INORGANIC Nomenclature Nomenclature Humor Fe 2 Fe 2








![Ionic Bonding Na. Cl n=3 - n=2 - - - - Na [Ne]3 s Ionic Bonding Na. Cl n=3 - n=2 - - - - Na [Ne]3 s](https://slidetodoc.com/presentation_image_h/c5581b3582d1a05a84f89975c81019a3/image-19.jpg)




































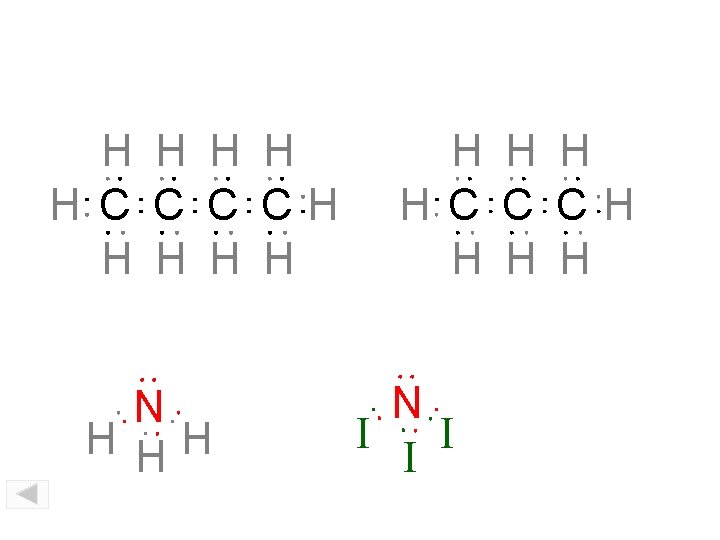


- Slides: 155

INORGANIC Nomenclature

Nomenclature - Humor Fe 2+ Fe 2+ “Ferrous Wheel” Fe = iron (Latin = ferrum) Fe 2+ = lower oxidation state = ferrous Fe 3+ = higher oxidation state = ferric Ba. Na 2 “Ba. Na” What weapon can you make from the elements nickel, potassium and iron? A KNi. Fe

Teacher: What is the formula for water? Student: H, I, J, K, L, M, N, O Teacher: That’s not what I taught you. Student: But you said the formula for water was…H to O. mis WATER? Website: Dihydrogen monoxide Information Campaign "H-O-H"? ! WHAT'S THAT SPELL? ! “Little Johnny took a drink, Now he shall drink no more. For what he thought was H 2 O, Was H 2 SO 4. ” Under aged Pb walks into a bar and the bartender turns to the gold Bouncer and says, “Au, get the lead out!”

Table of Contents ‘Nomenclature’ Binary Compounds - Metal (fixed oxidation) + Nonmetal Criss-Cross Rule Binary Compounds - Metal (variable oxidation) + Nonmetal Binary Compounds - Nonmetal + Nonmetal Ternary Compounds Binary Hydrogen Compounds Meaning of Suffixes Empirical Formulas Subscripts, Superscripts, and Coefficients Centrum Multivitamin Polyatomic Ions

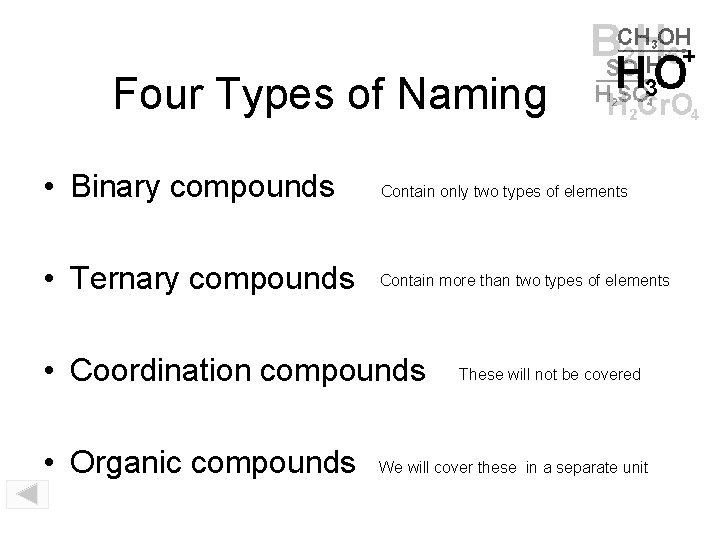
Four Types of Naming • Binary compounds Contain only two types of elements • Ternary compounds Contain more than two types of elements • Coordination compounds • Organic compounds These will not be covered We will cover these in a separate unit

1+ Binary Compounds H 2 3 4 Binary compounds that contain a metal of fixed oxidation number (group 1, group 2, Al, Zn, Ag, etc. ), and a non-metal. 5 6 7 He 2+ 3+ Be B C N O F 2 Ne 3 4 Na Mg 5 Al 6 Si 7 P 8 S 9 Cl 10 Ar 13 14 15 16 Cu Zn Ga Ge As Se 17 Br 18 Kr 36 Xe 1 1 Li 11 K 1+ 2+ 12 Ca Sc Ti V Cr Mn Fe Co Ni 19 20 Rb Sr 21 Y 22 23 24 25 26 27 28 29 30 31 Zr Nb Mo Tc Ru Rh Pd Ag Cd In 32 33 34 Sn Sb Te 35 I 37 38 Cs Ba 39 40 Hf 46 47 48 49 Pt Au Hg Tl 50 Pb 51 Bi 52 Po 53 54 At Rn 78 82 83 84 85 55 Fr 56 Ra 87 88 * W 41 Ta 42 W 43 44 45 Re Os Ir 72 73 74 75 76 77 Rf Db Sg Bh Hs Mt 79 80 81 104 105 106 107 108 109 To name these compounds, give the name of metal followed by the name of the non-metal, with the ending replaced by the suffix –ide. Examples: Na. Cl sodium chlor ide ine (Na 1+ Cl 1 -) Ca. S calcium sulf ide ur (Ca 2+ Al. I 3 aluminum iodide ine (Al 3+ 3 I 1 -) S 2 -) 86

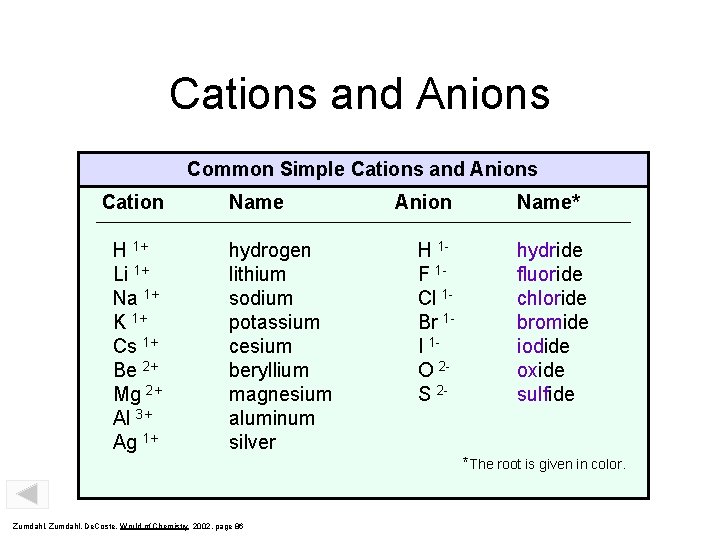
Cations and Anions Common Simple Cations and Anions Cation H 1+ Li 1+ Na 1+ K 1+ Cs 1+ Be 2+ Mg 2+ Al 3+ Ag 1+ Name hydrogen lithium sodium potassium cesium beryllium magnesium aluminum silver Anion H 1 F 1 Cl 1 Br 1 I 1 O 2 S 2 - Name* hydride fluoride chloride bromide iodide oxide sulfide *The root is given in color. Zumdahl, De. Coste, World of Chemistry 2002, page 86

Molecular Compound A compound containing atoms of two or more elements that are bonded together by sharing electrons. Silicon dioxide, Si. O 2, is a molecular compound. It is also a mineral called quartz (left). Quartz is found in nearly every type of rock. Most sand grains (center) are bits of quartz. Glass is made from sand.

“Perhaps one of you gentlemen would mind telling me just what is outside the window that you find so attractive. . ? ” Image courtesy Nearing. Zero. net

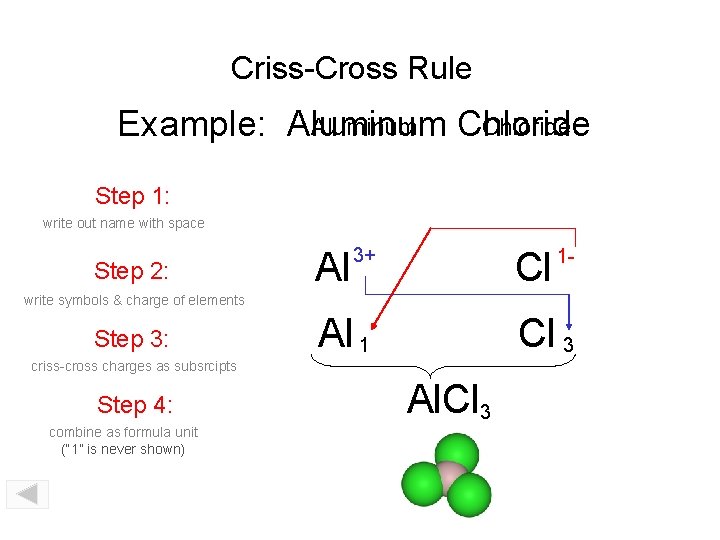
Criss-Cross Rule Aluminum Chloride Example: Aluminum Chloride Step 1: write out name with space Step 2: Al 3+ Cl 1 - write symbols & charge of elements Step 3: Al 1 Cl 3 criss-cross charges as subsrcipts Step 4: combine as formula unit (“ 1” is never shown) Al. Cl 3

Criss-Cross Rule Example: Aluminum Chloride Step 1: Aluminum Chloride Step 2: Al 3+ Cl 1 - Step 3: Al 1 Cl 3 Step 4: Al. Cl 3

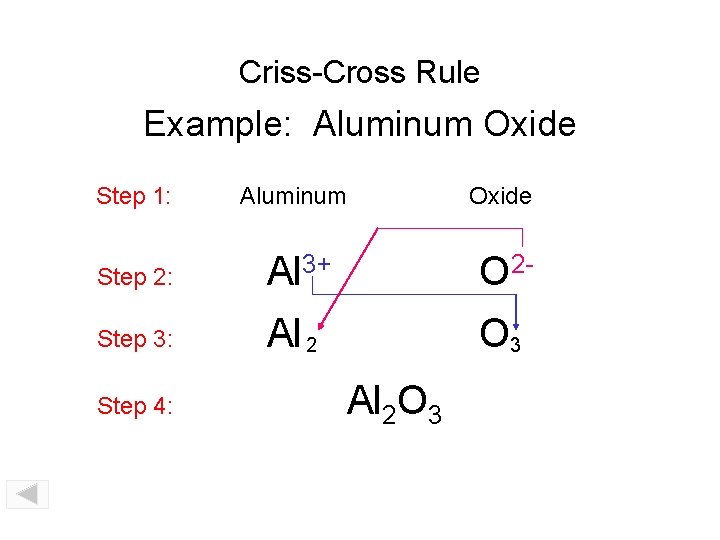
Criss-Cross Rule Example: Aluminum Oxide Step 1: Aluminum Oxide Step 2: Al 3+ O 2 - Step 3: Al 2 O 3 Step 4: Al 2 O 3

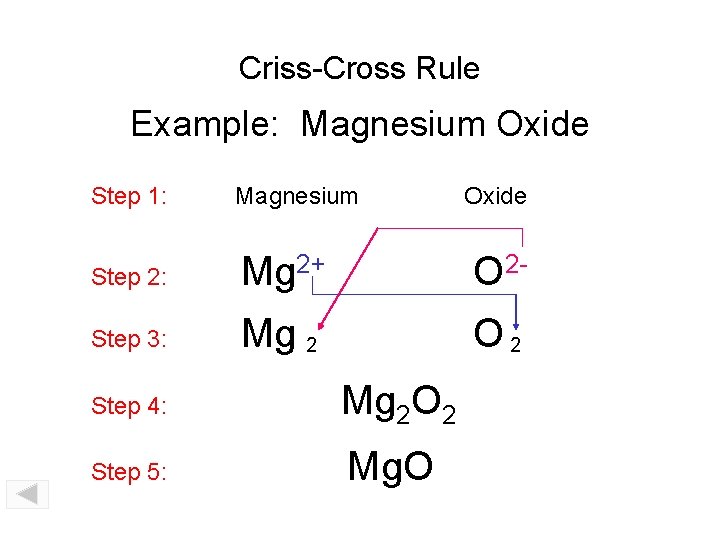
Criss-Cross Rule Example: Magnesium Oxide Step 1: Magnesium Step 2: Mg 2+ O 2 - Step 3: Mg 2 O 2 Step 4: Mg 2 O 2 Step 5: Mg. O Oxide

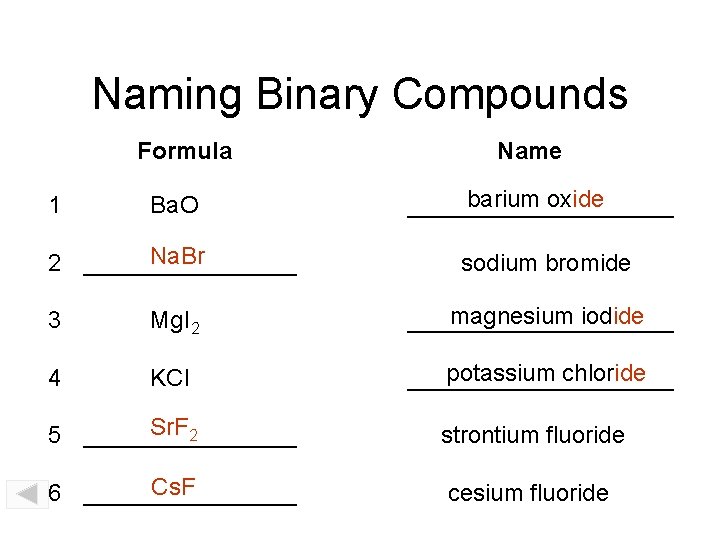
Naming Binary Compounds Formula Name Ba. O barium oxide __________ Na. Br 2 ________ sodium bromide 1 3 Mg. I 2 magnesium iodide __________ 4 KCl potassium chloride __________ Sr. F 2 5 ________ strontium fluoride Cs. F 6 ________ cesium fluoride

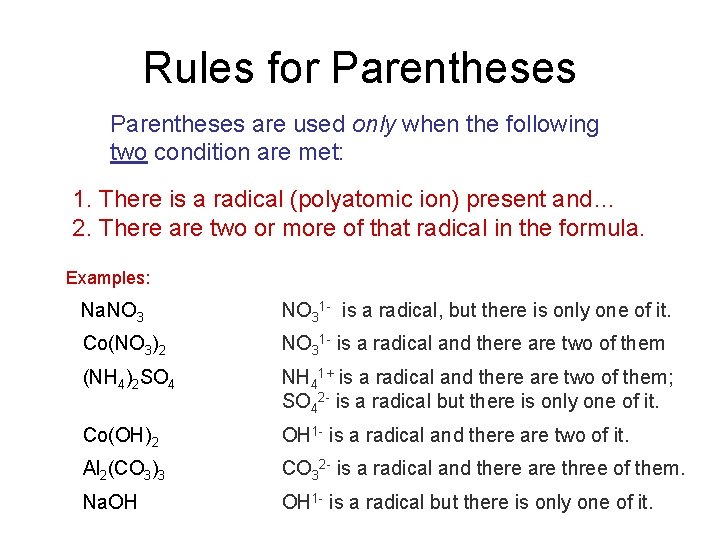
Rules for Parentheses are used only when the following two condition are met: 1. There is a radical (polyatomic ion) present and… 2. There are two or more of that radical in the formula. Examples: Na. NO 31 - is a radical, but there is only one of it. Co(NO 3)2 NO 31 - is a radical and there are two of them (NH 4)2 SO 4 NH 41+ is a radical and there are two of them; SO 42 - is a radical but there is only one of it. Co(OH)2 OH 1 - is a radical and there are two of it. Al 2(CO 3)3 CO 32 - is a radical and there are three of them. Na. OH OH 1 - is a radical but there is only one of it.

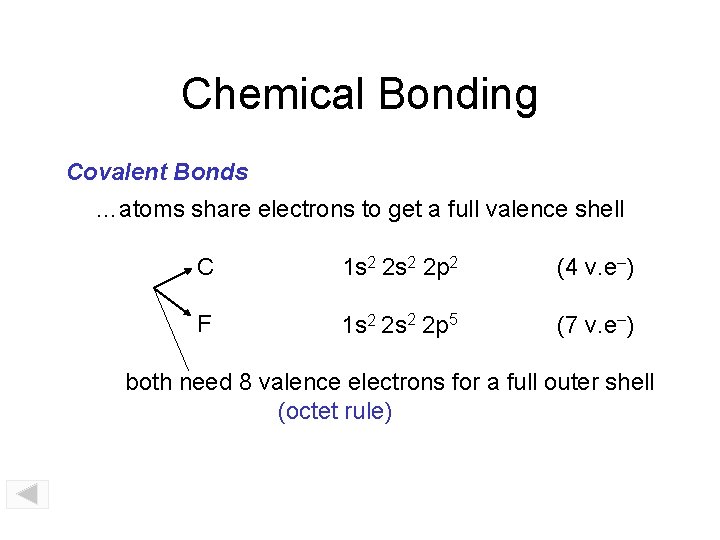
Chemical Bonding Covalent Bonds …atoms share electrons to get a full valence shell C 1 s 2 2 p 2 (4 v. e–) F 1 s 2 2 p 5 (7 v. e–) both need 8 valence electrons for a full outer shell (octet rule)

Covalent bonding • • Fluorine has seven valence electrons A second F atom also has seven By sharing electrons Both end with full orbitals (stable octets) F 8 Valence electrons

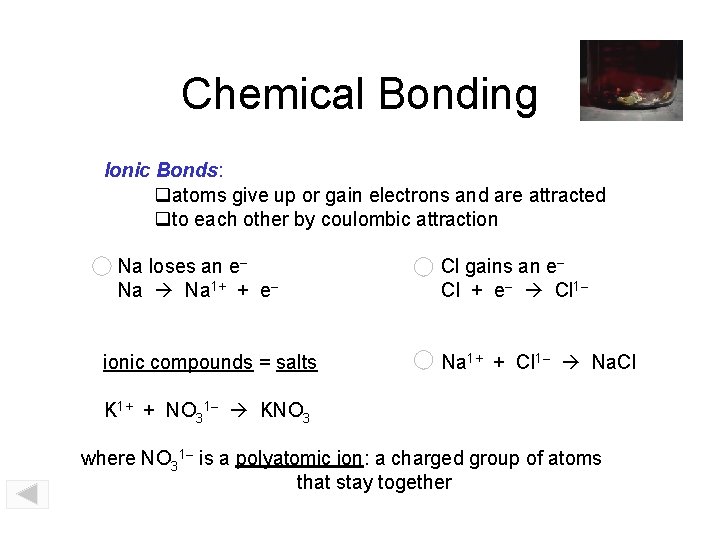
Chemical Bonding Ionic Bonds: atoms give up or gain electrons and are attracted to each other by coulombic attraction Na loses an e– Na 1+ + e– ionic compounds = salts Cl gains an e– Cl + e– Cl 1– Na 1+ + Cl 1– Na. Cl K 1+ + NO 31– KNO 3 where NO 31– is a polyatomic ion: a charged group of atoms that stay together
![Ionic Bonding Na Cl n3 n2 Na Ne3 s Ionic Bonding Na. Cl n=3 - n=2 - - - - Na [Ne]3 s](https://slidetodoc.com/presentation_image_h/c5581b3582d1a05a84f89975c81019a3/image-19.jpg)
Ionic Bonding Na. Cl n=3 - n=2 - - - - Na [Ne]3 s 1 - - - + - - - - - Cl [Ne]3 s 23 p 5 - - - Na+ [Ne] - - - Cl[Ne]3 s 23 p 6 Transfer of electrons to achieve a stable octet (8 electrons in valence shell).

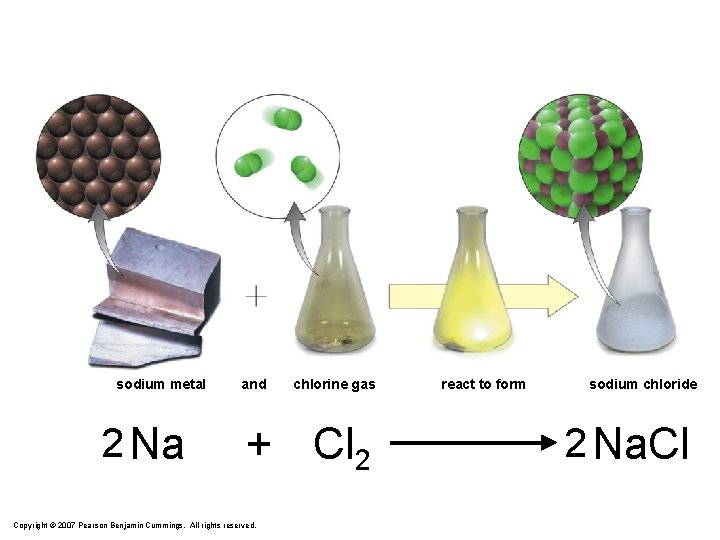
sodium metal 2 Na and chlorine gas + Cl 2 Copyright © 2007 Pearson Benjamin Cummings. All rights reserved. react to form sodium chloride 2 Na. Cl

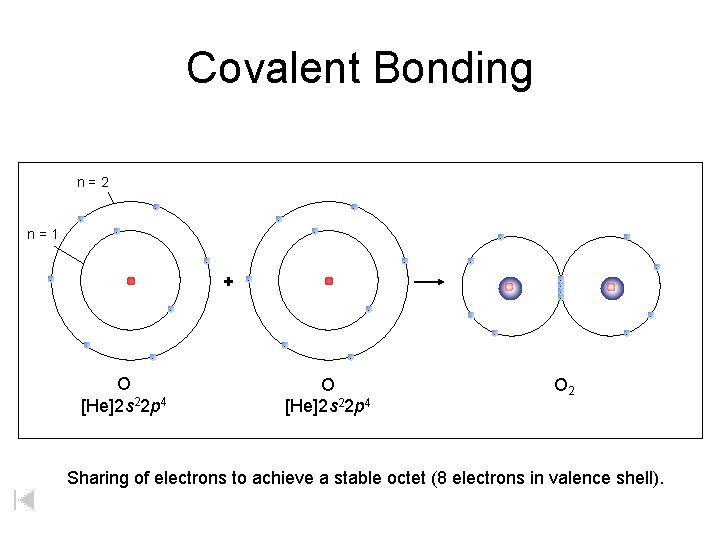
Covalent Bonding n=2 - - n=1 - - - + - - - - O [He]2 s 22 p 4 O 2 Sharing of electrons to achieve a stable octet (8 electrons in valence shell).

