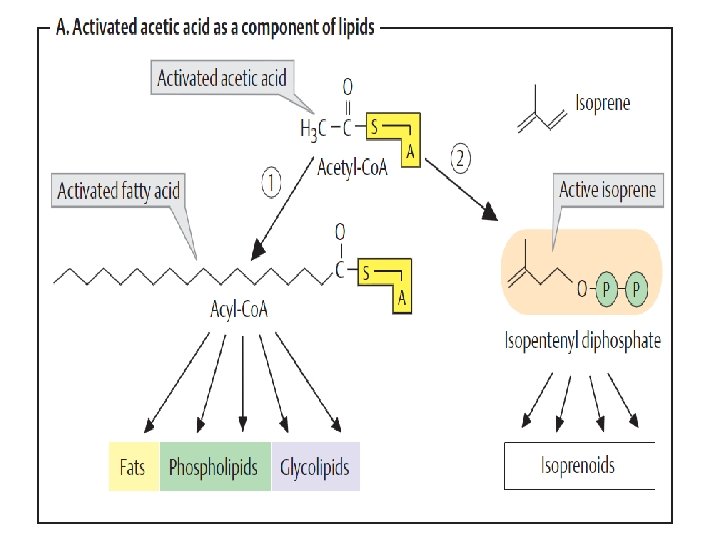
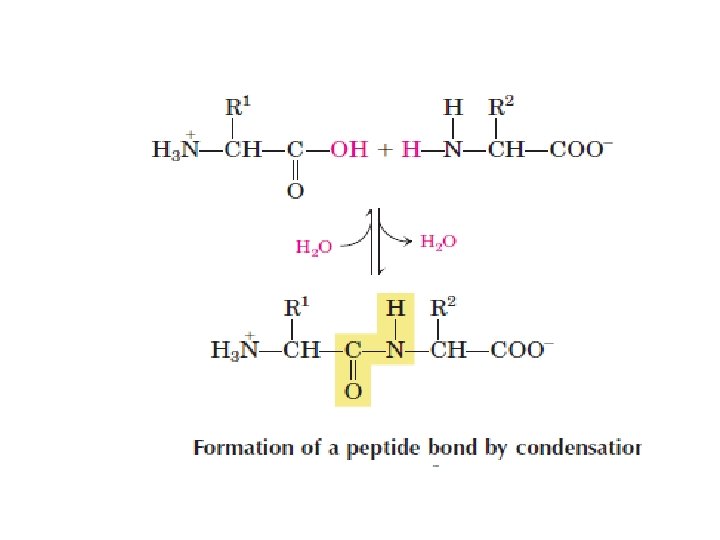
ﺍﻧﻮﺍﻉ ﻭﺍکﻨﺸﻬﺎی ﻣﺘﺎﺑﻮﻟیﺴﻤی monomers (mono = single; mer = segment or Piece • polymer (poly = many; mer = segments • The monomers in a polymer are usually combined by a dehydration • synthesis reaction (de = remove; hydro = water; synthesis = combine). The reverse of a dehydration synthesis reaction is known as hydrolysis (hydro = water; lyse = to split or break). Hydrolysis is the process of splitting a larger organic molecule into two or more component parts by the addition of water. Digestion of food molecules in the stomach is an important example of hydrolysis. • •


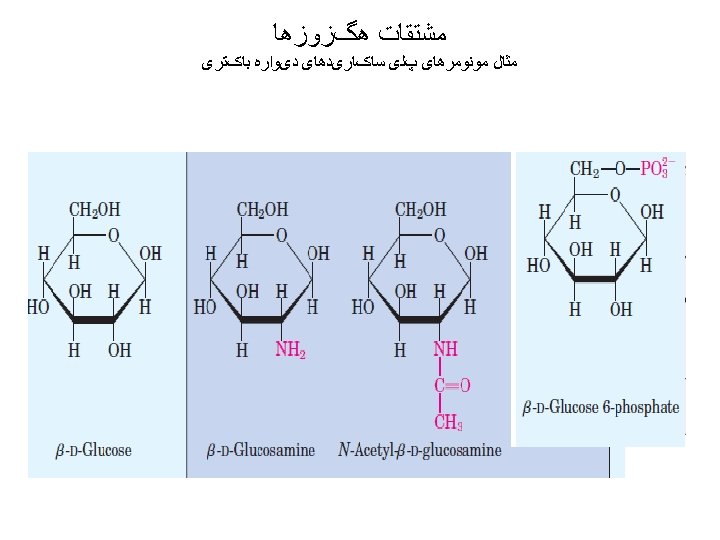
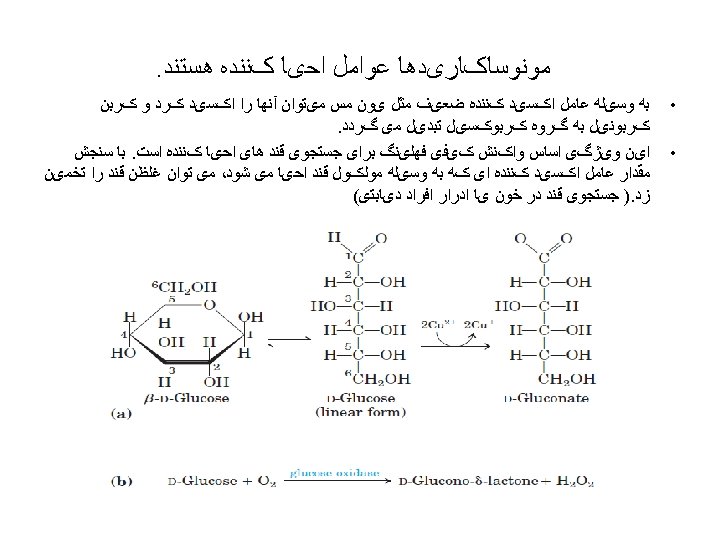
Carbohydrates • composed of carbon, hydrogen, and oxygen • atoms 1 - Monosaccharides (mono = single; saccharine = • sugar) ((CH 2 O)n ) sweet, 2 - Oligosaccharides: • When two simple sugars bond to each other with • glycosidic bonds, a disaccharide (di- = two) is formed; trisaccharide (tri- = three) is formed. • 3 - Polysaccharide (many sugar units). •

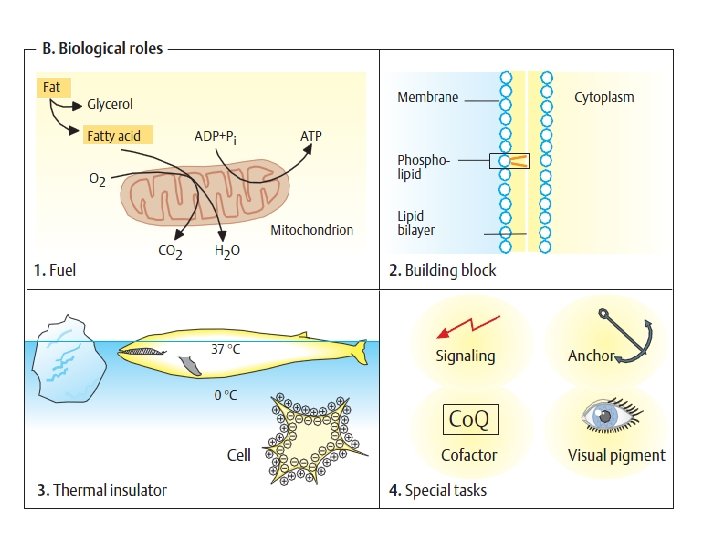
Carbohydrates play a number of roles in living • things. 1 - They serve as an immediate source of • energy (sugars), 2 -provide shape to certain cells (cellulose in • plant cell walls), 3 - are components of many antibiotics and • coenymes, 4 - and are an essential part of genes (DNA). •



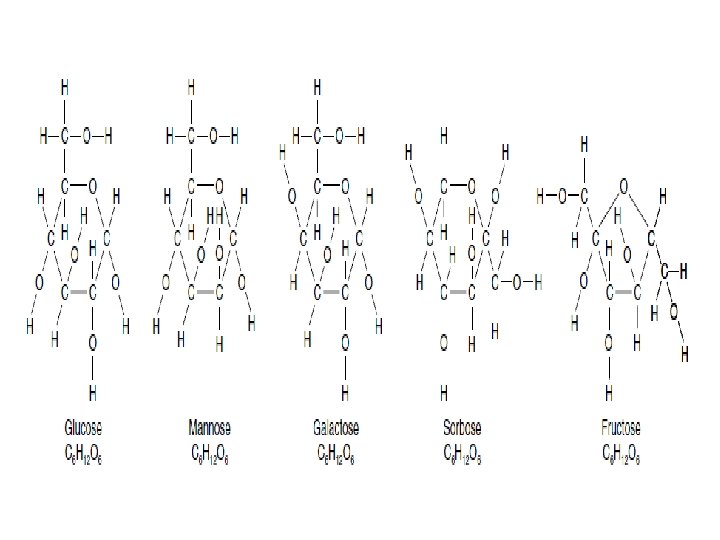
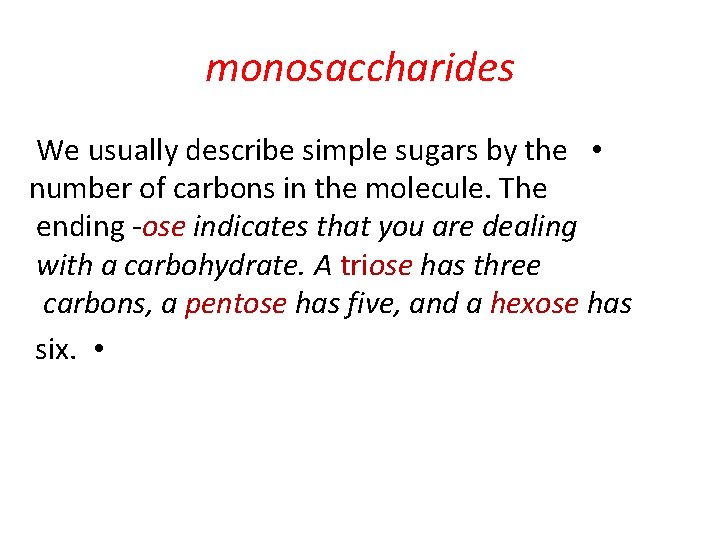
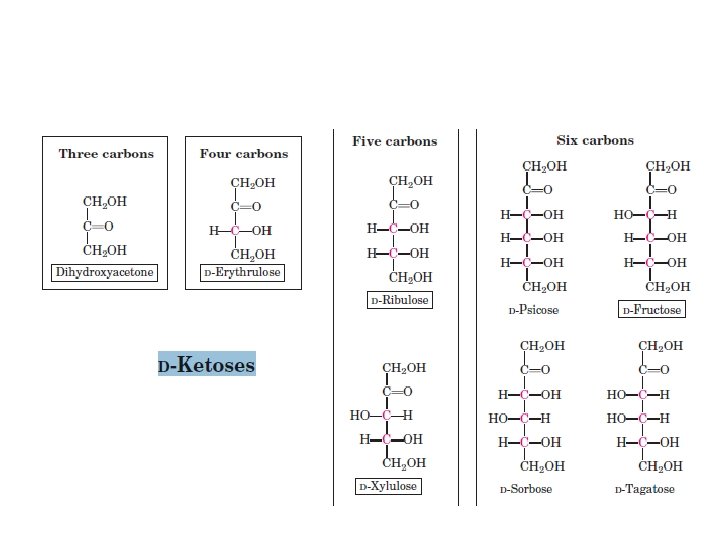
monosaccharides We usually describe simple sugars by the • number of carbons in the molecule. The ending -ose indicates that you are dealing with a carbohydrate. A triose has three carbons, a pentose has five, and a hexose has six. •