Properties of Salts VERY HARD each ion is bonded to several oppositelycharged ions HIGH MELTING POINTS many bonds must be broken BRITTLE with sufficient force, like atoms are brought next to each other and repel

Vocabulary l Chemical Bond – attractive force between atoms or ions that binds them together as a unit – bonds form in order to… • decrease potential energy (PE) • increase stability Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

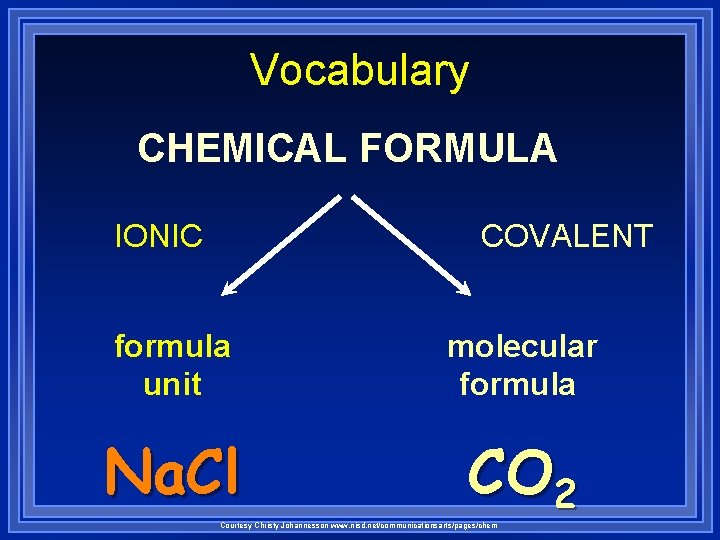
Vocabulary CHEMICAL FORMULA IONIC COVALENT formula unit molecular formula Na. Cl CO 2 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

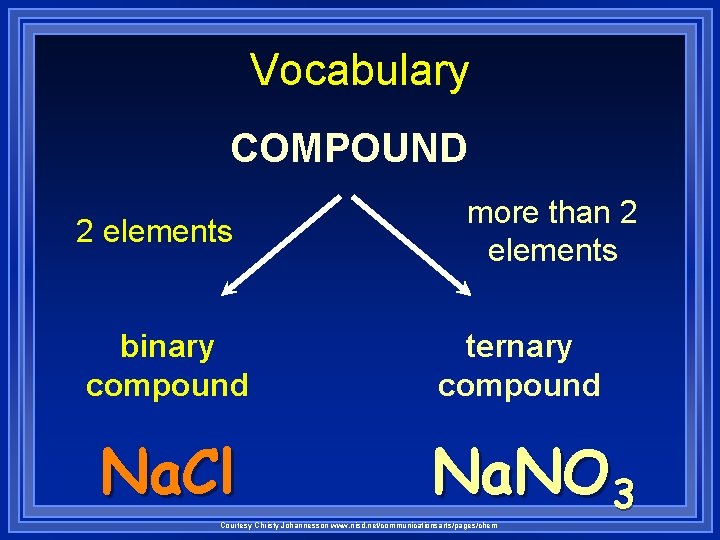
Vocabulary COMPOUND 2 elements binary compound Na. Cl more than 2 elements ternary compound Na. NO 3 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

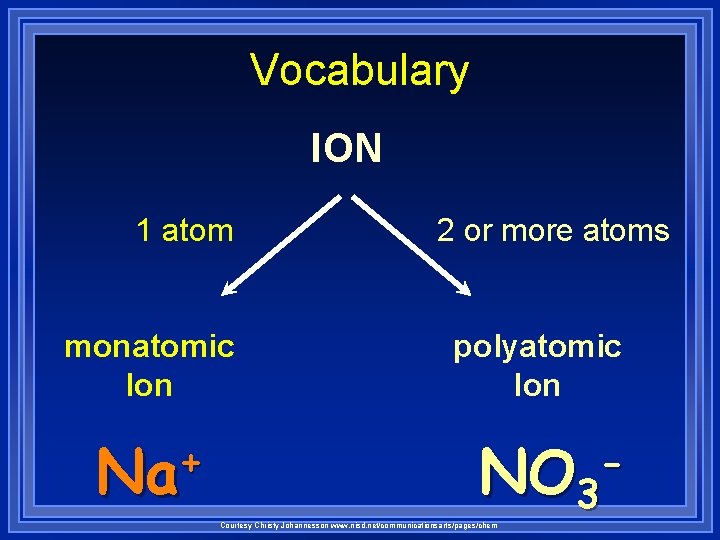
Vocabulary ION 1 atom monatomic Ion + Na 2 or more atoms polyatomic Ion NO 3 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

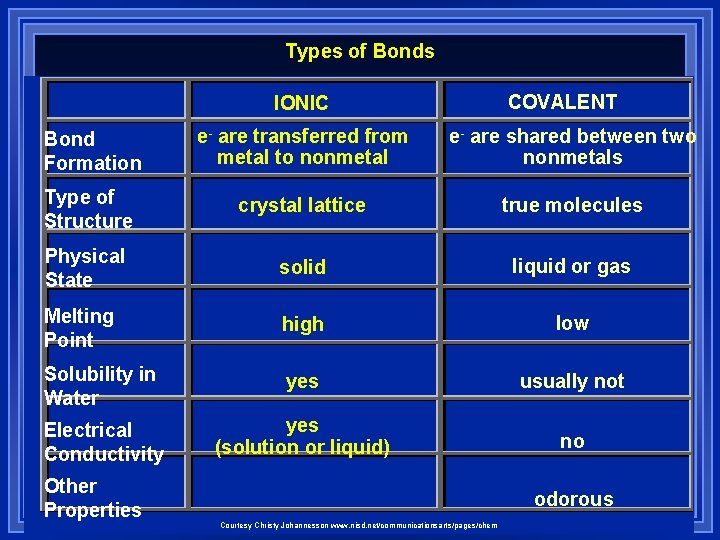
Types of Bonds COVALENT IONIC Bond Formation e- are transferred from metal to nonmetal e- are shared between two nonmetals Type of Structure crystal lattice true molecules Physical State solid liquid or gas Melting Point high low Solubility in Water yes usually not Electrical Conductivity yes (solution or liquid) no Other Properties odorous Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Types of Bonds METALLIC bond formation type of structure electrons are delocalized among metal atoms “electron sea” physical state solid melting point very high no solubility in water yes conductivity other properties (any form) malleable, ductile, lustrous Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

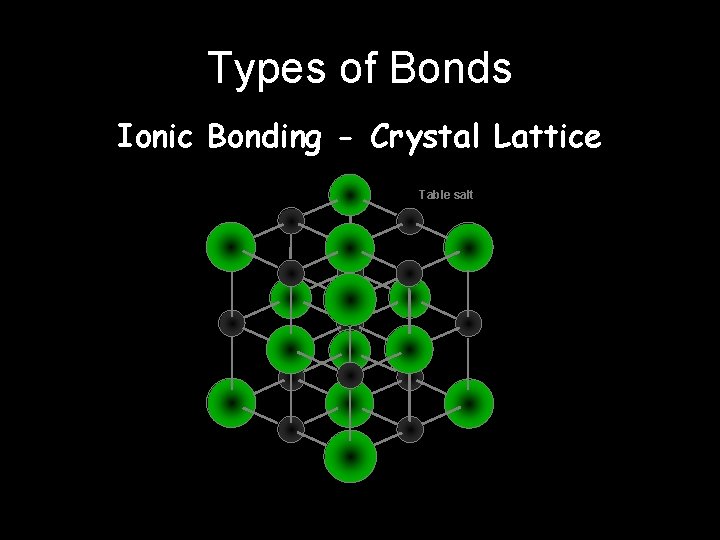
Types of Bonds Ionic Bonding - Crystal Lattice Table salt

Types of Bonds Ionic Bonding - Crystal Lattice Table salt

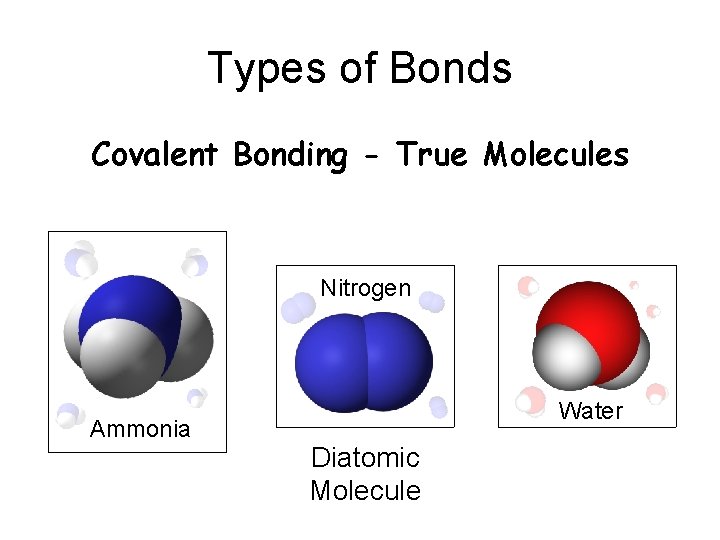
Types of Bonds Covalent Bonding - True Molecules Nitrogen Ammonia Water Diatomic Molecule

Lewis Structure Lewis structure: a model of a covalent molecule that shows all of the valence electrons 1. Two shared electrons make a single covalent bond, four make a double bond, etc. 2. unshared pairs: pairs of un-bonded valence electrons 3. Each atom needs a full outer shell, i. e. , 8 electrons. Exception: H needs 2 electrons

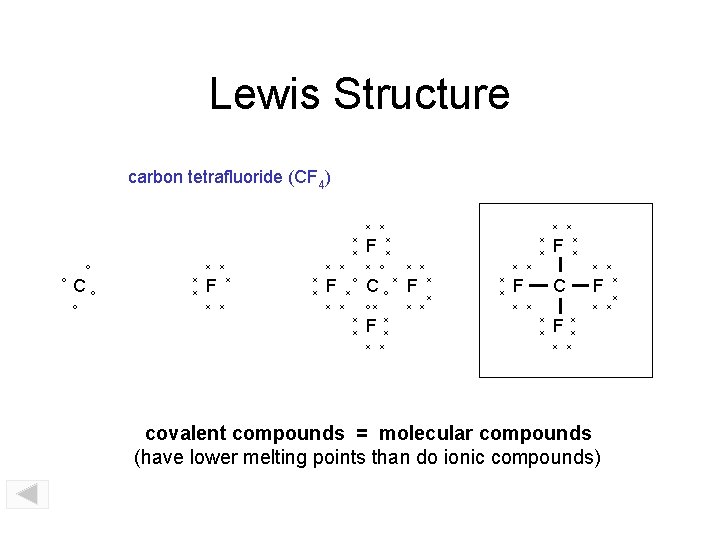
Lewis Structure carbon tetrafluoride (CF 4) x x x o o Co o x x F x x x x F x o x x x x o x x F x x Co F ox x x x x C x x F x x x covalent compounds = molecular compounds (have lower melting points than do ionic compounds) x x

Lewis Structure o o methane (CH 4) o o N o o C o I x x x Ox x x Ox o o o x x o I N I x x ox x x I x x x o o H Hx C ox H x x o o x o H o o nitrogen triiodide (NI 3) carbon dioxide (CO 2) o C o H H C H H H x x o o C o x x x x Ox x x I N x. Ix x x x I x xx x x o o xx O =C=O xx xx

Properties of Metals conduct heat and electricity; ductile; malleable Other Types of Bonds dipole-dipole forces hydrogen bonds London dispersion forces ion-dipole forces (solutions)

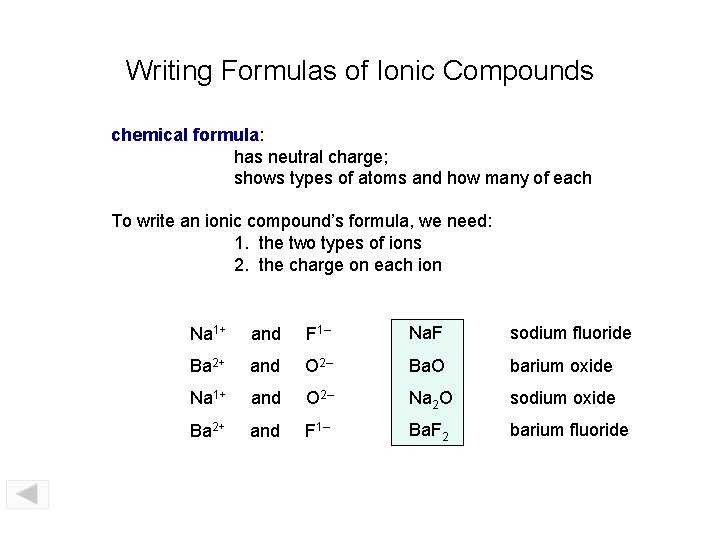
Writing Formulas of Ionic Compounds chemical formula: has neutral charge; shows types of atoms and how many of each To write an ionic compound’s formula, we need: 1. the two types of ions 2. the charge on each ion Na 1+ and F 1– Na. F sodium fluoride Ba 2+ and O 2– Ba. O barium oxide Na 1+ and O 2– Na 2 O sodium oxide Ba 2+ and F 1– Ba. F 2 barium fluoride

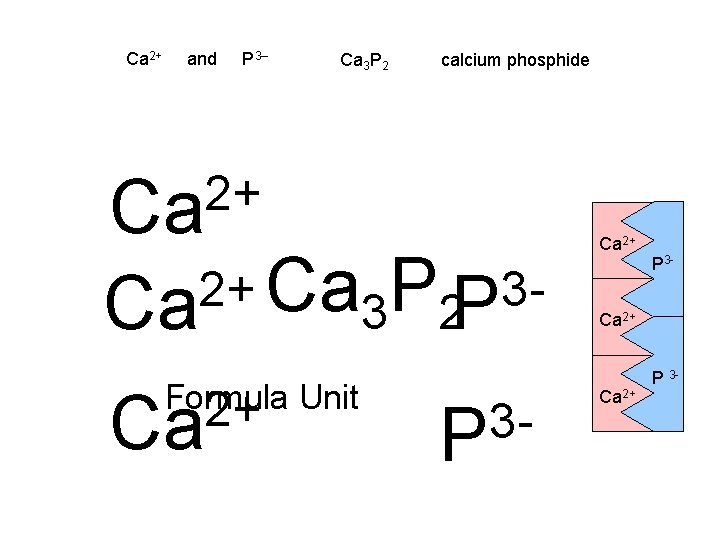
Ca 2+ and P 3– Ca 3 P 2 calcium phosphide 2+ Ca 2+ 3 Ca P Ca 3 2 P Formula Unit 2+ Ca 3 P Ca 2+ P 3 -

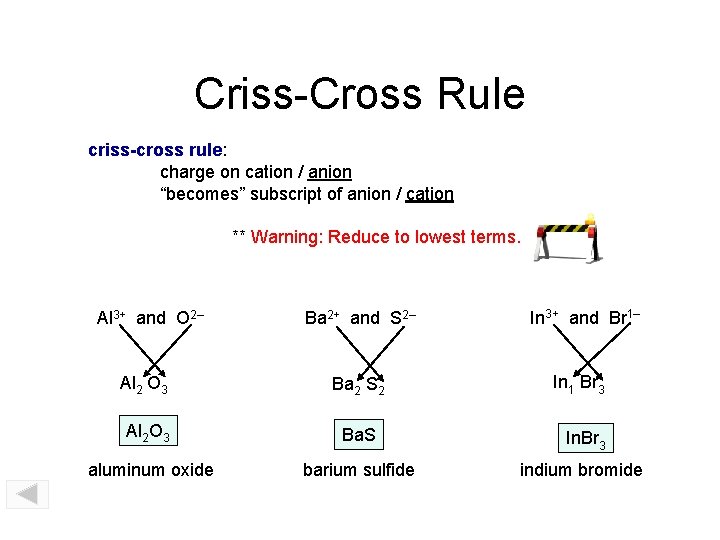
Criss-Cross Rule criss-cross rule: charge on cation / anion “becomes” subscript of anion / cation ** Warning: Reduce to lowest terms. Al 3+ and O 2– Ba 2+ and S 2– In 3+ and Br 1– In 1 Br 3 Al 2 O 3 Ba 2 S 2 Al 2 O 3 Ba. S In. Br 3 aluminum oxide barium sulfide indium bromide

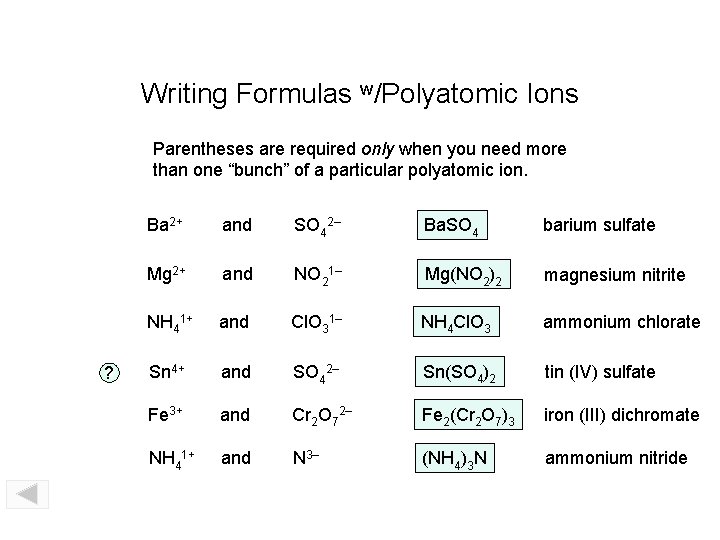
Writing Formulas w/Polyatomic Ions Parentheses are required only when you need more than one “bunch” of a particular polyatomic ion. ? Ba 2+ and SO 42– Ba. SO 4 barium sulfate Mg 2+ and NO 21– Mg(NO 2)2 magnesium nitrite NH 41+ and Cl. O 31– NH 4 Cl. O 3 ammonium chlorate Sn 4+ and SO 42– Sn(SO 4)2 tin (IV) sulfate Fe 3+ and Cr 2 O 72– Fe 2(Cr 2 O 7)3 iron (III) dichromate NH 41+ and N 3– (NH 4)3 N ammonium nitride

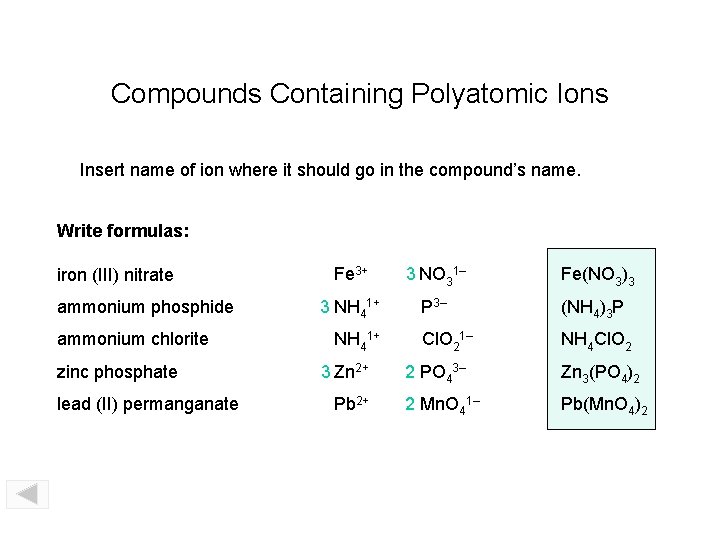
Compounds Containing Polyatomic Ions Insert name of ion where it should go in the compound’s name. Write formulas: iron (III) nitrate ammonium phosphide ammonium chlorite zinc phosphate lead (II) permanganate Fe 3+ 3 NO 31– Fe(NO 3)3 3 NH 41+ P 3– (NH 4)3 P Cl. O 21– NH 4 Cl. O 2 NH 41+ 3 Zn 2+ Pb 2+ 2 PO 43– Zn 3(PO 4)2 2 Mn. O 41– Pb(Mn. O 4)2

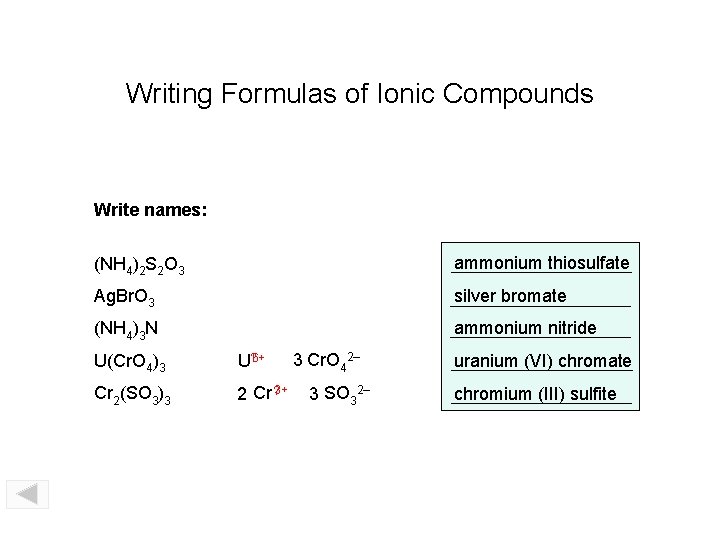
Writing Formulas of Ionic Compounds Write names: (NH 4)2 S 2 O 3 ammonium thiosulfate Ag. Br. O 3 silver bromate (NH 4)3 N ammonium nitride U(Cr. O 4)3 U? 6+ Cr 2(SO 3)3 2 Cr ? 3+ 3 Cr. O 42– 3 SO 32– uranium (VI) chromate chromium (III) sulfite