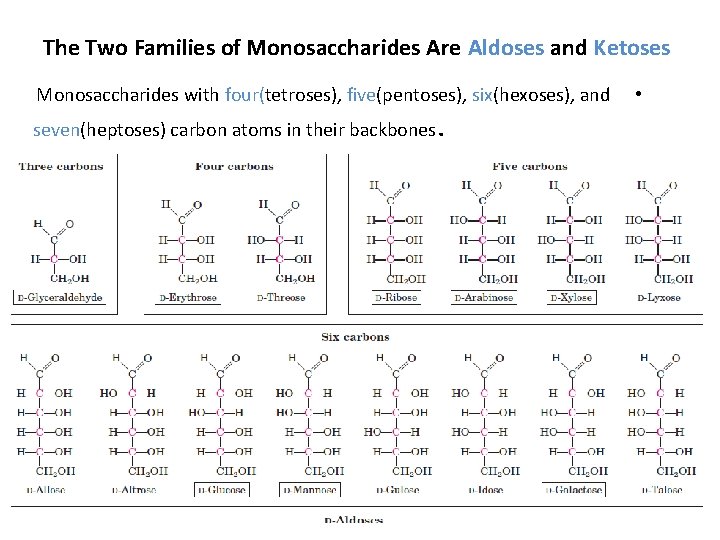
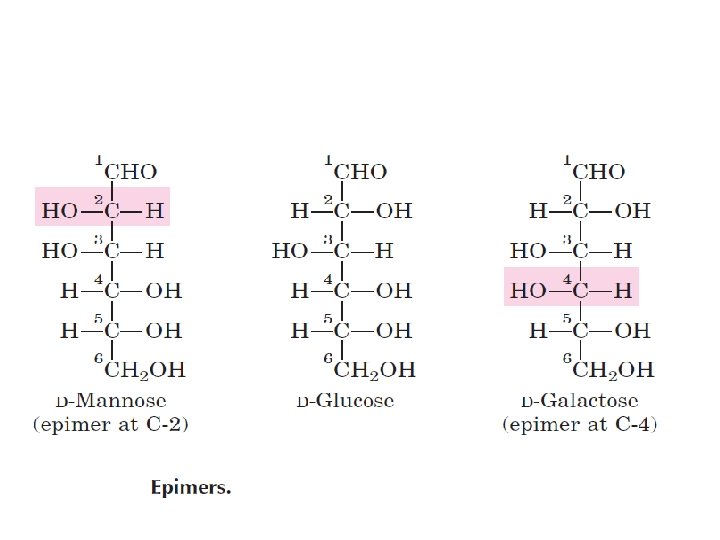
The Two Families of Monosaccharides Are Aldoses and Ketoses Monosaccharides with four(tetroses), five(pentoses), six(hexoses), and seven(heptoses) carbon atoms in their backbones . •








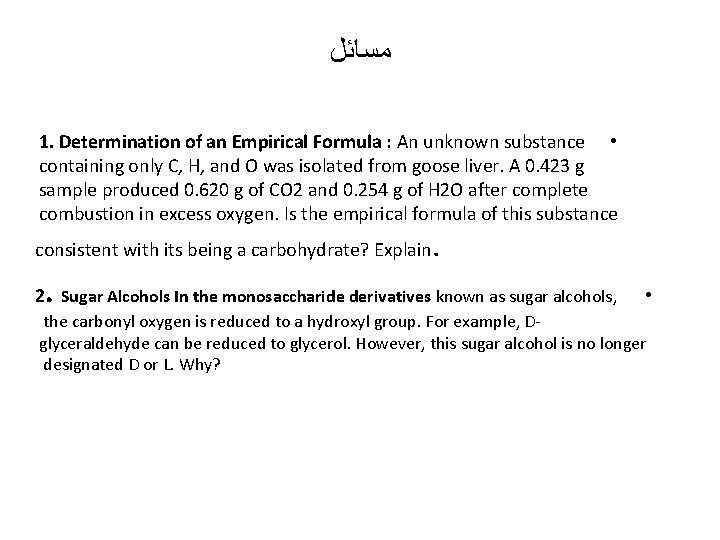
ﻣﺴﺎﺋﻞ 1. Determination of an Empirical Formula : An unknown substance • containing only C, H, and O was isolated from goose liver. A 0. 423 g sample produced 0. 620 g of CO 2 and 0. 254 g of H 2 O after complete combustion in excess oxygen. Is the empirical formula of this substance consistent with its being a carbohydrate? Explain . . 2 Sugar Alcohols In the monosaccharide derivatives known as sugar alcohols, • the carbonyl oxygen is reduced to a hydroxyl group. For example, Dglyceraldehyde can be reduced to glycerol. However, this sugar alcohol is no longer designated D or L. Why?