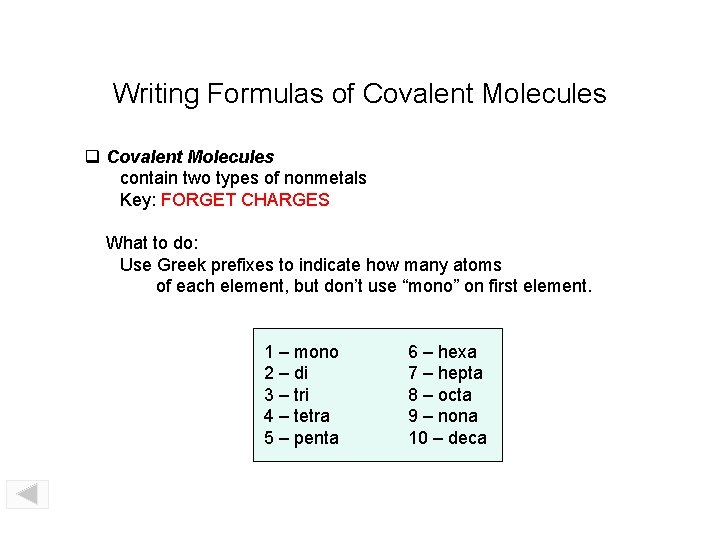
Writing Formulas of Covalent Molecules contain two types of nonmetals Key: FORGET CHARGES What to do: Use Greek prefixes to indicate how many atoms of each element, but don’t use “mono” on first element. 1 – mono 2 – di 3 – tri 4 – tetra 5 – penta 6 – hexa 7 – hepta 8 – octa 9 – nona 10 – deca

Writing Formulas of Covalent Molecules EXAMPLES: carbon dioxide CO dinitrogen trioxide N 2 O 5 carbon tetrachloride NI 3 CO 2 carbon monoxide N 2 O 3 dinitrogen pentoxide CCl 4 nitrogen triiodide

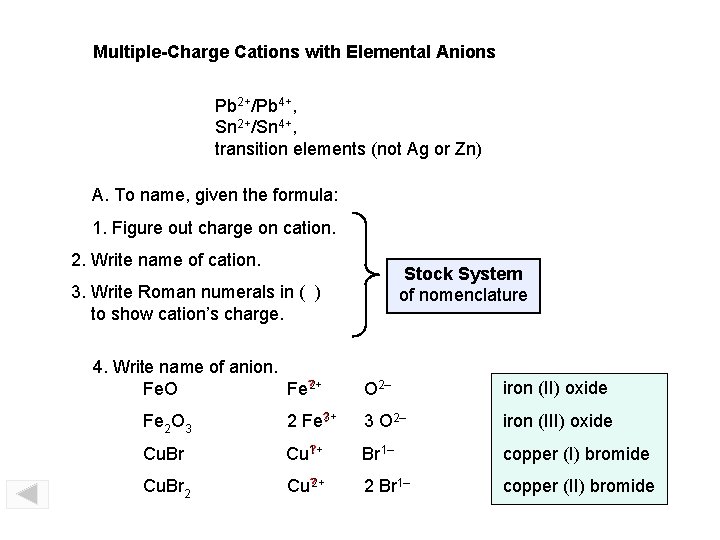
Multiple-Charge Cations with Elemental Anions Pb 2+/Pb 4+, Sn 2+/Sn 4+, transition elements (not Ag or Zn) A. To name, given the formula: 1. Figure out charge on cation. 2. Write name of cation. Stock System of nomenclature 3. Write Roman numerals in ( ) to show cation’s charge. 4. Write name of anion. Fe? 2+ Fe. O O 2– iron (II) oxide Fe 2 O 3 ? 2 Fe 3+ 3 O 2– iron (III) oxide Cu. Br ? Cu 1+ Br 1– copper (I) bromide Cu. Br 2 Cu? 2+ 2 Br 1– copper (II) bromide

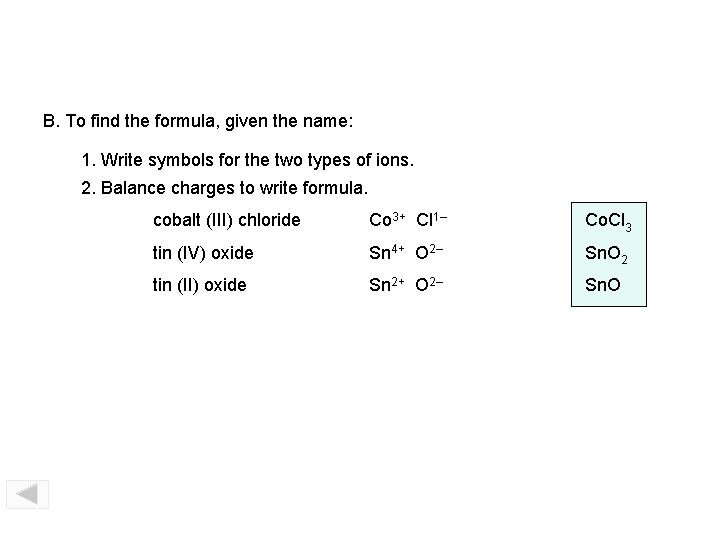
B. To find the formula, given the name: 1. Write symbols for the two types of ions. 2. Balance charges to write formula. cobalt (III) chloride Co 3+ Cl 1– Co. Cl 3 tin (IV) oxide Sn 4+ O 2– Sn. O 2 tin (II) oxide Sn 2+ O 2– Sn. O

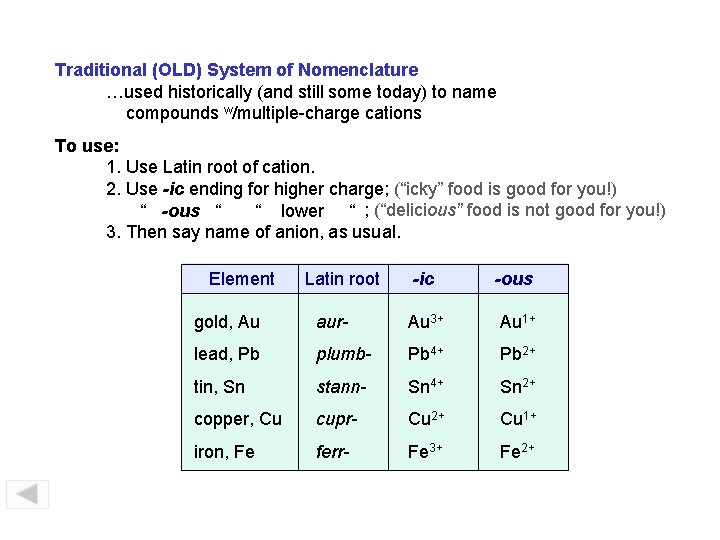
Traditional (OLD) System of Nomenclature …used historically (and still some today) to name compounds w/multiple-charge cations To use: 1. Use Latin root of cation. 2. Use -ic ending for higher charge; (“icky” food is good for you!) “ -ous “ “ lower “ ; (“delicious” food is not good for you!) 3. Then say name of anion, as usual. Element Latin root -ic -ous gold, Au aur- Au 3+ Au 1+ lead, Pb plumb- Pb 4+ Pb 2+ tin, Sn stann- Sn 4+ Sn 2+ copper, Cu cupr- Cu 2+ Cu 1+ iron, Fe ferr- Fe 3+ Fe 2+

Write formulas: cuprous sulfide Cu 1+ S 2– Write names: Cu 2 S Pb 3 P 4 copper (I) sulfide auric nitride Au 3+ N 3– lead (IV) phosphide Au. N Pb 3 P 2 lead (II) phosphide Sn. Cl 4 ferrous fluoride iron (II) fluoride 3 Pb? 2 P 3– plumbous phosphide gold (III) nitride Fe 2+ F 1– 3 Pb? 4 P 3– plumbic phosphide Fe. F 2 Sn? 4 Cl 1– stannic chloride tin (IV) chloride

K 1+ e- e- potassium atom 1 Br. Br bromine atom K Br e- bromine atom potassium atom K 1+ bromide ion potassium ion bromide potassium ion Br 1 bromide ion KBr Br 1 - K 1+ Mg 2+ O 2 Br 1 - magnesium bromide Mg. Br 2 K 1+ potassium oxide K 2 O

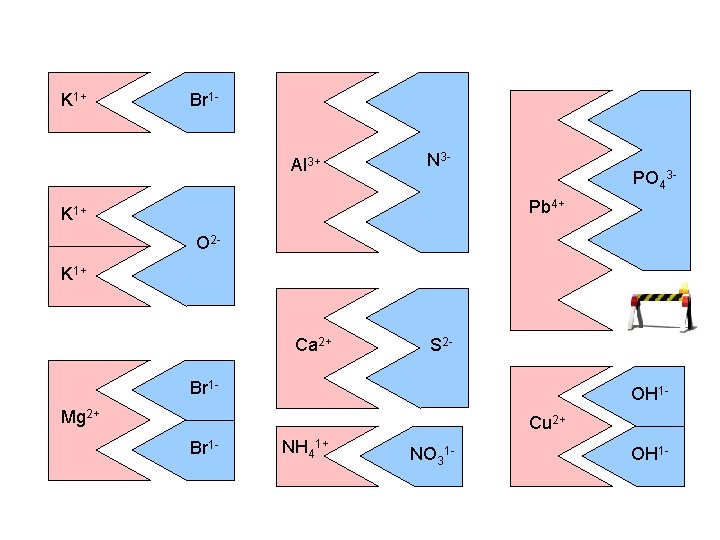
K 1+ Br 1 Al 3+ N 3 - PO 43 Pb 4+ K 1+ O 2 K 1+ ? Ca 2+ S 2 - Br 1 - OH 1 - Mg 2+ Cu 2+ Br 1 - NH 41+ NO 31 - OH 1 -

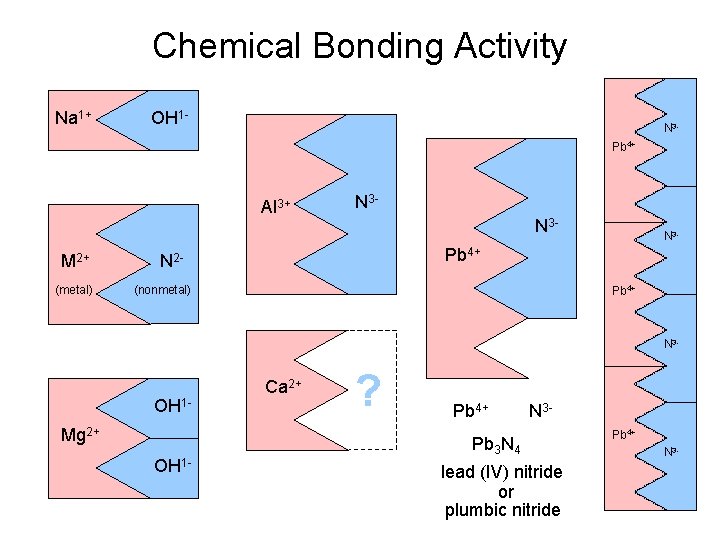
Chemical Bonding Activity Na 1+ OH 1 - N 3 Pb 4+ Al 3+ N 3 N 3 - M 1+ (metal) M 2+ (metal) M 1+ (metal) N 3 - Pb 4+ N 2(nonmetal) Pb 4+ N 3 - OH 1 Mg 2+ OH 1 - Ca 2+ ? Pb 4+ N 3 - Pb 3 N 4 lead (IV) nitride or plumbic nitride Pb 4+ N 3 -

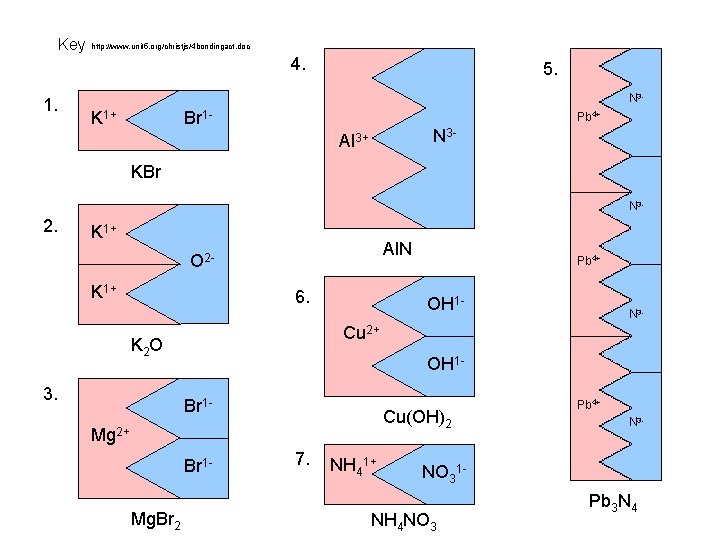
Key http: //www. unit 5. org/christjs/4 bondingact. doc 4. 1. 5. N 3 - K 1+ Br 1 - Pb 4+ N 3 - Al 3+ KBr N 3 - 2. K 1+ Al. N O 2 K 1+ 6. OH 1 - Br 1 - Cu(OH)2 Mg 2+ Br 1 Mg. Br 2 N 3 - Cu 2+ K 2 O 3. Pb 4+ 7. NH 41+ Pb 4+ N 3 - NO 31 - NH 4 NO 3 Pb 3 N 4

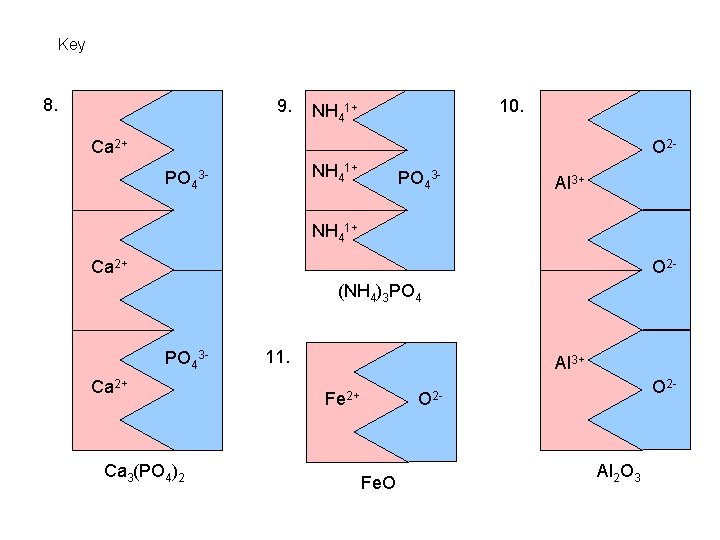
Key 8. 9. 10. NH 41+ Ca 2+ O 2 PO 4 NH 41+ 3 - PO 43 - Al 3+ NH 41+ Ca 2+ O 2(NH 4)3 PO 43 - Ca 2+ Ca 3(PO 4)2 11. Al 3+ Fe 2+ O 2 - Fe. O Al 2 O 3

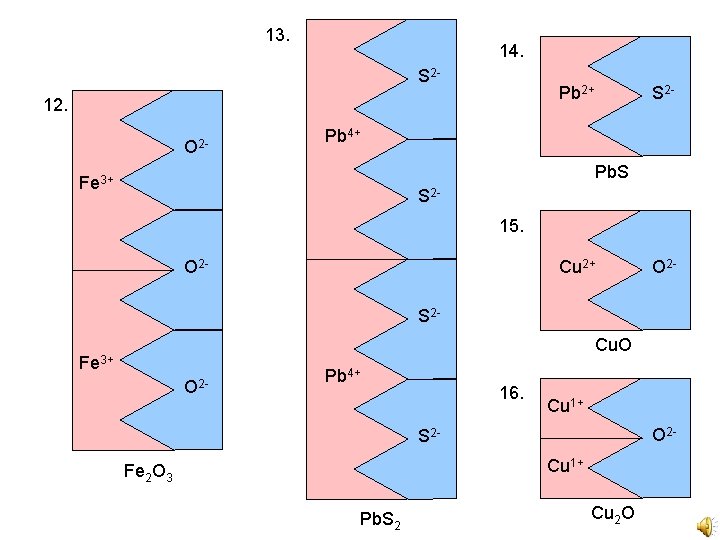
13. Key 14. S 2 - Pb 2+ 12. O 2 - S 2 - Pb 4+ Pb. S Fe 3+ S 215. O 2 - Cu 2+ O 2 - S 2 Cu. O Fe 3+ O 2 - Pb 4+ 16. Cu 1+ O 2 - S 2 Cu 1+ Fe 2 O 3 Pb Pb. S 2 S 24 3 Cu 2 O

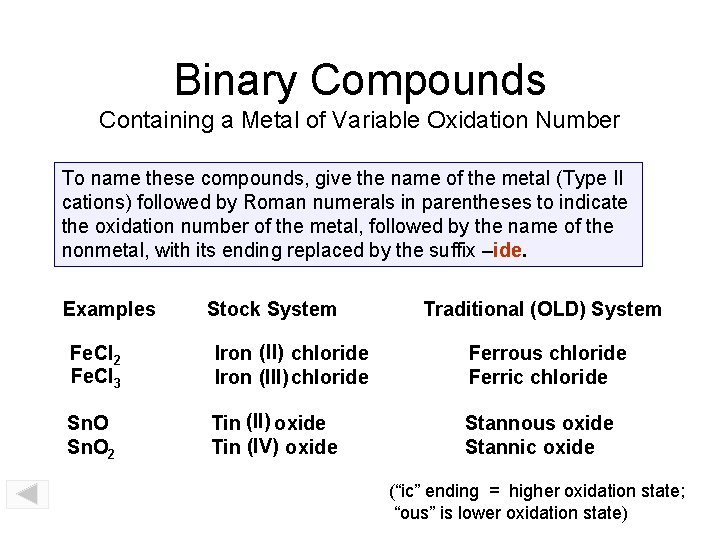
Binary Compounds Containing a Metal of Variable Oxidation Number To name these compounds, give the name of the metal (Type II cations) followed by Roman numerals in parentheses to indicate the oxidation number of the metal, followed by the name of the nonmetal, with its ending replaced by the suffix –ide. Examples Stock System Traditional (OLD) System Fe. Cl 2 Fe. Cl 3 Iron (II) chloride Iron (III) chloride Ferrous chloride Ferric chloride Sn. O 2 Tin (II) oxide Tin (IV) oxide Stannous oxide Stannic oxide (“ic” ending = higher oxidation state; “ous” is lower oxidation state)

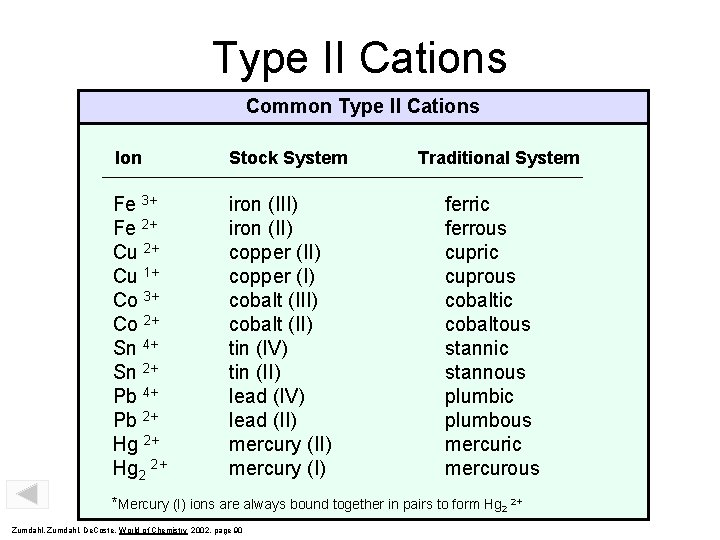
Type II Cations Common Type II Cations Ion Stock System Fe 3+ Fe 2+ Cu 1+ Co 3+ Co 2+ Sn 4+ Sn 2+ Pb 4+ Pb 2+ Hg 2 2+ iron (III) iron (II) copper (I) cobalt (II) tin (IV) tin (II) lead (IV) lead (II) mercury (I) Traditional System ferric ferrous cupric cuprous cobaltic cobaltous stannic stannous plumbic plumbous mercuric mercurous *Mercury (I) ions are always bound together in pairs to form Hg 2 2+ Zumdahl, De. Coste, World of Chemistry 2002, page 90

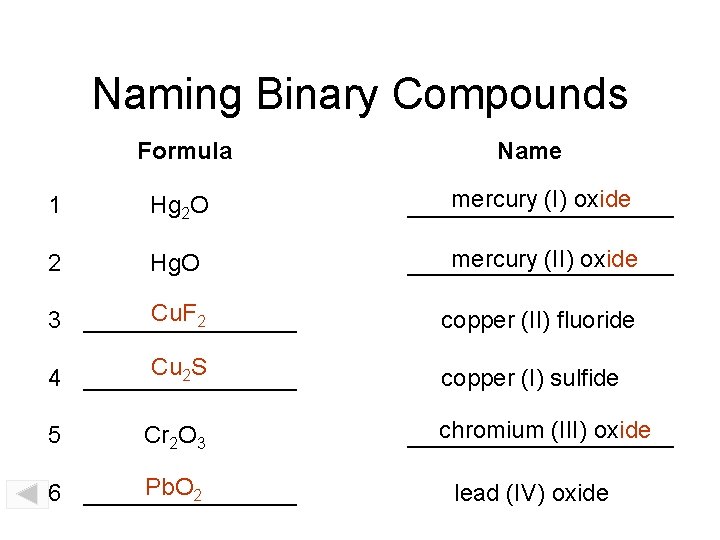
Naming Binary Compounds Formula Name 1 Hg 2 O mercury (I) oxide __________ 2 Hg. O mercury (II) oxide __________ Cu. F 2 3 ________ copper (II) fluoride Cu 2 S 4 ________ copper (I) sulfide 5 Cr 2 O 3 Pb. O 2 6 ________ chromium (III) oxide __________ lead (IV) oxide