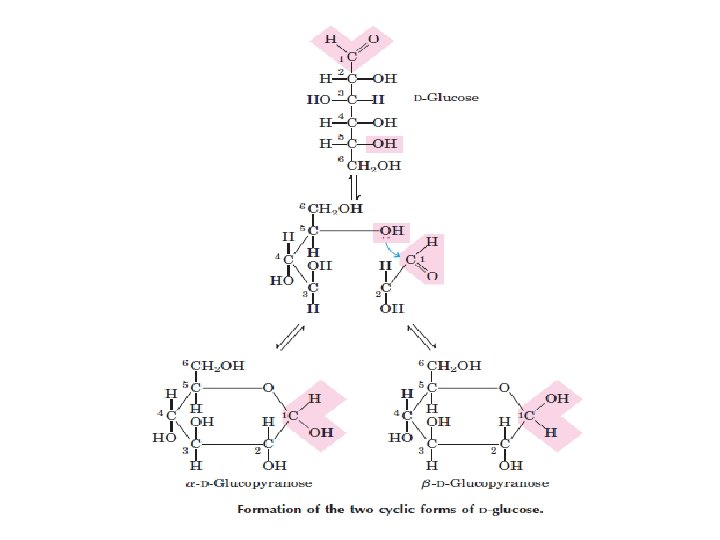
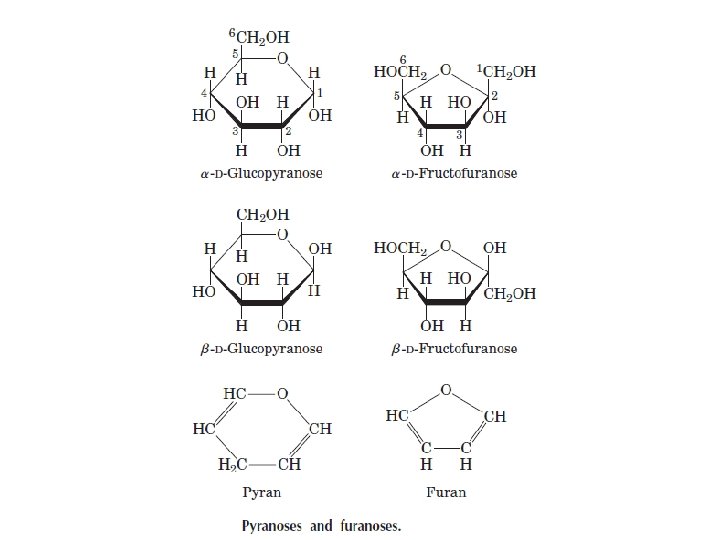
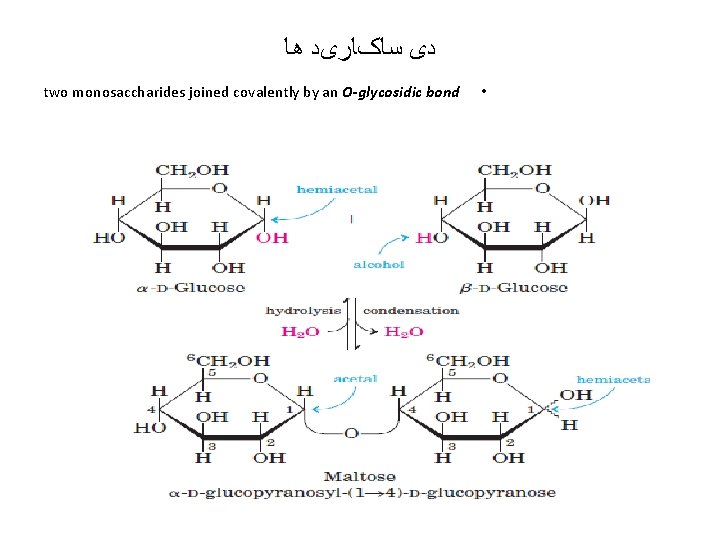
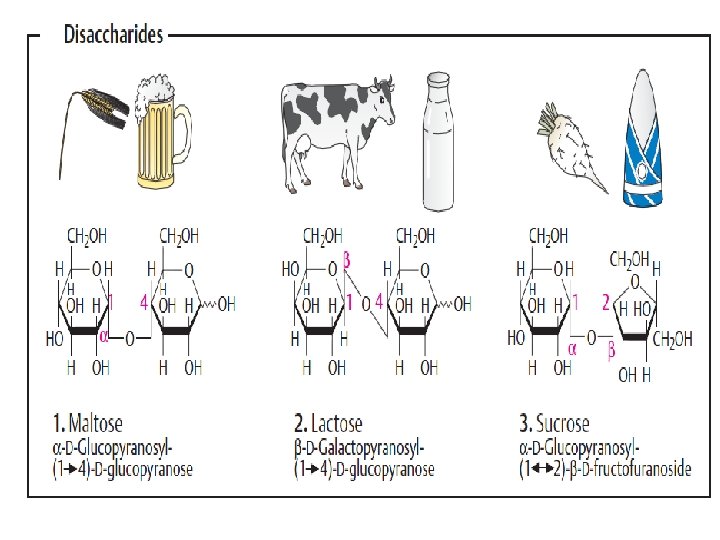
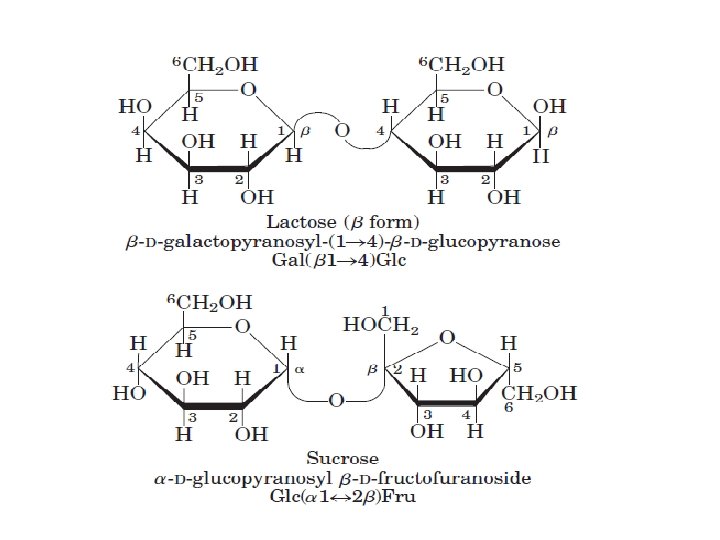