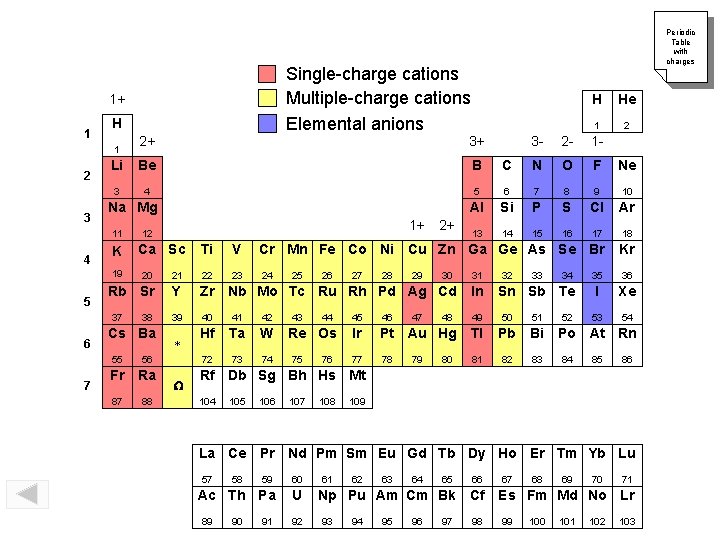
Single-charge cations Multiple-charge cations Elemental anions 1+ 1 H 2+ 3+ Li Be B 3 4 1 2 3 Na Mg 11 4 K 19 5 7 1+ 12 Ca Sc 2+ N O F Ne 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 Hf Ta W 72 73 74 55 56 Fr Ra 87 88 W 2 C Rb Sr * 1 1 - 22 Cs Ba He 2 - 21 38 H 3 - 20 37 6 Periodic Table with charges 25 43 26 44 Re Os 75 76 27 28 29 47 30 32 33 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 80 34 Sn Sb Te 45 79 48 31 52 Po At Rn 84 85 86 Rf Db Sg Bh Hs Mt 104 105 106 107 108 109 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 57 59 60 Ac Th Pa U 89 58 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103

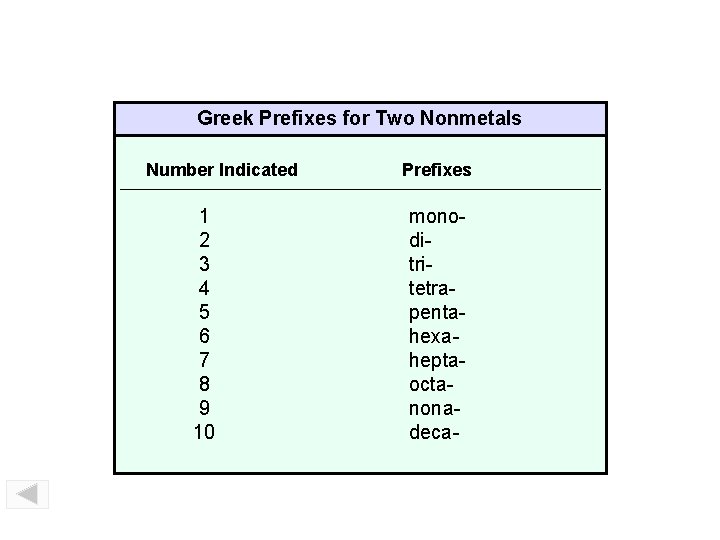
Binary Compounds Containing Two Nonmetals To name these compounds, give the name of the less electronegative element first with the Greek prefix indicating the number of atoms of that element present, followed by the name of the more electronegative nonmetal with the Greek prefix indicating the number of atoms of that element present and with its ending replaced by the suffix –ide. Prefixes you should know: Mono Di Tri Tetra Penta Hexa Hepta Octa Nona Deca 1 2 3 4 5 6 7 8 9 10

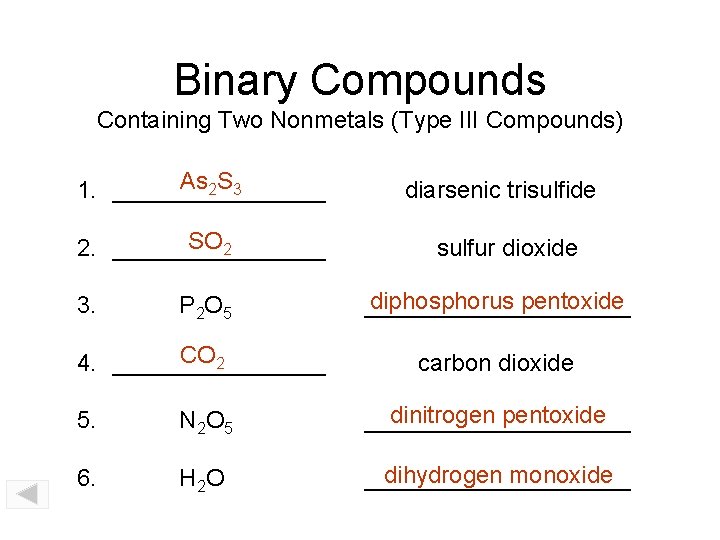
Binary Compounds Containing Two Nonmetals (Type III Compounds) As 2 S 3 1. ________ diarsenic trisulfide SO 2 2. ________ sulfur dioxide P 2 O 5 diphosphorus pentoxide __________ CO 2 4. ________ carbon dioxide 3. 5. N 2 O 5 dinitrogen pentoxide __________ 6. H 2 O dihydrogen monoxide __________

Greek Prefixes for Two Nonmetals Prefixes – Binary Molecular Compounds Number Indicated 1 2 3 4 5 6 7 8 9 10 Prefixes monoditritetrapentahexaheptaoctanonadeca-

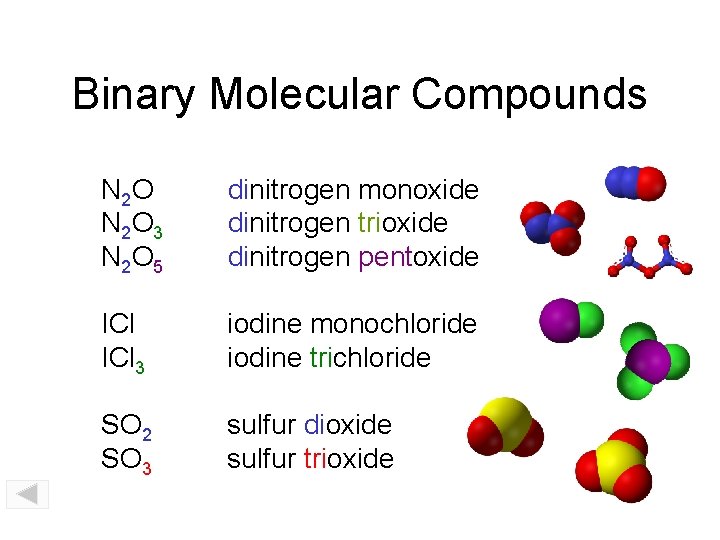
Binary Molecular Compounds N 2 O 3 N 2 O 5 dinitrogen monoxide dinitrogen trioxide dinitrogen pentoxide ICl 3 iodine monochloride iodine trichloride SO 2 SO 3 sulfur dioxide sulfur trioxide

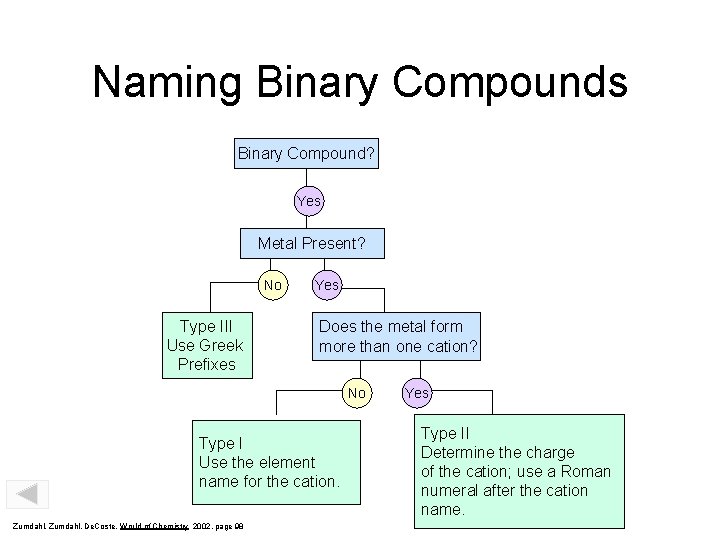
Naming Binary Compounds Binary Compound? Yes Metal Present? No Type III Use Greek Prefixes Yes Does the metal form more than one cation? No Type I Use the element name for the cation. Zumdahl, De. Coste, World of Chemistry 2002, page 98 Yes Type II Determine the charge of the cation; use a Roman numeral after the cation name.

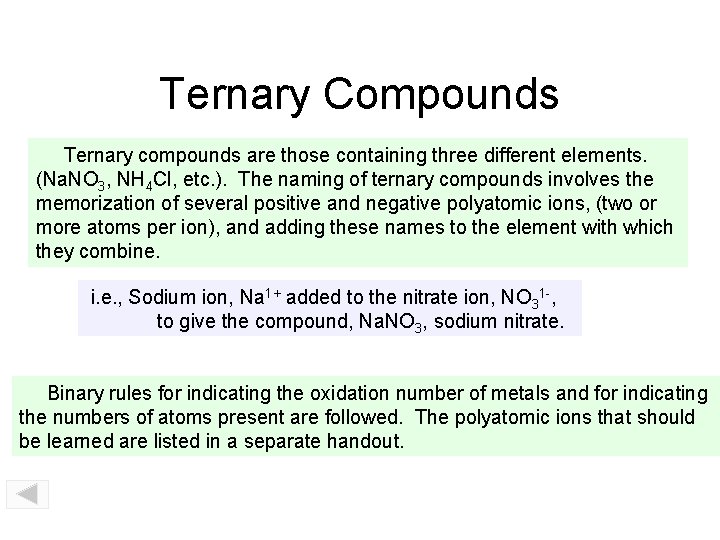
Ternary Compounds Ternary compounds are those containing three different elements. (Na. NO 3, NH 4 Cl, etc. ). The naming of ternary compounds involves the memorization of several positive and negative polyatomic ions, (two or more atoms per ion), and adding these names to the element with which they combine. i. e. , Sodium ion, Na 1+ added to the nitrate ion, NO 31 -, to give the compound, Na. NO 3, sodium nitrate. Binary rules for indicating the oxidation number of metals and for indicating the numbers of atoms present are followed. The polyatomic ions that should be learned are listed in a separate handout.

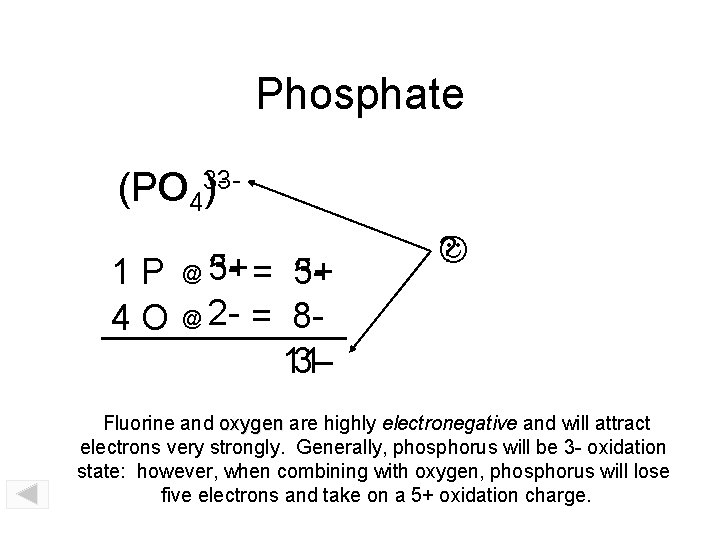
Phosphate (PO PO 43)31 P 4 O 5+ = 335+ @ 2 - = 8113@ ? Fluorine and oxygen are highly electronegative and will attract electrons very strongly. Generally, phosphorus will be 3 - oxidation state: however, when combining with oxygen, phosphorus will lose five electrons and take on a 5+ oxidation charge.

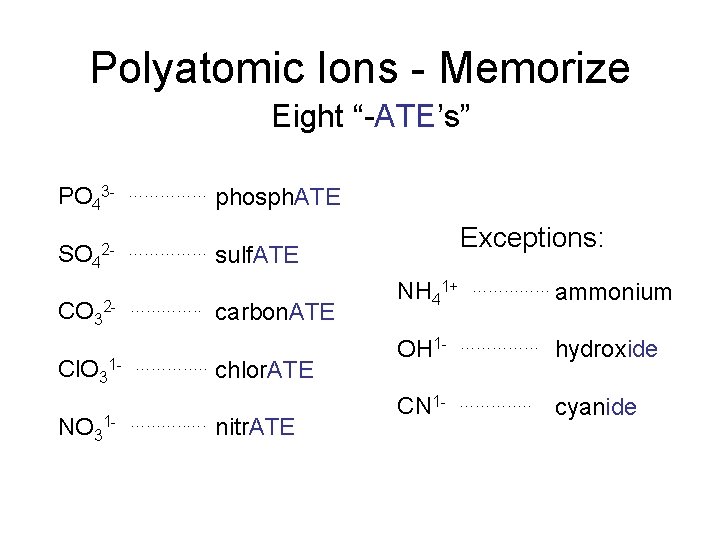
Polyatomic Ions - Memorize Eight “-ATE’s” PO 43 SO 4 …………… 2 - …………… CO 32 Cl. O 3 NO 3 …………. . 1 - ………. . …. phosphate phosph. ATE Exceptions: sulfate sulf. ATE carbonate carbon. ATE chlorate chlor. ATE nitrate nitr. ATE NH 41+ …………… ammonium OH 1 - …………… hydroxide CN 1 - …………. . cyanide

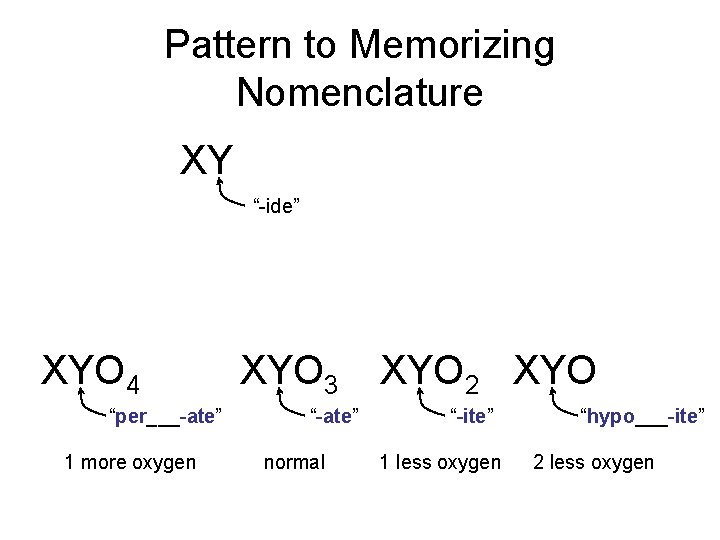
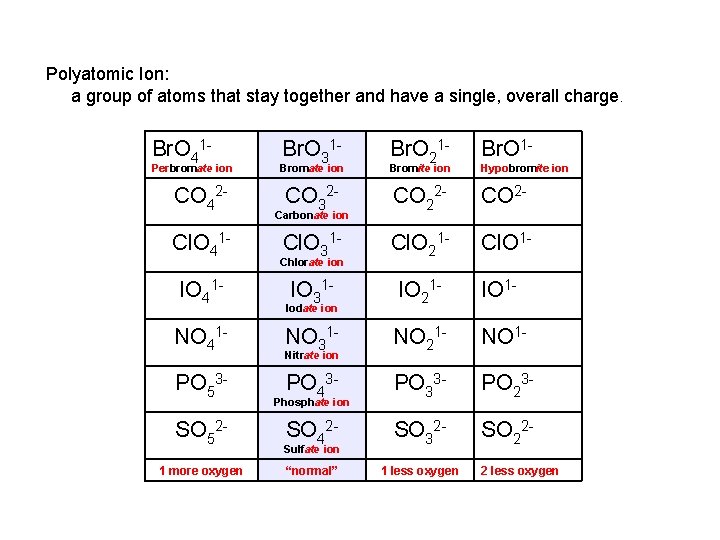
Pattern to Memorizing Nomenclature XY “-ide” XYO 4 “per___-ate” 1 more oxygen XYO 3 “-ate” normal XYO 2 XYO “-ite” 1 less oxygen “hypo___-ite” 2 less oxygen

Polyatomic Ion: a group of atoms that stay together and have a single, overall charge. Br. O 41 - Perbromate ion CO 42 Cl. O 41 IO 41 NO 41 PO 53 SO 521 more oxygen Br. O 31 - Br. O 1 - Bromate ion Br. O 21 - Bromite ion CO 32 - CO 2 - Cl. O 31 - Cl. O 21 - Cl. O 1 - IO 31 - IO 21 - IO 1 - NO 31 - NO 21 - NO 1 - PO 43 - PO 33 - PO 23 - SO 42 - SO 32 - SO 22 - “normal” 1 less oxygen Carbonate ion Chlorate ion Iodate ion Nitrate ion Phosphate ion Sulfate ion Hypobromite ion 2 less oxygen

Polyatomic Ion: a group of atoms that stay together and have a single, overall charge. Br. O 41 - Perbromate ion CO 42 Cl. O 41 IO 41 NO 41 PO 53 SO 521 more oxygen Br. O 31 - Br. O 1 - Bromate ion Br. O 21 - Bromite ion CO 32 - CO 2 - Cl. O 31 - Cl. O 21 - Cl. O 1 - IO 31 - IO 21 - IO 1 - NO 31 - NO 21 - NO 1 - PO 43 - PO 33 - PO 23 - SO 42 - SO 32 - SO 22 - “normal” 1 less oxygen Carbonate ion Chlorate ion Iodate ion Nitrate ion Phosphate ion Sulfate ion Hypobromite ion 2 less oxygen

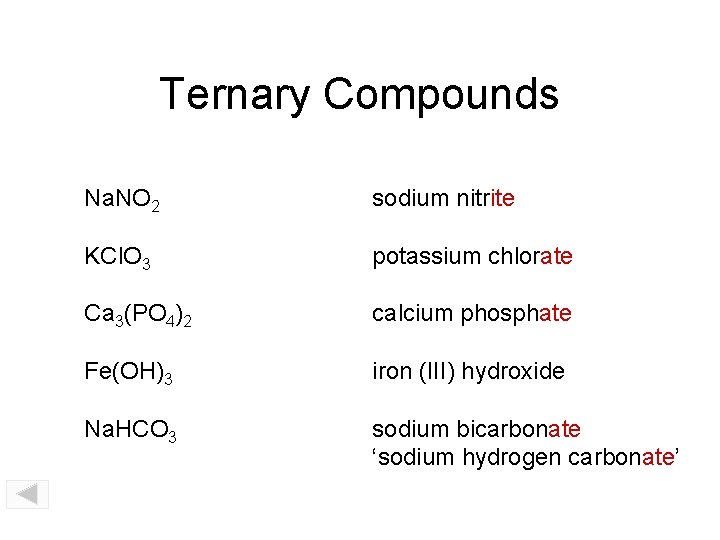
Ternary Compounds Na. NO 2 sodium nitrite KCl. O 3 potassium chlorate Ca 3(PO 4)2 calcium phosphate Fe(OH)3 iron (III) hydroxide Na. HCO 3 sodium bicarbonate ‘sodium hydrogen carbonate’

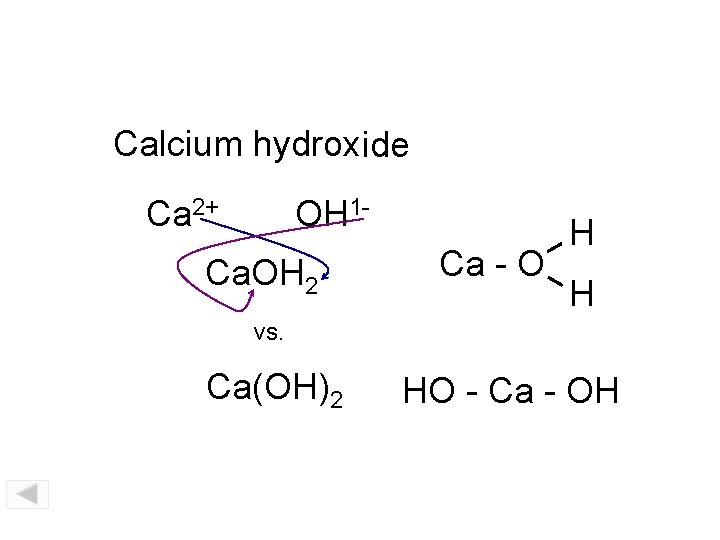
Calcium hydroxide Ca 2+ OH 1 - Ca. OH 2 Ca - O H H vs. Ca(OH)2 HO - Ca - OH

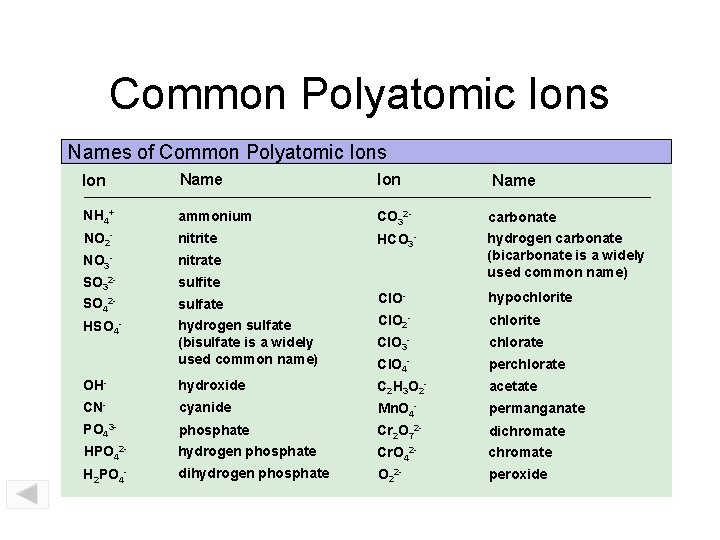
Common Polyatomic Ions Names of Common Polyatomic Ions Ion Name NH 4 1+ NO 2 1 NO 3 1 SO 3 2 SO 4 2 HSO 4 1 - ammonium nitrite nitrate sulfite sulfate hydrogen sulfate (“bisulfate” is a widely used common name) hydroxide cyanide phosphate hydrogen phosphate dihydrogen phosphate CO 3 2 HCO 3 1 - carbonate hydrogen carbonate (“bicarbonate” is a widely used common name) hypochlorite chlorate perchlorate acetate permanganate dichromate peroxide OH 1 CN 1 PO 4 3 HPO 4 2 H 2 PO 4 1 - Zumdahl, De. Coste, World of Chemistry 2002, page 100 Cl. O 1 Cl. O 2 1 Cl. O 3 1 Cl. O 4 1 C 2 H 3 O 2 2 Mn. O 4 1 Cr 2 O 7 2 Cr. O 4 2 O 2 2 - Print Version

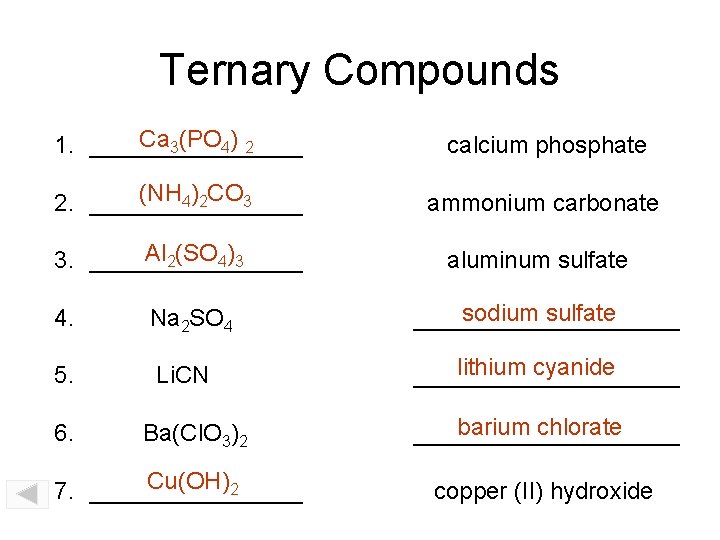
Ternary Compounds Ca 3(PO 4) 2 1. ________ calcium phosphate (NH 4)2 CO 3 2. ________ ammonium carbonate Al 2(SO 4)3 3. ________ aluminum sulfate 4. Na 2 SO 4 sodium sulfate __________ 5. Li. CN lithium cyanide __________ 6. Ba(Cl. O 3)2 Cu(OH)2 7. ________ barium chlorate __________ copper (II) hydroxide

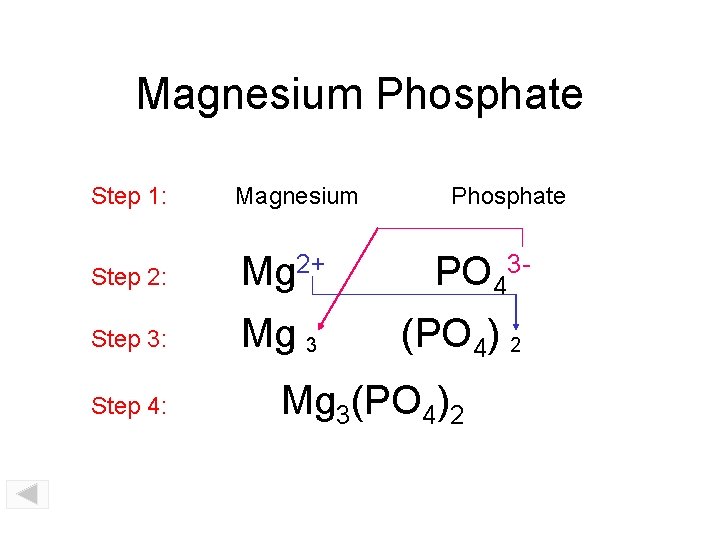
Magnesium Phosphate Step 1: Magnesium Step 2: Mg 2+ PO 43 - Step 3: Mg 3 (PO 4) 2 Step 4: Phosphate Mg 3(PO 4)2

variable Ir 2+, 3+, 4+, 6+ Ir 2 F(Cr 3 2 O 7 )3 iridium (III) dichromate fluoride Ca (OH) S 2 calcium hydroxide sulfide Ti (Cr. O S 2 4 )2 titanium (IV) chromate sulfide variable Ti 3+, 4+ Pt (CH Cl 2 3 COO)2 platinum (II) acetate chloride variable Pt 2+, 4+ Ba(Br. O Br 2 3)2 barium bromate bromide fixed Ba 2+ Sr 3 SO P 2 4 strontium sulfate phosphide fixed Sr 2+ KCN F potassium cyanide fluoride fixed K 1+ Zn (NO I 2 2 )2 zinc nitrite iodide fixed Zn 2+ Mn (Cl. O Cl 4 3)4 manganese (IV) chlorate chloride variable Mn 2, 3, 4, 6, 7+ Au PO 2 O 34 gold (III) phosphate oxide Na 3 NO P 3 sodium nitrate phosphide fixed Ca 2+ variable Au 1+, 3+ fixed Na 1+

variable Ir 2+, 3+, 4+, 6+ Ir F 3 iridium (III) fluoride Ca S calcium sulfide Ti S 2 titanium (IV) sulfide variable Ti 3+, 4+ Pt Cl 2 platinum (II) chloride variable Pt 2+, 4+ Ba. Br 2 barium bromide fixed Ba 2+ Sr 3 P 2 strontium phosphide fixed Sr 2+ KF potassium fluoride fixed K 1+ Zn I 2 zinc iodide fixed Zn 2+ Mn Cl 4 manganese (IV) chloride variable Mn 2, 3, 4, 6, 7+ Au 2 O 3 gold (III) oxide Na 3 P sodium phosphide fixed Ca 2+ variable Au 1+, 3+ fixed Na 1+

variable Ir 2+, 3+, 4+, 6+ Ir 2(Cr 2 O 7)3 iridium (III) dichromate Ca (OH)2 calcium hydroxide Ti (Cr. O 4)2 titanium (IV) chromate variable Ti 3+, 4+ Pt (CH 3 COO)2 platinum (II) acetate variable Pt 2+, 4+ Ba(Br. O 3)2 barium bromate fixed Ba 2+ Sr 3 SO 4 strontium sulfate fixed Sr 2+ KCN potassium cyanide fixed K 1+ Zn (NO 2)2 zinc nitrite fixed Zn 2+ Mn (Cl. O 3)4 manganese (IV) chlorate variable Mn 2, 3, 4, 6, 7+ Au PO 4 gold (III) phosphate Na NO 3 sodium nitrate fixed Ca 2+ variable Au 1+, 3+ fixed Na 1+

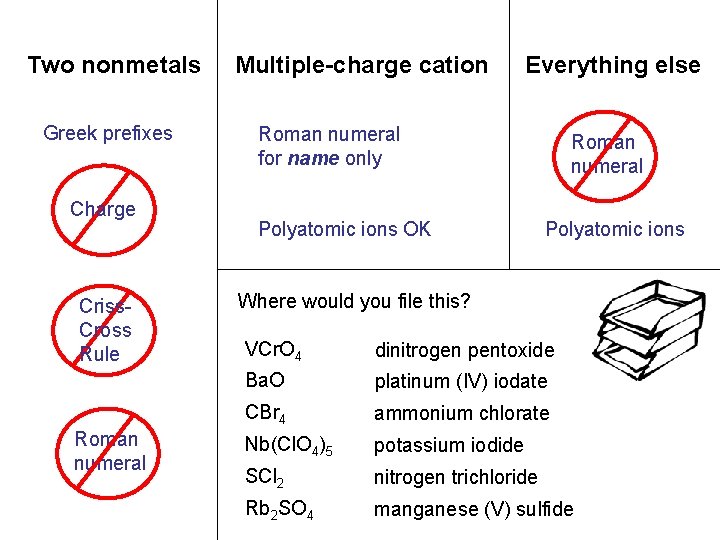
Two nonmetals Multiple-charge cation Everything else carbon sulfur. N tetrabromide dichloride NCl O 35 2 vanadium niobium. Mn Pt(IO (V) (II) perchlorate )4 2 S 53 chromate rubidium sulfate NH 4 KI Cl. O barium oxide 3 Greek prefixes Charge Criss. Cross Rule Roman numeral for name only Polyatomic ions OK Roman numeral Polyatomic ions OK Where would you file this? VCr. O 4 dinitrogen pentoxide Ba. O platinum (IV) iodate CBr 4 ammonium chlorate Nb(Cl. O 4)5 potassium iodide SCl 2 nitrogen trichloride Rb 2 SO 4 manganese (V) sulfide

Two nonmetals Greek prefixes Charge Criss. Cross Rule Roman numeral Multiple-charge cation Everything else Roman numeral for name only Polyatomic ions OK Roman numeral Polyatomic ions Where would you file this? VCr. O 4 dinitrogen pentoxide Ba. O platinum (IV) iodate CBr 4 ammonium chlorate Nb(Cl. O 4)5 potassium iodide SCl 2 nitrogen trichloride Rb 2 SO 4 manganese (V) sulfide

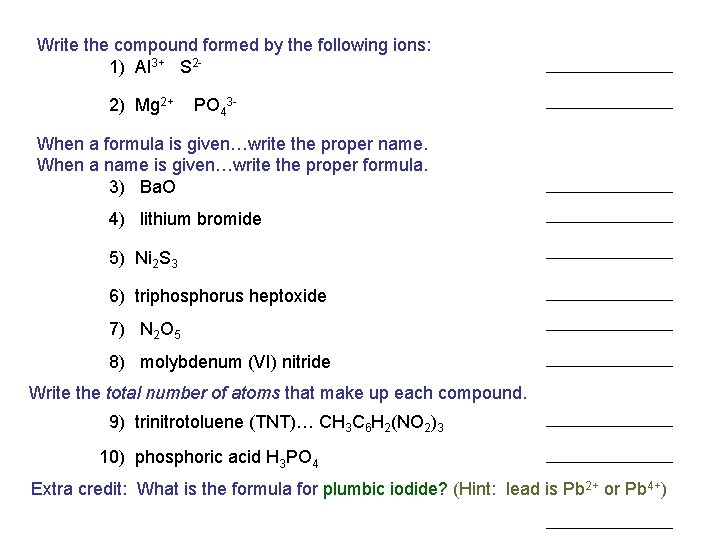
Write the compound formed by the following ions: 1) Al 3+ S 22) Mg 2+ PO 43 - When a formula is given…write the proper name. When a name is given…write the proper formula. 3) Ba. O 4) lithium bromide 5) Ni 2 S 3 6) triphosphorus heptoxide 7) N 2 O 5 8) molybdenum (VI) nitride Write the total number of atoms that make up each compound. 9) trinitrotoluene (TNT)… CH 3 C 6 H 2(NO 2)3 10) phosphoric acid H 3 PO 4 Extra credit: What is the formula for plumbic iodide? (Hint: lead is Pb 2+ or Pb 4+)

Write the compound formed by the following ions: 1) Al 3+ S 22) Mg 2+ PO 43 - When a formula is given…write the proper name. When a name is given…write the proper formula. 3) Ba. O POP QUIZ 4) lithium bromide 5) Ni 2 S 3 6) triphosphorus heptoxide 7) N 2 O 5 8) molybdenum (VI) nitride Write the total number of atoms that make up each compound. 9) trinitrotoluene (TNT)… CH 3 C 6 H 2(NO 2)3 10) phosphoric acid H 3 PO 4 Extra credit: What is the formula for plumbic iodide? (Hint: lead is Pb 2+ or Pb 4+)

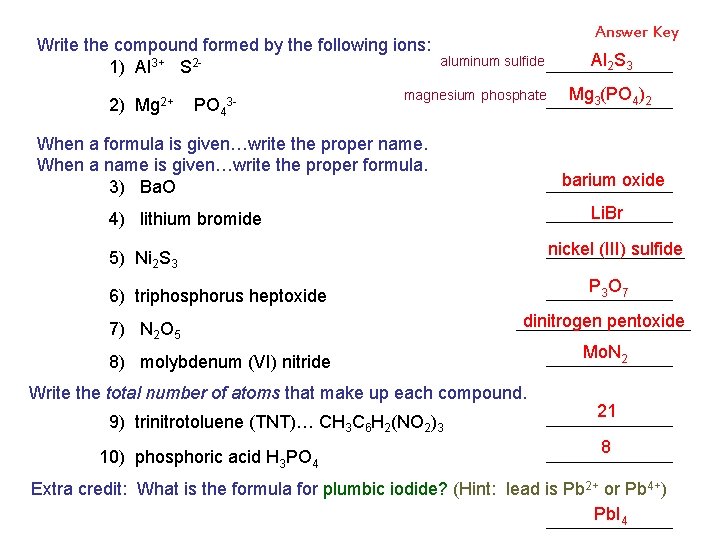
Write the compound formed by the following ions: 1) Al 3+ S 22) Mg 2+ PO 43 - Answer Key aluminum sulfide magnesium phosphate When a formula is given…write the proper name. When a name is given…write the proper formula. 3) Ba. O Li. Br nickel (III) sulfide 5) Ni 2 S 3 P 3 O 7 6) triphosphorus heptoxide dinitrogen pentoxide 8) molybdenum (VI) nitride Write the total number of atoms that make up each compound. 9) trinitrotoluene (TNT)… CH 3 C 6 H 2(NO 2)3 10) phosphoric acid H 3 PO 4 Mg 3(PO 4)2 barium oxide 4) lithium bromide 7) N 2 O 5 Al 2 S 3 Mo. N 2 21 8 Extra credit: What is the formula for plumbic iodide? (Hint: lead is Pb 2+ or Pb 4+) Pb. I 4

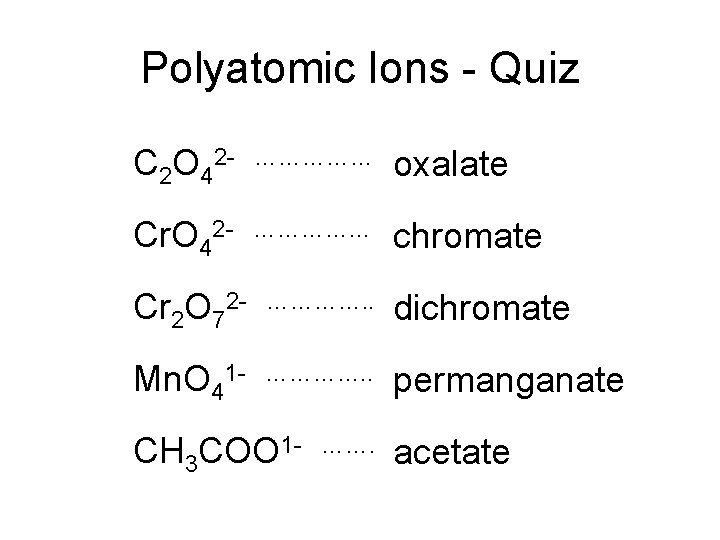
Polyatomic Ions - Quiz C 2 O 42 - …………… oxalate Cr. O 42 - …………… chromate Cr 2 O 72 - …………. . dichromate Mn. O 41 - …………. . permanganate CH 3 COO 1 - ……. acetate

Exceptions! Two exceptions to the simple –ide ending are the diatomic oxide ions, O 22 - and O 21 -. O 22 - is called peroxide Note the differences. O 21 - is called superoxide. barium oxide barium peroxide Ba. O _____ Ba. O 2 _____ sodium oxide sodium peroxide Na 2 O _____ Na 2 O 2 _____ potassium oxide potassium superoxide K 2 O _____ KO 2 _____ Ba 2+ Na 1+ Do Not Reduce to lowest terms! K 1+

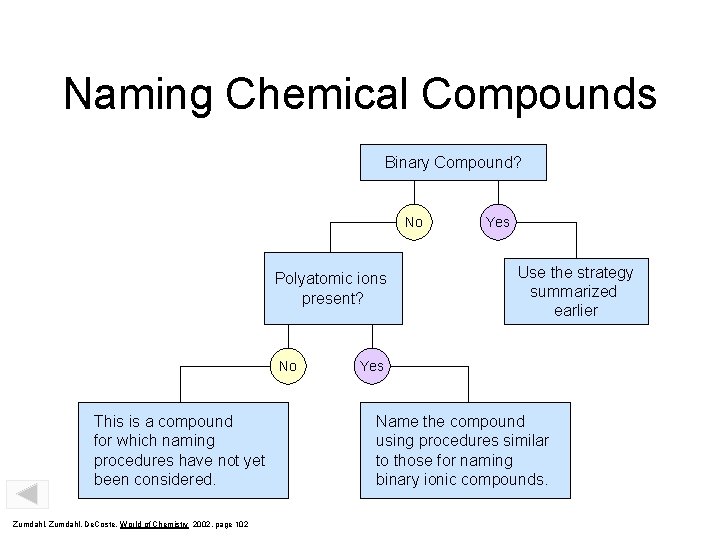
Naming Chemical Compounds Binary Compound? No Polyatomic ions present? No This is a compound for which naming procedures have not yet been considered. Zumdahl, De. Coste, World of Chemistry 2002, page 102 Yes Use the strategy summarized earlier Yes Name the compound using procedures similar to those for naming binary ionic compounds.

Nomenclature Review Flow Chart

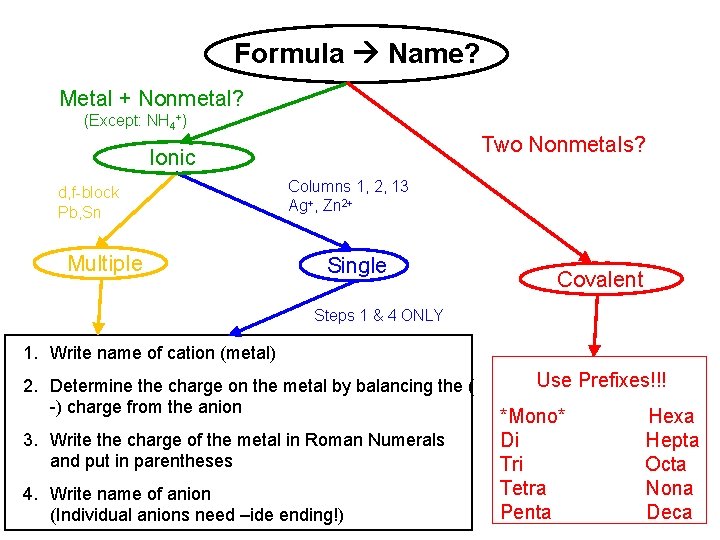
Formula Name? Metal + Nonmetal? (Except: NH 4+) Two Nonmetals? Ionic d, f-block Pb, Sn Multiple Columns 1, 2, 13 Ag+, Zn 2+ Single Covalent Steps 1 & 4 ONLY 1. Write name of cation (metal) 2. Determine the charge on the metal by balancing the ( -) charge from the anion 3. Write the charge of the metal in Roman Numerals and put in parentheses 4. Write name of anion (Individual anions need –ide ending!) Use Prefixes!!! *Mono* Di Tri Tetra Penta Hexa Hepta Octa Nona Deca

Name Formula? No Prefixes? Ionic Prefixes? Covalent 1. Determine the ions present and the charge on each (Roman Numeral = cation charge, otherwise use PT) 1. FORGET CHARGES!!! 2. Balance formula (criss-cross) 3. Do NOT reduce subscripts! 3. Reduce subscripts (if needed) 2. Use prefixes to determine subscripts

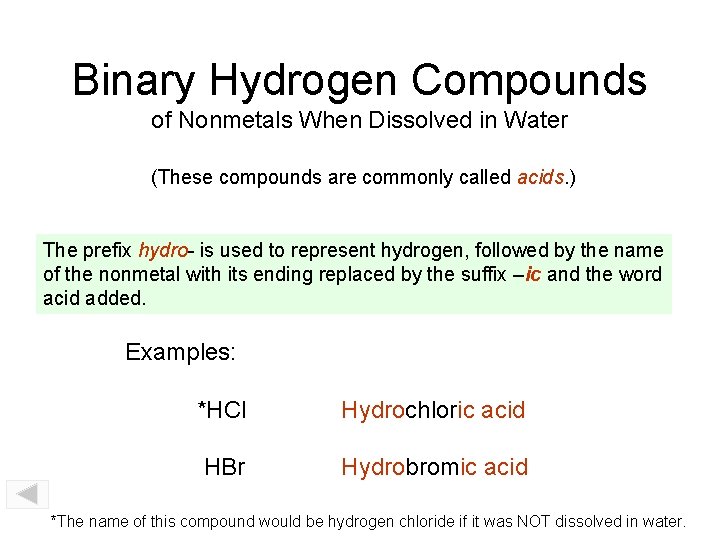
Binary Hydrogen Compounds of Nonmetals When Dissolved in Water (These compounds are commonly called acids. ) The prefix hydro- is used to represent hydrogen, followed by the name of the nonmetal with its ending replaced by the suffix –ic and the word acid added. Examples: *HCl Hydrochloric acid HBr Hydrobromic acid *The name of this compound would be hydrogen chloride if it was NOT dissolved in water.