ﺩی ﺳﺎکﺎﺭیﺪ ﻫﺎ two monosaccharides joined covalently by an O-glycosidic bond •



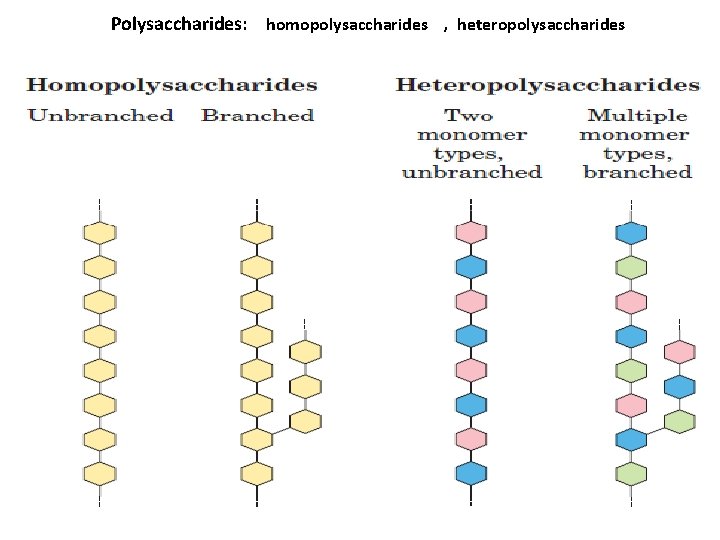
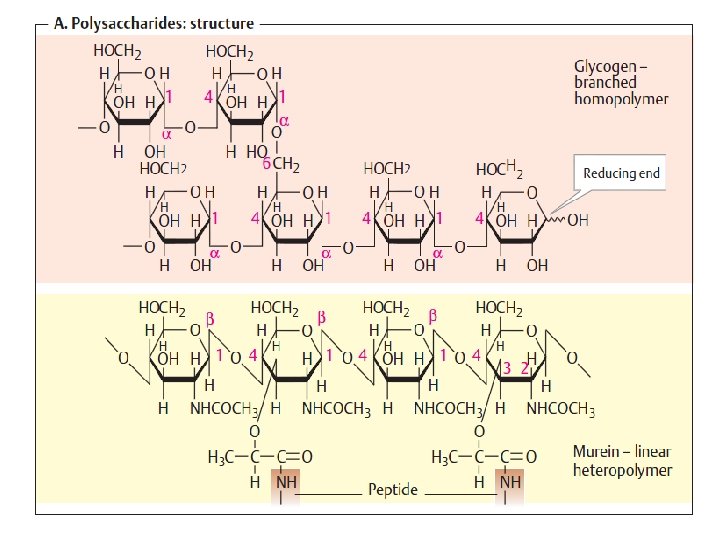
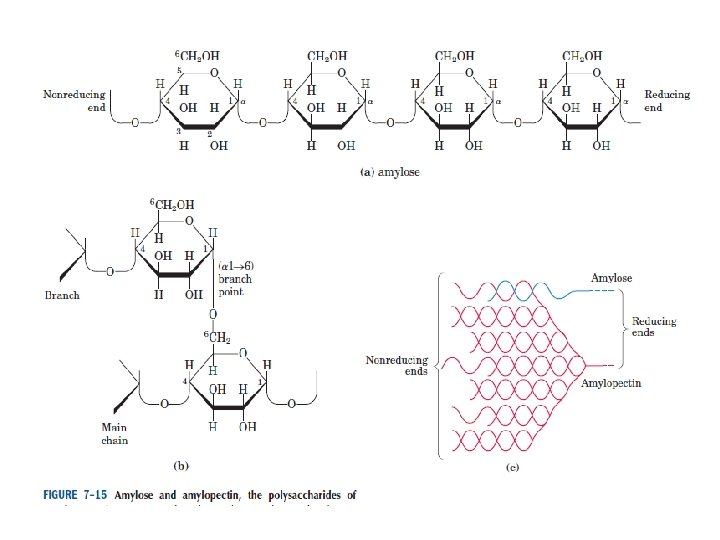
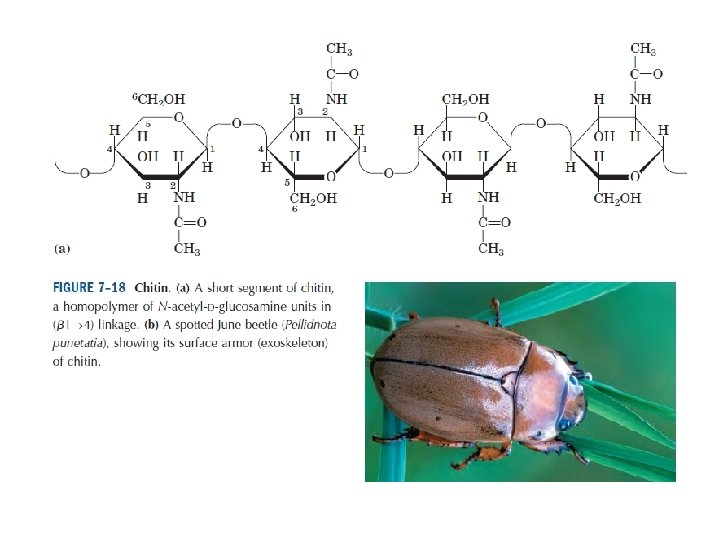
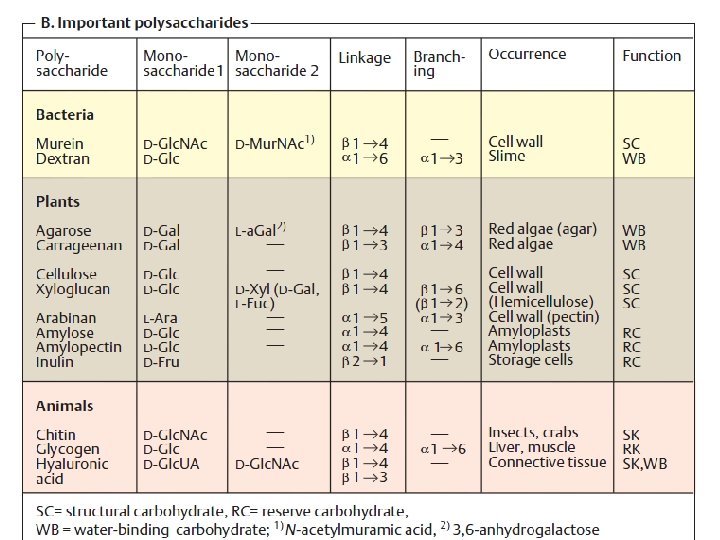
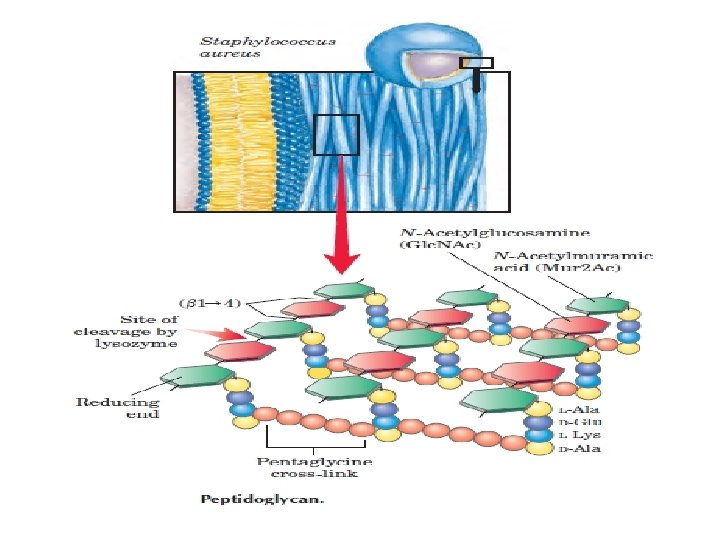
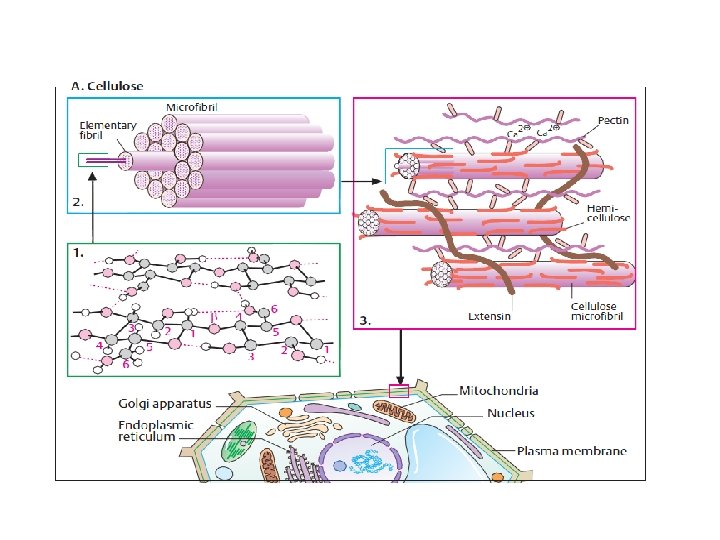
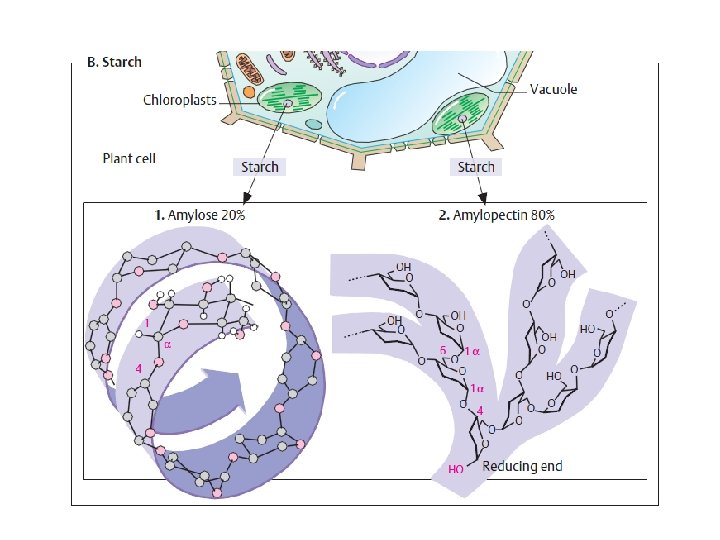
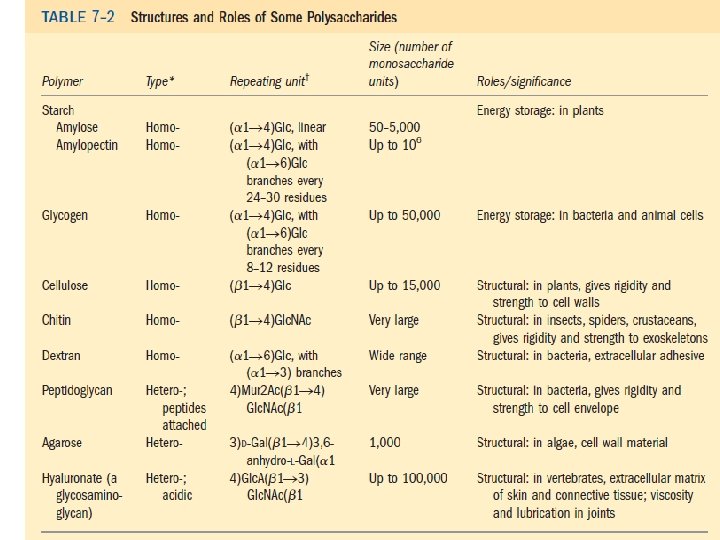
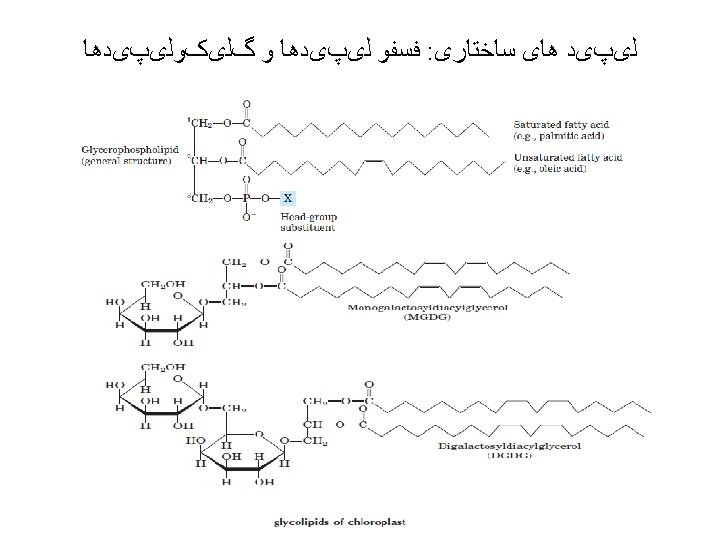