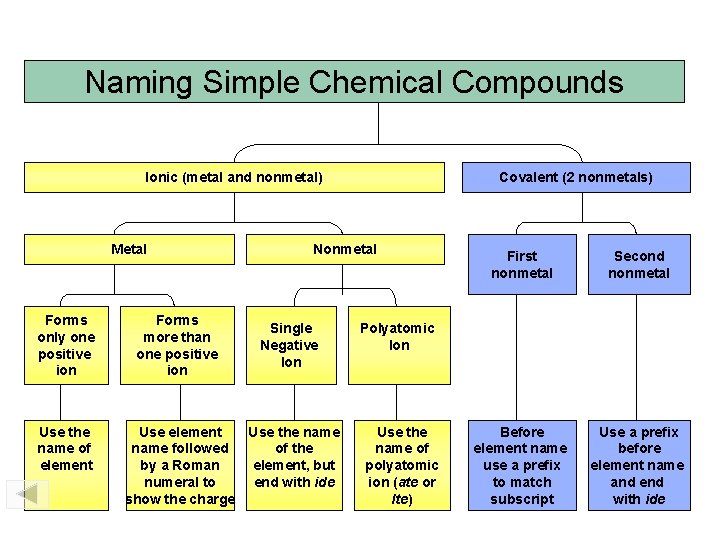
Naming Simple Chemical Compounds Ionic (metal and nonmetal) Metal Forms only one positive ion Use the name of element Forms more than one positive ion Covalent (2 nonmetals) Nonmetal Single Negative Ion Use element Use the name followed of the by a Roman element, but numeral to end with ide show the charge First nonmetal Second nonmetal Before element name use a prefix to match subscript Use a prefix before element name and end with ide Polyatomic Ion Use the name of polyatomic ion (ate or Ite)

Naming Ternary Compounds from Oxyacids The following table lists the most common families of oxy acids. one more oxygen atom HCl. O 4 perchloric acid most “common” HCl. O 3 chloric acid H 2 SO 4 sulfuric acid H 3 PO 4 phosphoric acid HNO 3 nitric acid one less oxygen HCl. O 2 chlorous acid H 2 SO 3 sulfurous acid H 3 PO 3 phosphorous acid HNO 2 nitrous acid two less oxygen HCl. O hypochlorous acid H 3 PO 2 hypophosphorous acid (HNO)2 hyponitrous acid

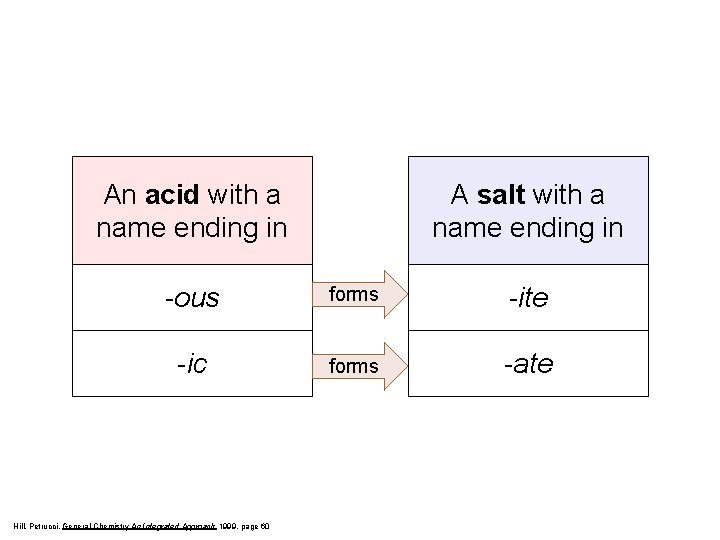
An acid with a name ending in A salt with a name ending in -ous forms -ite -ic forms -ate Hill, Petrucci, General Chemistry An Integrated Approach 1999, page 60

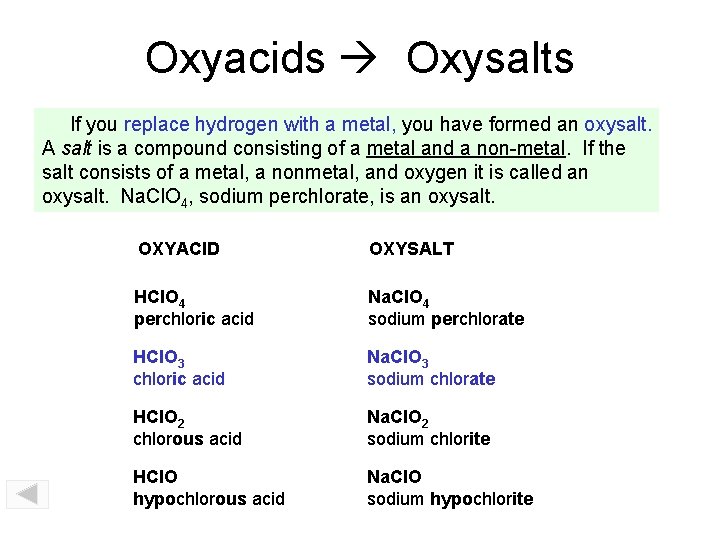
Oxyacids Oxysalts If you replace hydrogen with a metal, you have formed an oxysalt. A salt is a compound consisting of a metal and a non-metal. If the salt consists of a metal, a nonmetal, and oxygen it is called an oxysalt. Na. Cl. O 4, sodium perchlorate, is an oxysalt. OXYACID OXYSALT HCl. O 4 perchloric acid Na. Cl. O 4 sodium perchlorate HCl. O 3 chloric acid Na. Cl. O 3 sodium chlorate HCl. O 2 chlorous acid Na. Cl. O 2 sodium chlorite HCl. O hypochlorous acid Na. Cl. O sodium hypochlorite

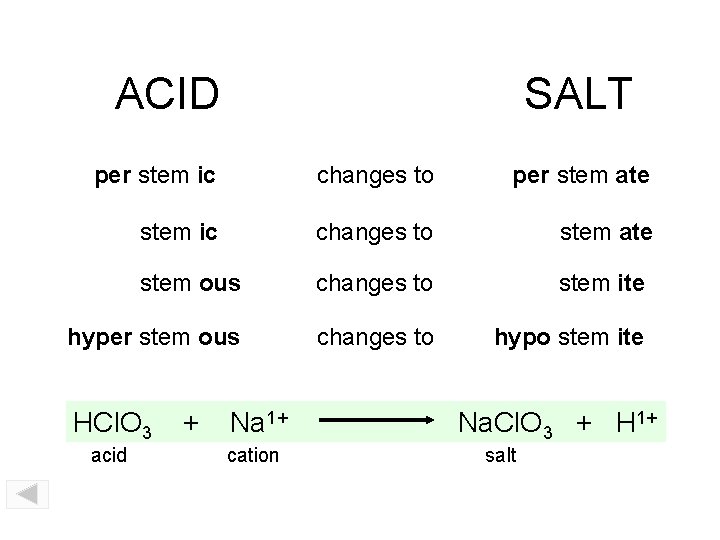
ACID SALT per stem ic changes to per stem ate stem ic changes to stem ate stem ous changes to stem ite hyper stem ous changes to hypo stem ite HCl. O 3 acid + Na 1+ cation Na. Cl. O 3 + H 1+ salt

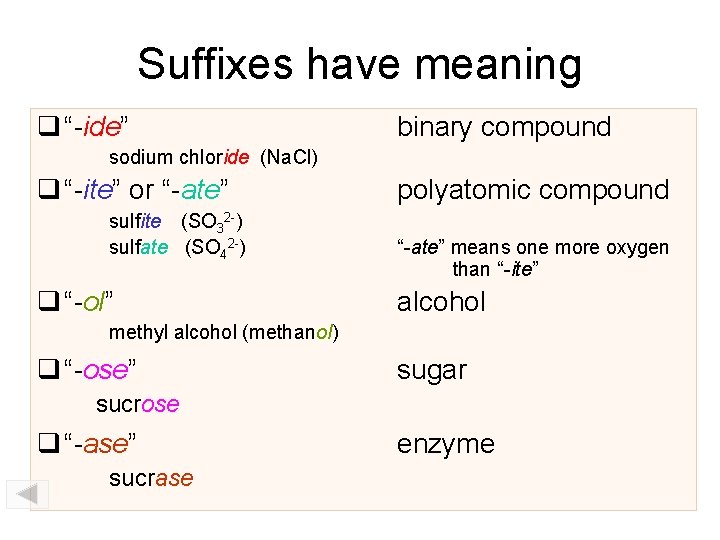
Suffixes have meaning “-ide” binary compound sodium chloride (Na. Cl) “-ite” or “-ate” sulfite (SO 32 -) sulfate (SO 42 -) “-ol” polyatomic compound “-ate” means one more oxygen than “-ite” alcohol methyl alcohol (methanol) “-ose” sugar sucrose “-ase” sucrase enzyme

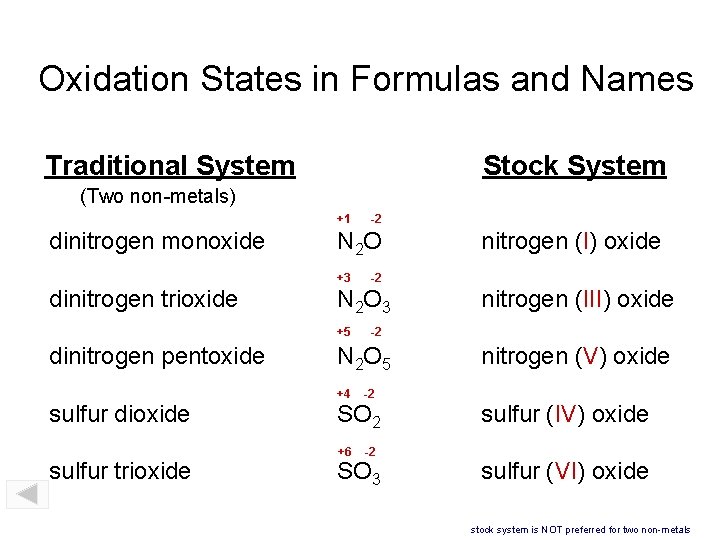
Oxidation States in Formulas and Names Traditional System Stock System (Two non-metals) +1 dinitrogen monoxide N 2 O +3 dinitrogen trioxide sulfur dioxide sulfur trioxide nitrogen (I) oxide -2 N 2 O 3 +5 dinitrogen pentoxide -2 nitrogen (III) oxide -2 N 2 O 5 +4 -2 +6 -2 SO 3 nitrogen (V) oxide sulfur (IV) oxide sulfur (VI) oxide stock system is NOT preferred for two non-metals

Percentage Composition (by mass. . . not atoms) 24. 305 35. 453 Mg Cl 12 17 magnesium chlorine partg 24 % Mg % = whole x 100 95 g 25. 52% Mg Mg 2+ Cl 174. 48% Cl Mg. Cl 2 It is not 33% Mg and 66% Cl 1 Mg @ 24. 305 amu = 24. 305 amu 2 Cl @ 35. 453 amu = 70. 906 amu 95. 211 amu

Empirical and Molecular Formulas A pure compound always consists of the same elements combined in the same proportions by weight. Therefore, we can express molecular composition as PERCENT BY WEIGHT Ethanol, C 2 H 6 O 52. 13% C 13. 15% H 34. 72% O

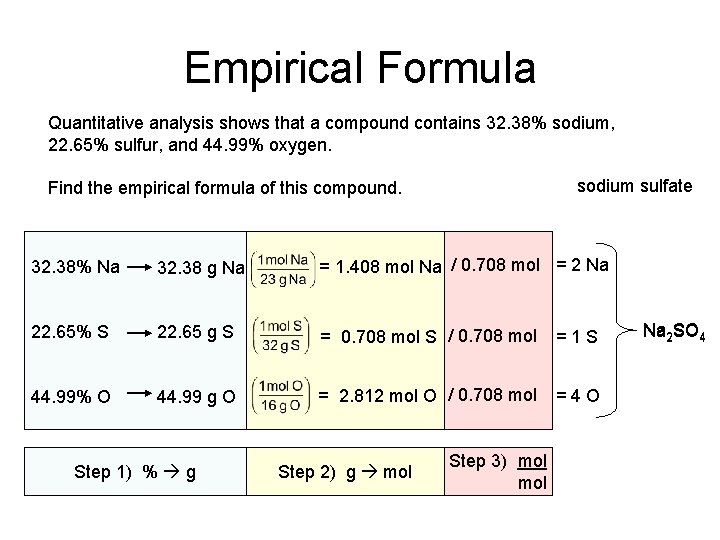
Empirical Formula Quantitative analysis shows that a compound contains 32. 38% sodium, 22. 65% sulfur, and 44. 99% oxygen. sodium sulfate Find the empirical formula of this compound. 32. 38% Na 32. 38 g Na = 1. 408 mol Na / 0. 708 mol = 2 Na 22. 65% S 22. 65 g S = 0. 708 mol S / 0. 708 mol =1 S 44. 99% O 44. 99 g O = 2. 812 mol O / 0. 708 mol =4 O Step 1) % g Step 2) g mol Step 3) mol Na 2 SO 4

Empirical Formula A sample weighing 250. 0 g is analyzed and found to contain the following: 27. 38 g 27. 38% 1. 19 g 14. 29% 14. 29 g 57. 14% 57. 14 g Na sodium H hydrogen C carbon O oxygen Assume sample is 100 g. Determine the empirical formula of this compound. Step 1) convert % gram Step 2) gram moles / 1. 19 mol = 1 Na / 1. 19 mol = 1 H / 1. 19 mol = 1 C / 1. 19 mol = 3 O Step 3) mol / mol Na. HCO 3

Empirical & Molecular Formula (contains only hydrogen + carbon) (~17% hydrogen) A 175 g hydrocarbon sample is analyzed and found to contain ~83% carbon. The molar mass of the sample is determined to be 58 g/mol. Determine the empirical and molecular formula for this sample. Determine the empirical formula of this compound. Step 1) convert % gram Assume sample is 100 g. Then, 83 g carbon and 17 g hydrogen. Step 2) gram moles 2 C @ 12 g = 24 g 5 H@ 1 g = 5 g 29 g MMempirical = 29 g/mol / 6. 917 mol = 1 C / 6. 917 mol = 2. 5 H (2. 4577 H) CH 2. 5 C 2 H 5 MMmolecular = 58 g/mol Step 3) mol / mol 58/29 = 2 Therefore 2(C 2 H 5) = C 4 H 10 butane

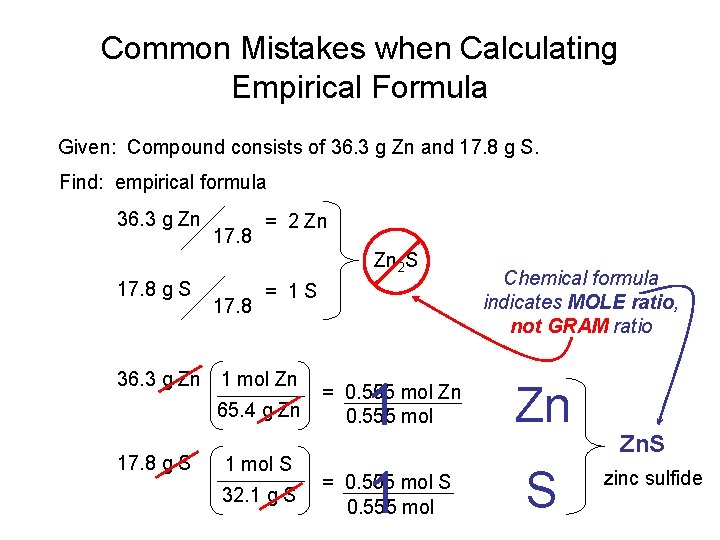
Common Mistakes when Calculating Empirical Formula Given: Compound consists of 36. 3 g Zn and 17. 8 g S. Find: empirical formula 36. 3 g Zn 17. 8 = 2 Zn Zn 2 S 17. 8 g S 17. 8 = 1 S 36. 3 g Zn 1 mol Zn 65. 4 g Zn 17. 8 g S 1 mol S 32. 1 g S 1 1 = 0. 555 mol Zn 0. 555 mol = 0. 555 mol S 0. 555 mol Chemical formula indicates MOLE ratio, not GRAM ratio Zn S Zn. S zinc sulfide

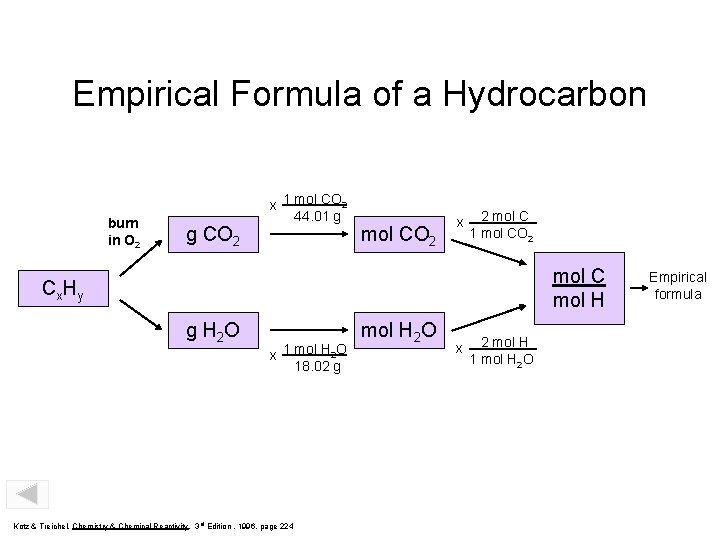
Empirical Formula of a Hydrocarbon burn in O 2 g CO 2 x 1 mol CO 2 44. 01 g mol CO 2 x 2 mol C 1 mol CO 2 mol C mol H Cx Hy g H 2 O x 1 mol H 2 O 18. 02 g Kotz & Treichel, Chemistry & Chemical Reactivity, 3 rd Edition , 1996, page 224 mol H 2 O x 2 mol H 1 mol H 2 O Empirical formula

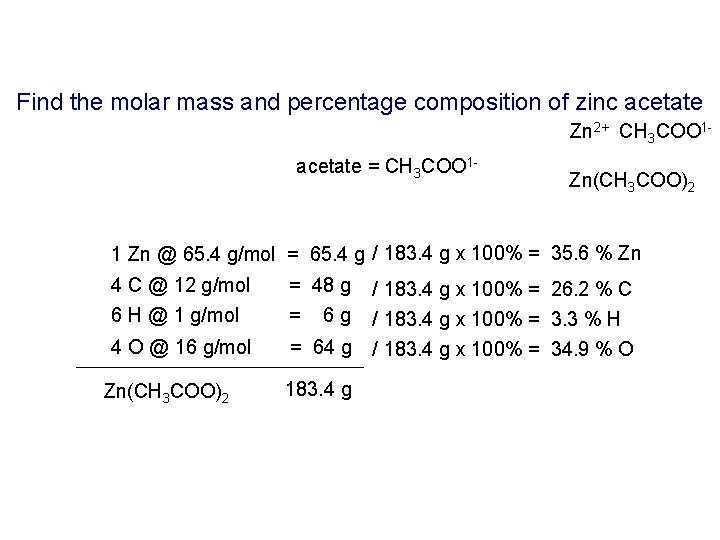
Find the molar mass and percentage composition of zinc acetate Zn 2+ CH 3 COO 1 acetate = CH 3 COO 1 - Zn(CH 3 COO)2 1 Zn @ 65. 4 g/mol = 65. 4 g / 183. 4 g x 100% = 35. 6 % Zn 4 C @ 12 g/mol 6 H @ 1 g/mol = 48 g = 6 g 4 O @ 16 g/mol = 64 g Zn(CH 3 COO)2 183. 4 g / 183. 4 g x 100% = 26. 2 % C / 183. 4 g x 100% = 3. 3 % H / 183. 4 g x 100% = 34. 9 % O

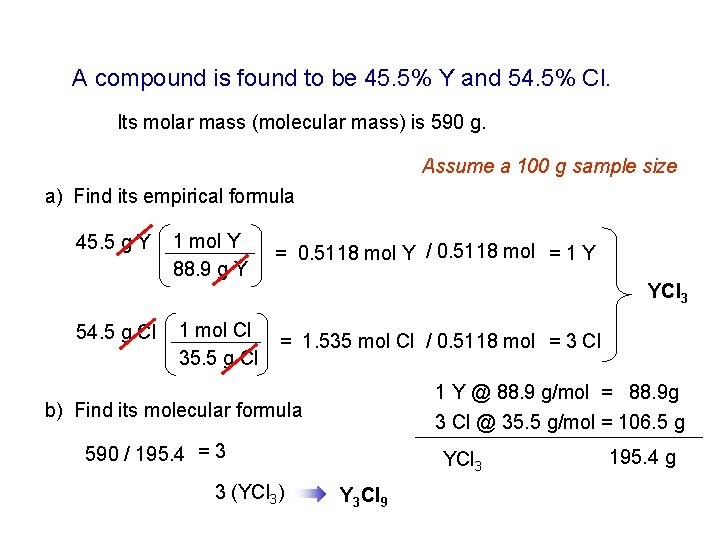
A compound is found to be 45. 5% Y and 54. 5% Cl. Its molar mass (molecular mass) is 590 g. Assume a 100 g sample size a) Find its empirical formula 45. 5 g Y 54. 5 g Cl 1 mol Y 88. 9 g Y = 0. 5118 mol Y / 0. 5118 mol = 1 Y 1 mol Cl 35. 5 g Cl = 1. 535 mol Cl / 0. 5118 mol = 3 Cl YCl 3 1 Y @ 88. 9 g/mol = 88. 9 g 3 Cl @ 35. 5 g/mol = 106. 5 g b) Find its molecular formula 590 / 195. 4 = 3 3 (YCl 3) YCl 3 Y 3 Cl 9 195. 4 g