Polysaccharides: homopolysaccharides , heteropolysaccharides







































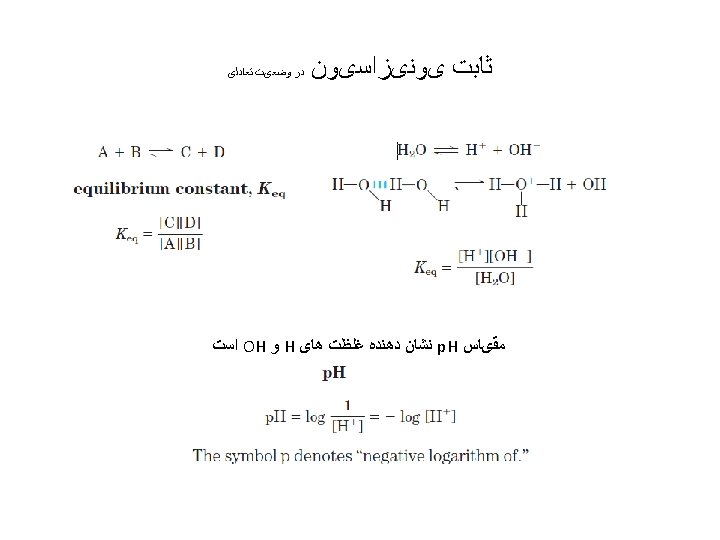
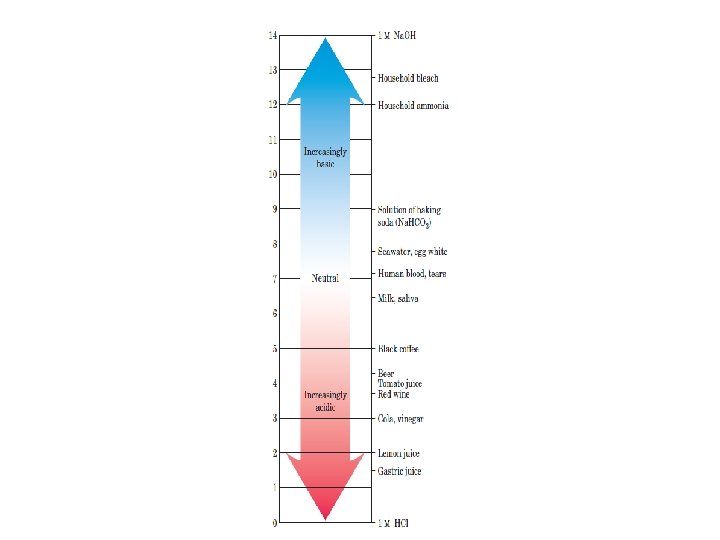
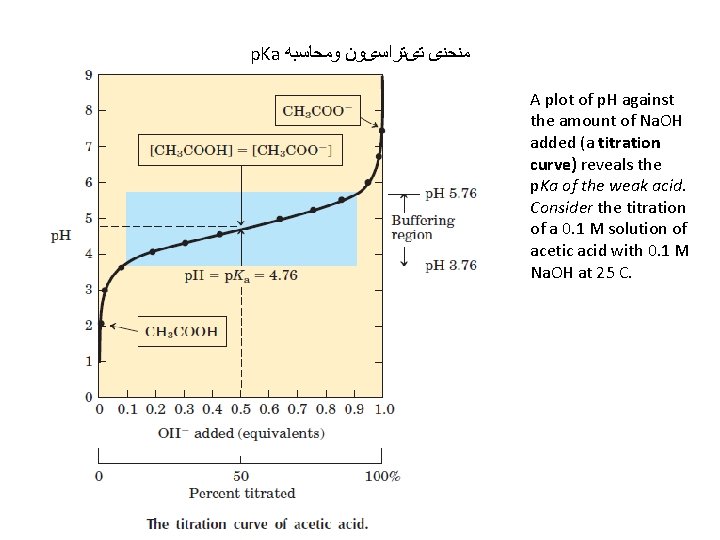
p. Ka ﻣﻨﺤﻨی ﺗیﺘﺮﺍﺳیﻮﻥ ﻭﻣﺤﺎﺳﺒﻪ A plot of p. H against the amount of Na. OH added (a titration curve) reveals the p. Ka of the weak acid. Consider the titration of a 0. 1 M solution of acetic acid with 0. 1 M Na. OH at 25 C.