6. 02 x 1023 Molar Mass Atomic Mass vs. 2 g H 2 = _______ 2 amu 18 g H 2 O = ________ 18 amu 120 g Mg. SO 4 = ________ 120 amu g (NH 4)3 PO 4 = 149 _____ (NH 4)3 PO 4 = ____ 149 amu Percentage Composition (by mass) % = part x 100 % whole Empirical vs. (lowest ratio) Molecular Formula Empirical Formula % g g mol mol

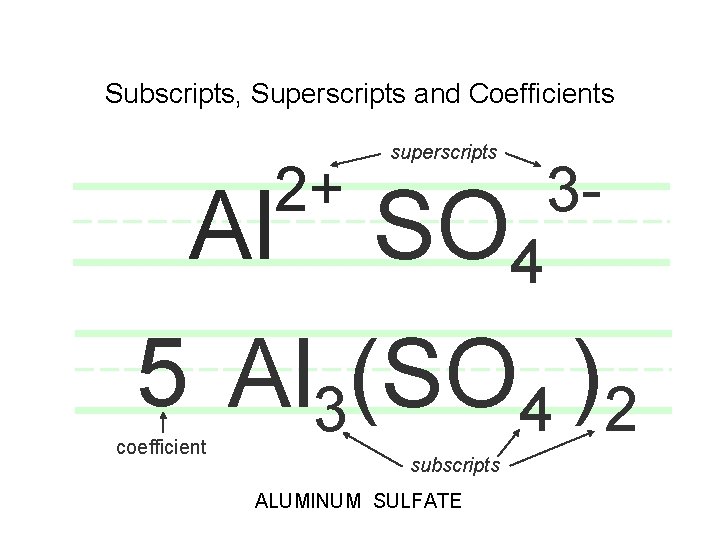
Subscripts, Superscripts and Coefficients Al 2+ superscripts SO 4 3 - 5 Al 3(SO 4 )2 coefficient subscripts ALUMINUM SULFATE

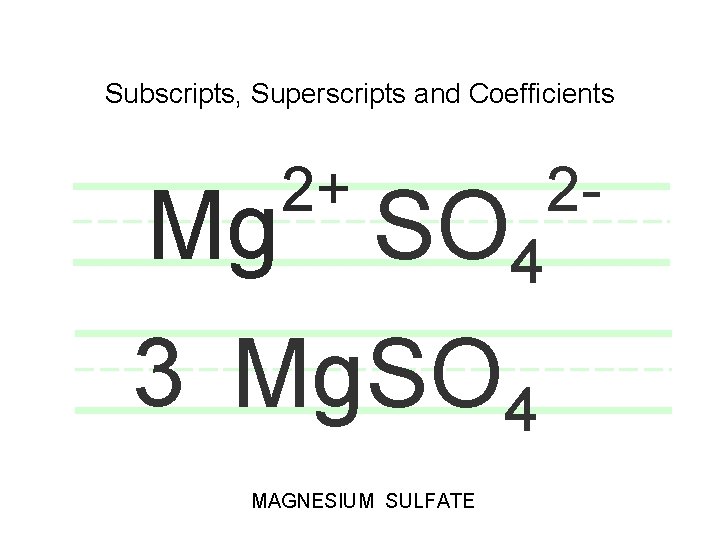
Subscripts, Superscripts and Coefficients 2+ Mg SO 4 3 Mg. SO 4 MAGNESIUM SULFATE 2 -

Subscripts, Superscripts and Coefficients 2+ Mg NO 3 1 - 4 Mg(NO Mg. NO 3)22 subscript MAGNESIUM NITRATE

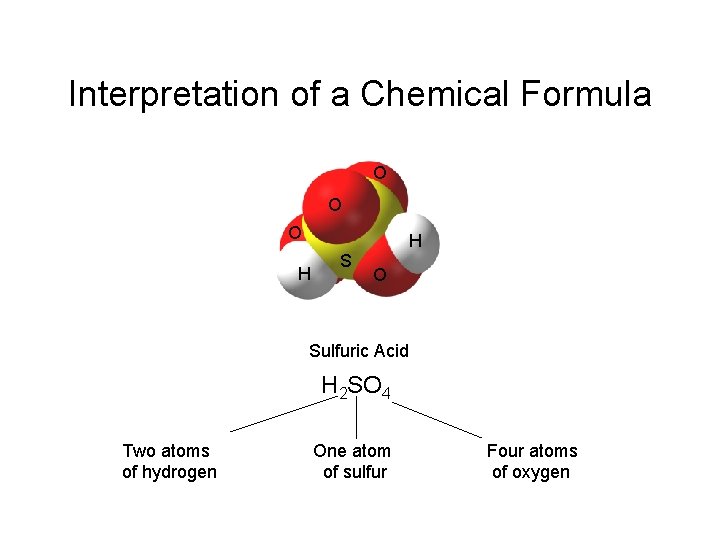
Interpretation of a Chemical Formula O O O H S H O Sulfuric Acid H 2 SO 4 Two atoms of hydrogen One atom of sulfur Four atoms of oxygen

Chemical Formulas C 8 H 18 Subscript indicates that there are 8 carbon atoms in a molecule of octane. Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 203 Subscript indicates that there are 18 hydrogen atoms in a molecule of octane.

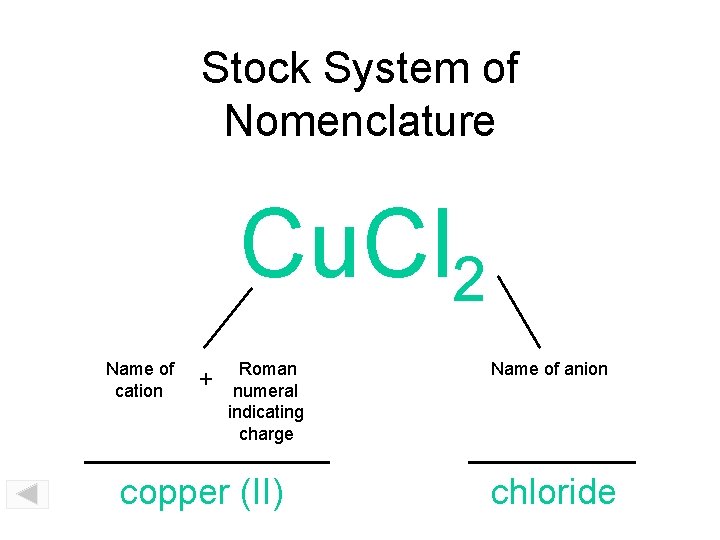
Stock System of Nomenclature Cu. Cl 2 Name of cation + Roman numeral indicating charge copper (II) Name of anion chloride

Chemical Formulas Al 2(SO 4)3 Subscript 2 refers to 2 aluminum atoms. Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 204 Subscript 4 refers to 4 oxygen atoms in sulfate ion. Subscript 3 refers to everything inside parentheses. Here there are 3 sulfate ions, with a total of 3 sulfur atoms and 12 oxygen atoms.

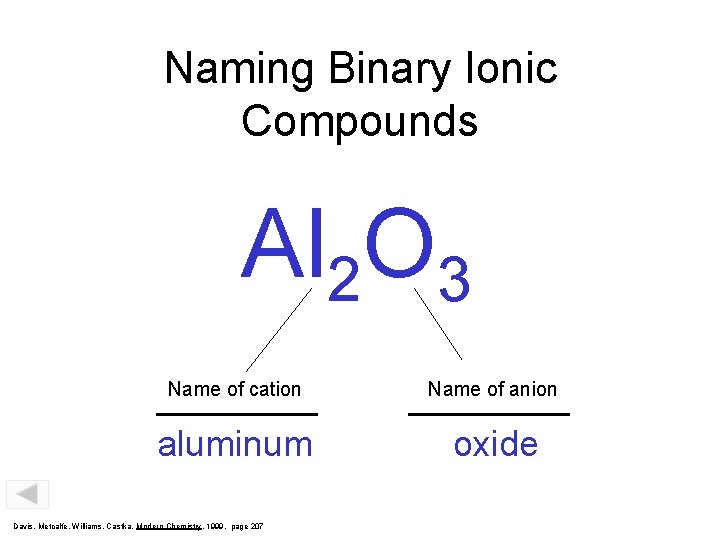
Naming Binary Ionic Compounds Al 2 O 3 Name of cation Name of anion aluminum oxide Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 207

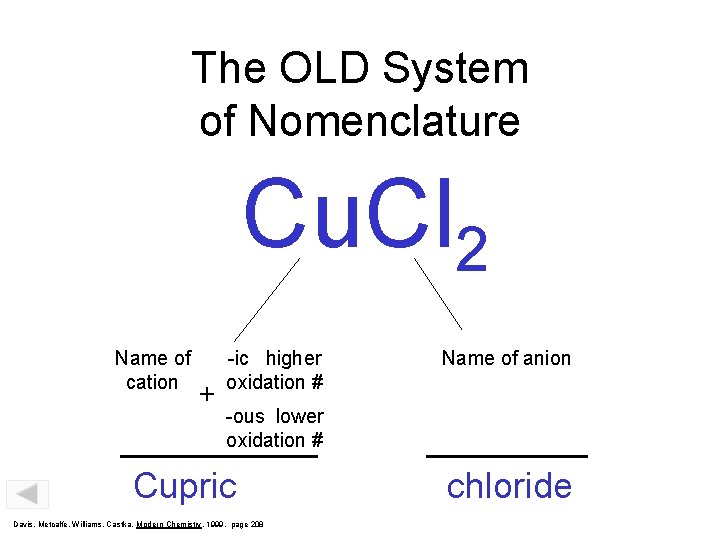
The OLD System of Nomenclature Cu. Cl 2 Name of cation + -ic higher oxidation # Name of anion -ous lower oxidation # Cupric Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 208 chloride

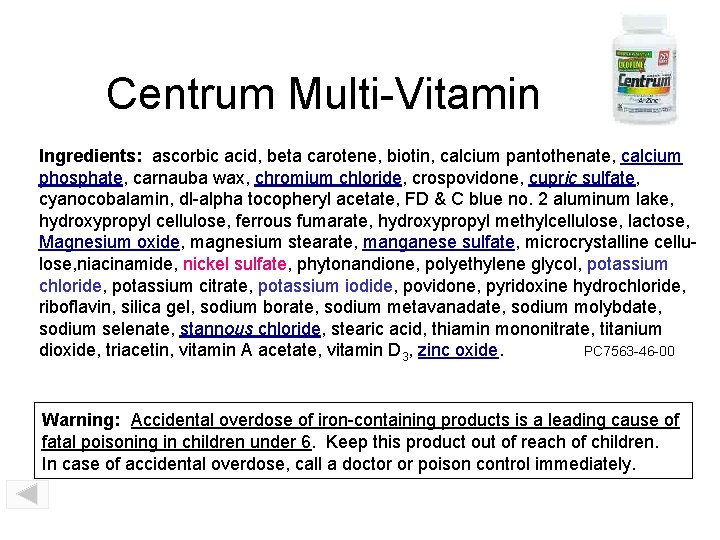
Centrum Multi-Vitamin Ingredients: ascorbic acid, beta carotene, biotin, calcium pantothenate, calcium phosphate, carnauba wax, chromium chloride, crospovidone, cupric sulfate, cyanocobalamin, dl-alpha tocopheryl acetate, FD & C blue no. 2 aluminum lake, hydroxypropyl cellulose, ferrous fumarate, hydroxypropyl methylcellulose, lactose, Magnesium oxide, magnesium stearate, manganese sulfate, microcrystalline cellulose, niacinamide, nickel sulfate, phytonandione, polyethylene glycol, potassium chloride, potassium citrate, potassium iodide, povidone, pyridoxine hydrochloride, riboflavin, silica gel, sodium borate, sodium metavanadate, sodium molybdate, sodium selenate, stannous chloride, stearic acid, thiamin mononitrate, titanium dioxide, triacetin, vitamin A acetate, vitamin D 3, zinc oxide. PC 7563 -46 -00 Warning: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control immediately.

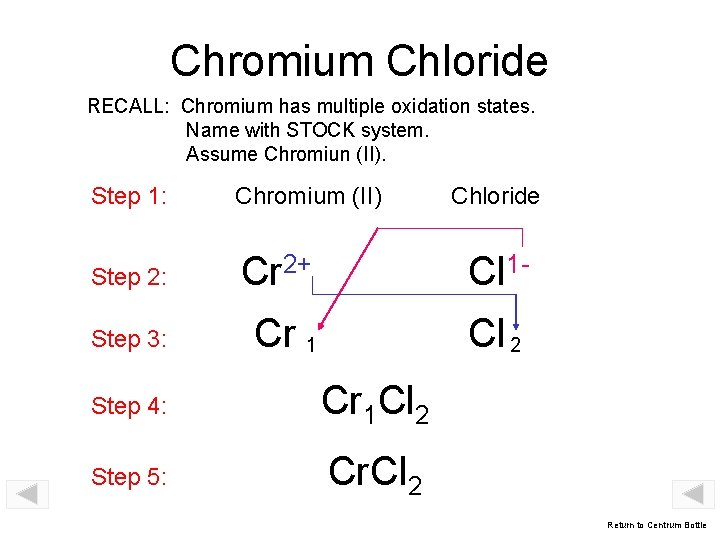
Chromium (III) Chloride RECALL: Chromium forms oxides in which metal exhibits oxidation states of +3 and +2. STOCK system indicates oxidation state of compound. Assume Cr 3+ (chromium (III) chloride). Step 1: Chromium (III) Step 2: Cr 3+ Cl 1 - Step 3: Cr 1 Cl 3 Step 4: Chloride Cr. Cl 3 Return to Centrum Bottle

Cupric Sulfate RECALL: “ic” higher oxidation & Cu 2+ (higher) “ous” lower oxidation Cu 1+ (lower) Step 1: Cupric Sulfate Step 2: Cu 2+ SO 42 - Step 3: Cu Step 4: Step 5: 2 (SO 4) 2 Cu 2(SO 4)2 Cu. SO 4 Return to Centrum Bottle

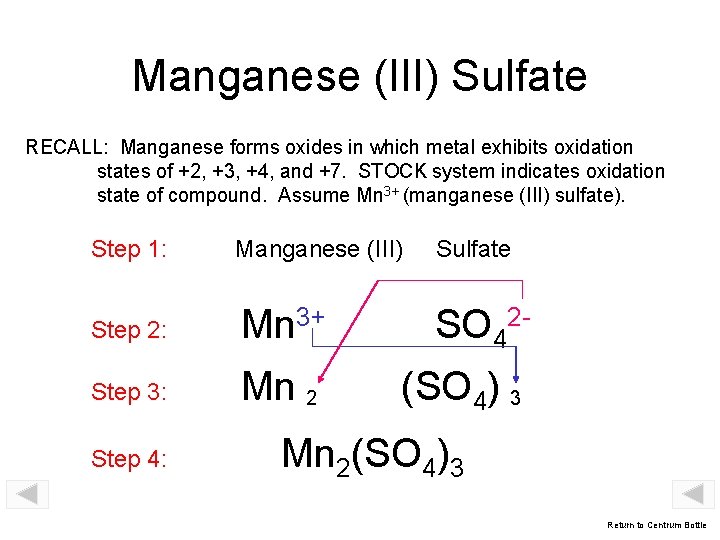
Manganese (III) Sulfate RECALL: Manganese forms oxides in which metal exhibits oxidation states of +2, +3, +4, and +7. STOCK system indicates oxidation state of compound. Assume Mn 3+ (manganese (III) sulfate). Step 1: Manganese (III) Sulfate Step 2: Mn 3+ SO 42 - Step 3: Mn 2 (SO 4) 3 Step 4: Mn 2(SO 4)3 Return to Centrum Bottle

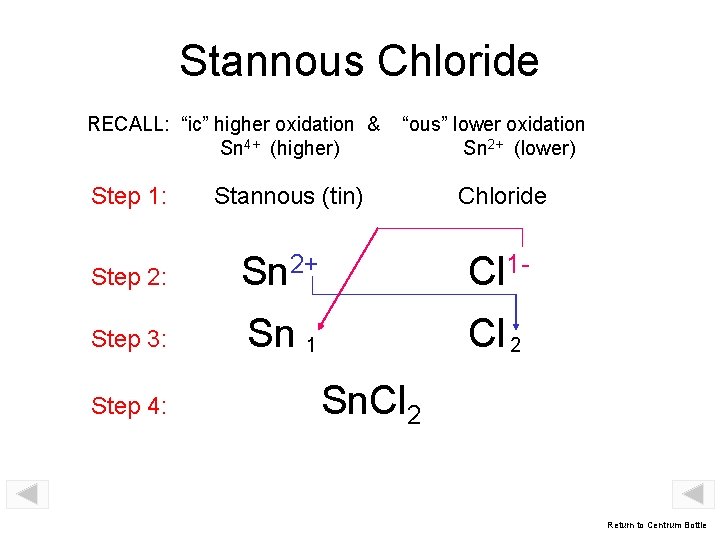
Stannous Chloride RECALL: “ic” higher oxidation & Sn 4+ (higher) Step 1: “ous” lower oxidation Sn 2+ (lower) Stannous (tin) Chloride Step 2: Sn 2+ Cl 1 - Step 3: Sn 1 Cl 2 Step 4: Sn. Cl 2 Return to Centrum Bottle

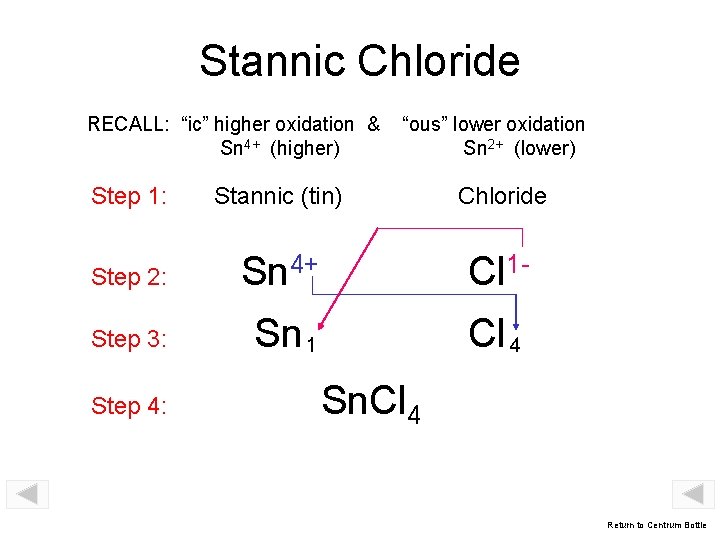
Stannic Chloride RECALL: “ic” higher oxidation & Sn 4+ (higher) “ous” lower oxidation Sn 2+ (lower) Step 1: Stannic (tin) Chloride Step 2: Sn 4+ Cl 1 - Step 3: Sn 1 Cl 4 Step 4: Sn. Cl 4 Return to Centrum Bottle

Chromium Chloride RECALL: Chromium has multiple oxidation states. Name with STOCK system. Assume Chromiun (II). Step 1: Chromium (II) Step 2: Cr 2+ Cl 1 - Step 3: Cr 1 Cl 2 Step 4: Cr 1 Cl 2 Step 5: Cr. Cl 2 Chloride Return to Centrum Bottle

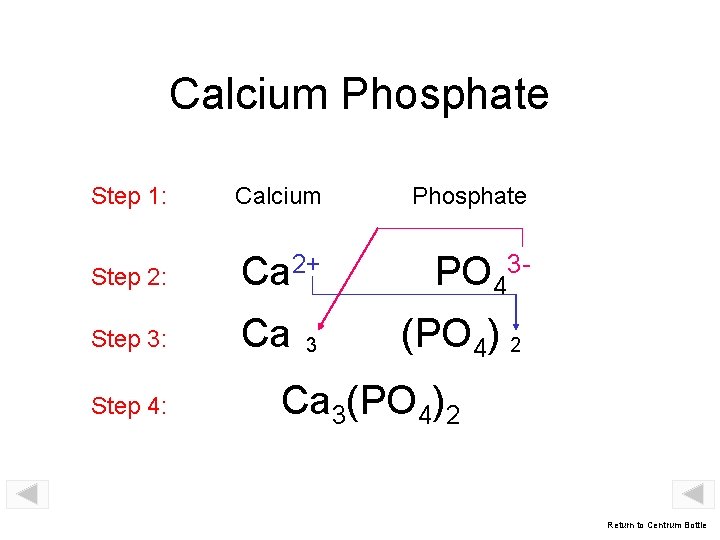
Calcium Phosphate Step 1: Calcium Phosphate Step 2: Ca 2+ PO 43 - Step 3: Ca Step 4: 3 (PO 4) 2 Ca 3(PO 4)2 Return to Centrum Bottle

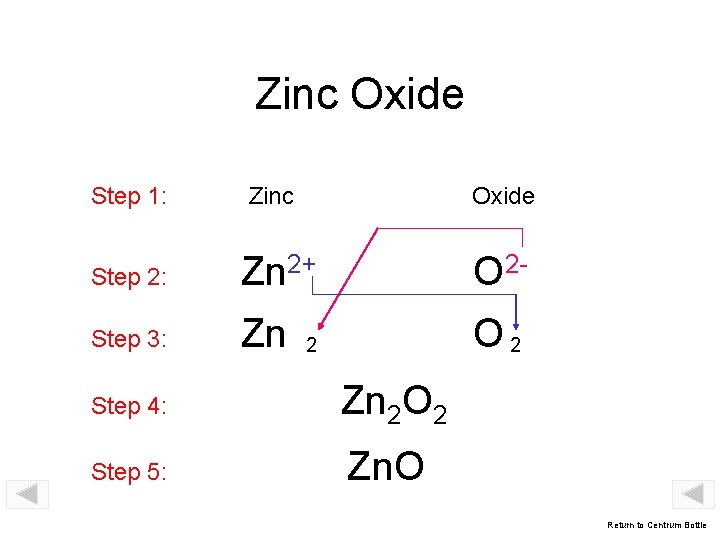
Zinc Oxide Step 1: Zinc Oxide Step 2: Zn 2+ O 2 - Step 3: Zn O 2 2 Step 4: Zn 2 O 2 Step 5: Zn. O Return to Centrum Bottle

Common Polyatomic Ions Names of Common Polyatomic Ions Ion Name NH 4+ ammonium CO 32 - carbonate NO 2 - nitrite HCO 3 - NO 3 - nitrate SO 32 - sulfite hydrogen carbonate (bicarbonate is a widely used common name) SO 42 - sulfate Cl. O- hypochlorite HSO 4 - hydrogen sulfate (bisulfate is a widely used common name) Cl. O 2 - chlorite Cl. O 3 - chlorate Cl. O 4 - perchlorate OH- hydroxide C 2 H 3 O 2 - acetate CN- cyanide Mn. O 4 - permanganate PO 43 - phosphate Cr 2 O 72 - dichromate HPO 42 - hydrogen phosphate Cr. O 42 - chromate H 2 PO 4 - dihydrogen phosphate O 22 - peroxide

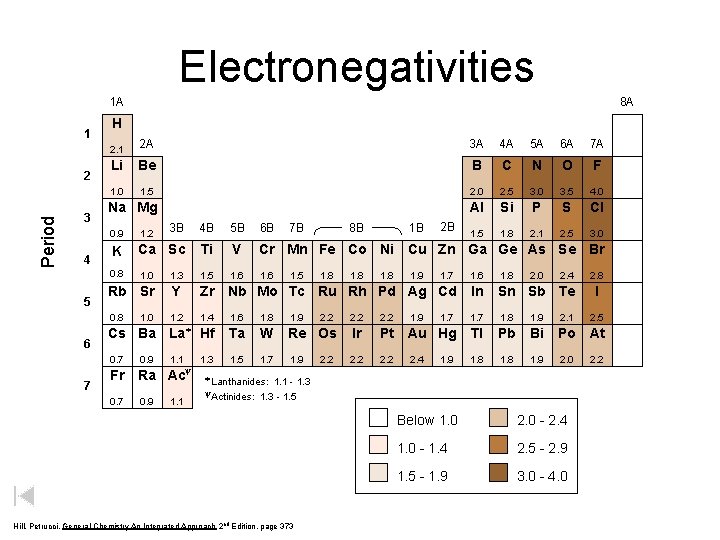
Electronegativities 1 A 1 Period 2 3 4 5 6 7 8 A H 2. 1 2 A 3 A 4 A 5 A 6 A 7 A Li Be B C N O F 1. 0 1. 5 2. 0 2. 5 3. 0 3. 5 4. 0 Al Si P S Cl 1. 5 1. 8 2. 1 2. 5 3. 0 Na Mg 1. 2 3 B 4 B 5 B 6 B K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 0. 8 1. 0 1. 3 1. 5 1. 6 1. 7 1. 6 1. 8 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te 0. 8 1. 2 1. 4 1. 6 1. 8 1. 9 2. 2 1. 7 1. 8 1. 9 2. 1 Cs Ba La* Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At 0. 7 1. 1 1. 3 1. 5 1. 7 1. 9 2. 2 1. 8 1. 9 2. 0 1. 0 0. 9 y Fr Ra Ac 0. 7 0. 9 1. 1 8 B 7 B 1. 5 1. 8 2. 2 1. 8 1 B 2 B 0. 9 1. 8 1. 9 2. 4 1. 9 2. 0 2. 4 * Lanthanides: 1. 1 - 1. 3 y. Actinides: 1. 3 - 1. 5 Hill, Petrucci, General Chemistry An Integrated Approach 2 nd Edition, page 373 Below 1. 0 2. 0 - 2. 4 1. 0 - 1. 4 2. 5 - 2. 9 1. 5 - 1. 9 3. 0 - 4. 0 2. 8 I 2. 5 2. 2

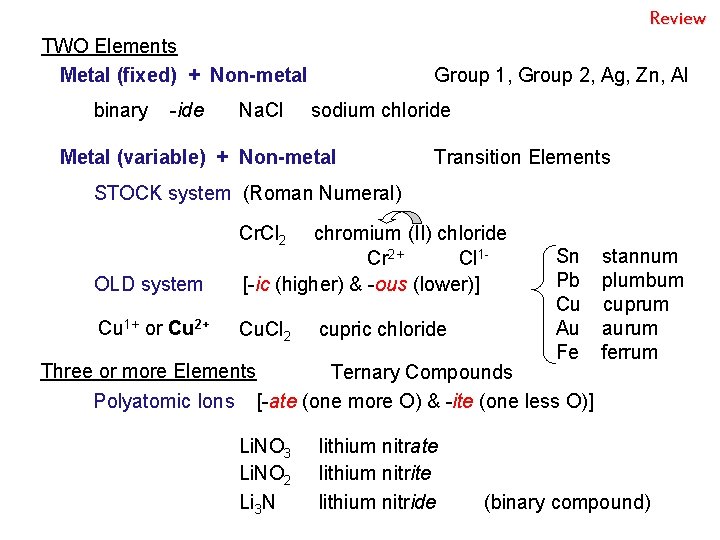
Review TWO Elements Metal (fixed) + Non-metal binary -ide Na. Cl Group 1, Group 2, Ag, Zn, Al sodium chloride Metal (variable) + Non-metal Transition Elements STOCK system (Roman Numeral) Cr. Cl 2 OLD system chromium (II) chloride Cr 2+ Cl 1[-ic (higher) & -ous (lower)] Cu 1+ or Cu 2+ Cu. Cl 2 cupric chloride Sn stannum Pb plumbum Cu cuprum Au aurum Fe ferrum Three or more Elements Ternary Compounds Polyatomic Ions [-ate (one more O) & -ite (one less O)] Li. NO 3 Li. NO 2 Li 3 N lithium nitrate lithium nitride (binary compound)

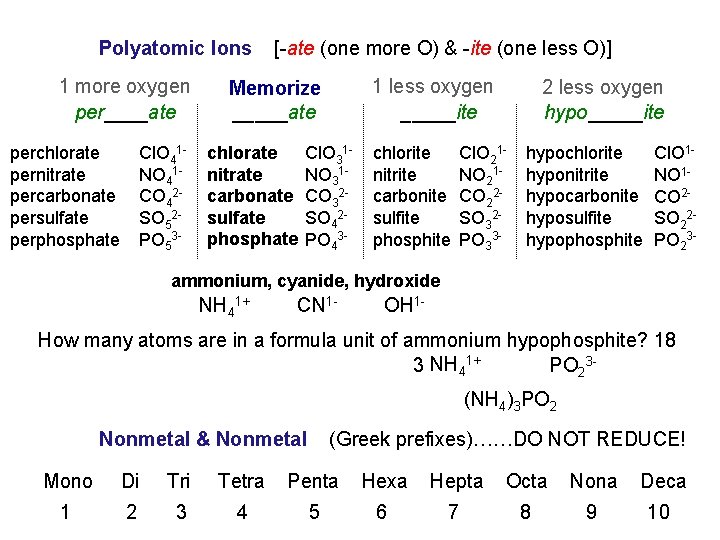
Polyatomic Ions 1 more oxygen per____ate perchlorate pernitrate percarbonate persulfate perphosphate Cl. O 41 NO 41 CO 42 SO 52 PO 53 - [-ate (one more O) & -ite (one less O)] 1 less oxygen _____ite Memorize NORMAL _____ate chlorate nitrate carbonate sulfate phosphate Cl. O 31 NO 31 CO 32 SO 42 PO 43 - chlorite nitrite carbonite sulfite phosphite 2 less oxygen hypo_____ite Cl. O 21 NO 21 CO 22 SO 32 PO 33 - hypochlorite hyponitrite hypocarbonite hyposulfite hypophosphite Cl. O 1 NO 1 CO 2 SO 22 PO 23 - ammonium, cyanide, hydroxide NH 41+ CN 1 - OH 1 - How many atoms are in a formula unit of ammonium hypophosphite? 18 3 NH 41+ PO 23(NH 4)3 PO 2 Nonmetal & Nonmetal (Greek prefixes)……DO NOT REDUCE! Mono Di Tri Tetra Penta Hexa Hepta Octa Nona Deca 1 2 3 4 5 6 7 8 9 10

Molecular Models Activity carbon tetrachloride ammonia methane hydrogen monochloride water trichloromethane urea ethyne propane dihydrogen monosulfide butane carbon dioxide nitrogen triiodide (video) supplies

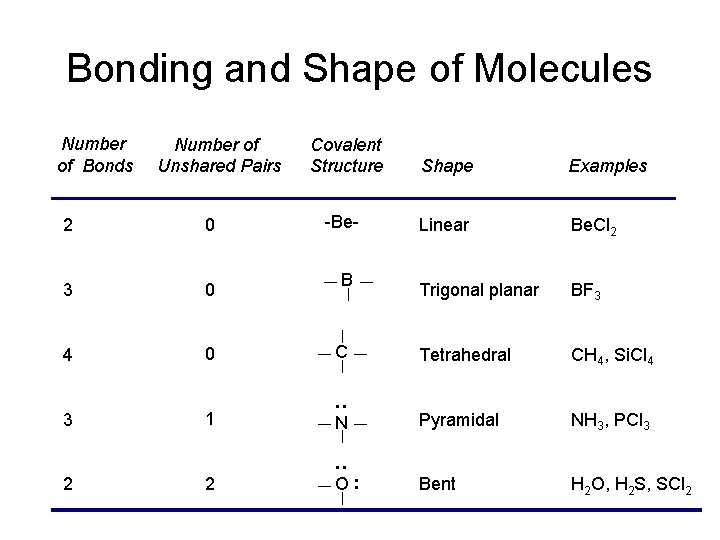
Bonding and Shape of Molecules Number of Unshared Pairs Covalent Structure Shape Examples -Be- Linear Be. Cl 2 Trigonal planar BF 3 2 0 3 0 4 0 C Tetrahedral CH 4, Si. Cl 4 3 1 : Number of Bonds Pyramidal NH 3, PCl 3 2 2 Bent H 2 O, H 2 S, SCl 2 B : N O:

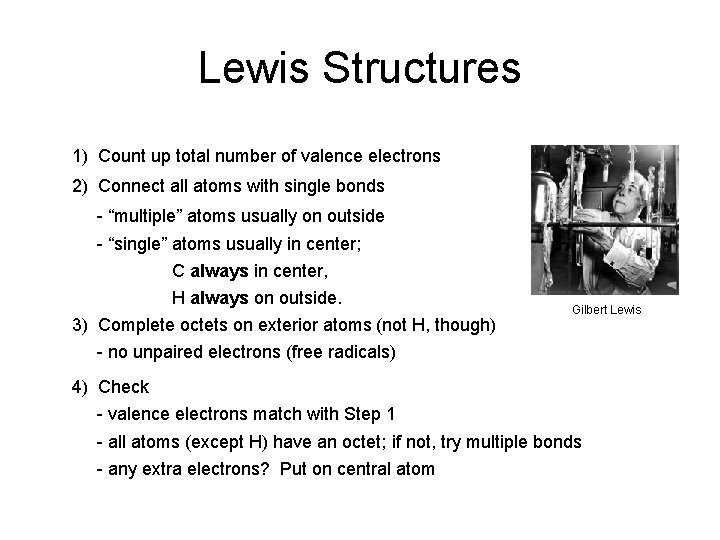
Lewis Structures 1) Count up total number of valence electrons 2) Connect all atoms with single bonds - “multiple” atoms usually on outside - “single” atoms usually in center; C always in center, H always on outside. 3) Complete octets on exterior atoms (not H, though) Gilbert Lewis - no unpaired electrons (free radicals) 4) Check - valence electrons match with Step 1 - all atoms (except H) have an octet; if not, try multiple bonds - any extra electrons? Put on central atom

Carbon tetrachloride Cl Cl CCl 4 Cl Cl 109. 5 o Cl Tetrahedral geometry Carbon tetrachloride – “carbon tet” had been used as dry cleaning solvent because of its extreme non-polarity.

Methane H H CH H H C H H 109. 5 o H Tetrahedral geometry Methane –The first member of the paraffin (alkane) hydrocarbons series. a. k. a. (marsh gas, CH 4).

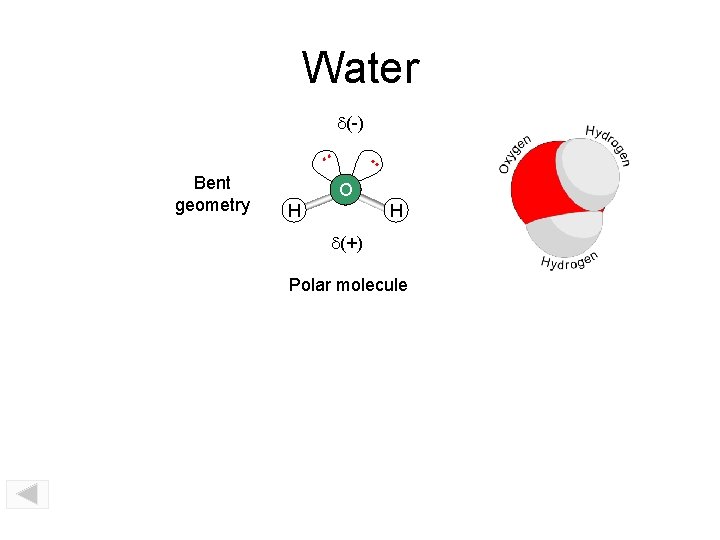
Water d(-) . . Bent geometry . . SO 2 O H H d(+) Polar molecule

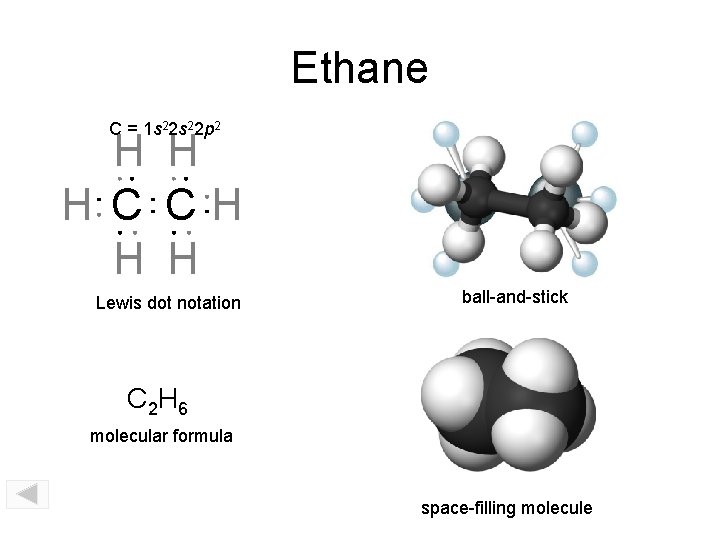
Ethane C = 1 s 22 p 2 H H H C CH H H Lewis dot notation ball-and-stick C 2 H 6 molecular formula space-filling molecule

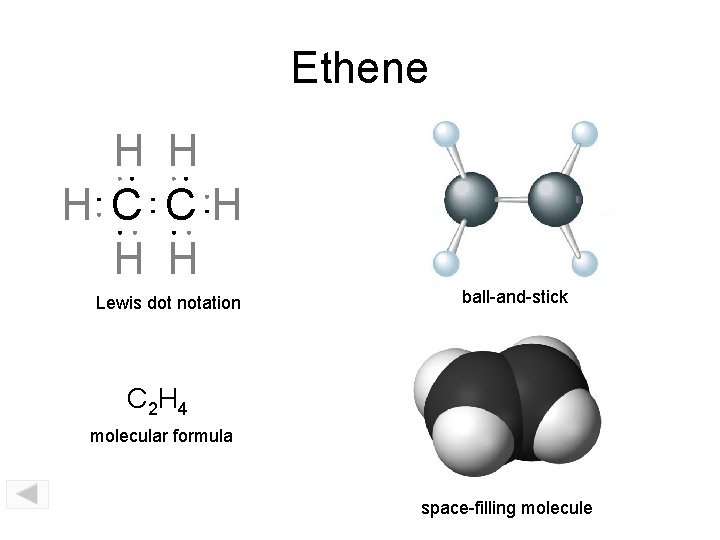
Ethene H H H C CH H H Lewis dot notation ball-and-stick C 2 H 4 molecular formula space-filling molecule

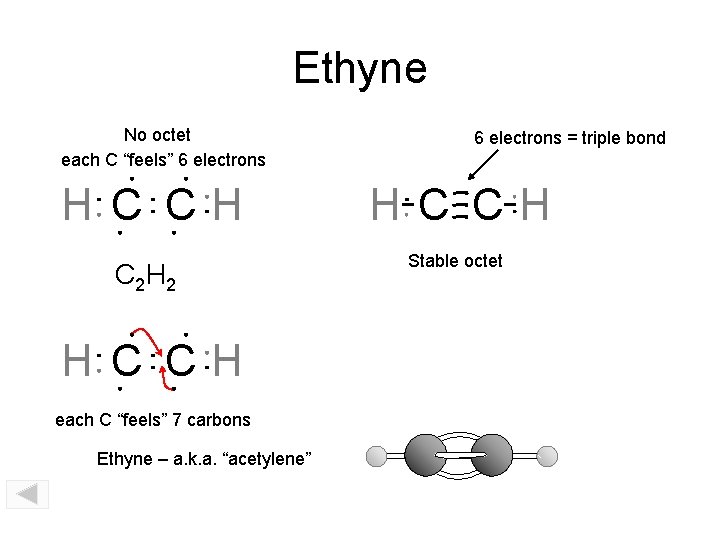
Ethyne No octet each C “feels” 6 electrons H C CH C 2 H 2 H C CH each C “feels” 7 carbons Ethyne – a. k. a. “acetylene” 6 electrons = triple bond H C CH Stable octet

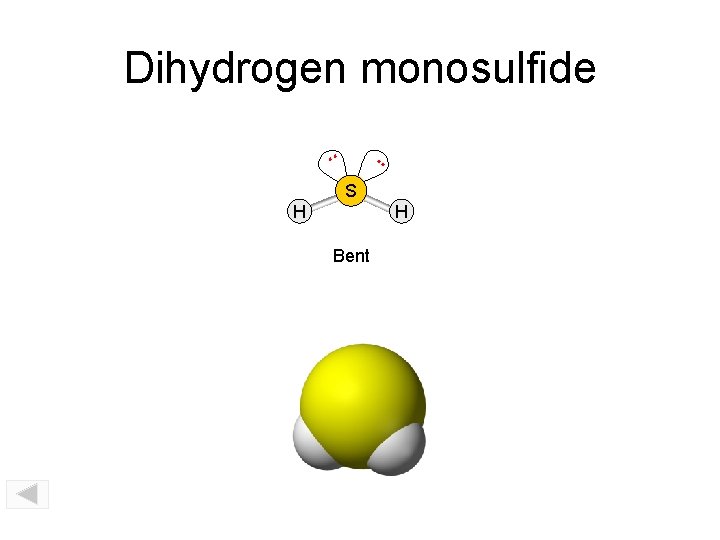
Dihydrogen monosulfide. . SO 2 S H H Bent

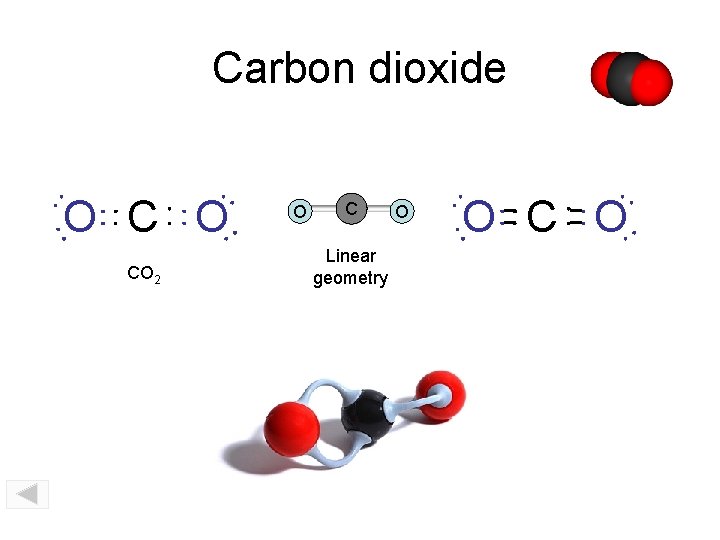
Carbon dioxide O CO 2 O C Linear geometry O O C O

Ammonia. . NH 3 N HH H N H H H Trigonal Pyramidal geometry N H H 107 o H

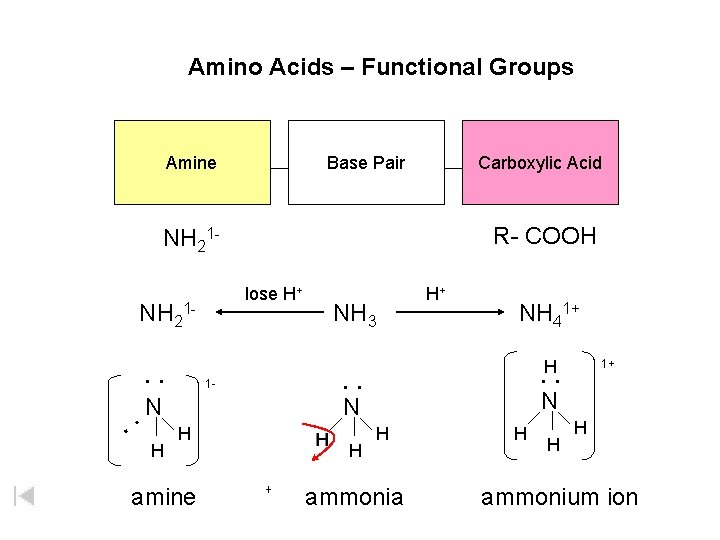
Amino Acids – Functional Groups Amine Base Pair Carboxylic Acid R- COOH NH 21 lose H+ NH 21 - NH 3 H+ NH 41+ H : N N N H : : : 1 - H amine H + H 1+ H ammonia H H H ammonium ion

Hydrogen monochloride H Cl HCl d(+) d(-) HCl(g) + H 2 O(l) HCl(aq) hydrogen chloride Polar molecule water hydrochloric acid

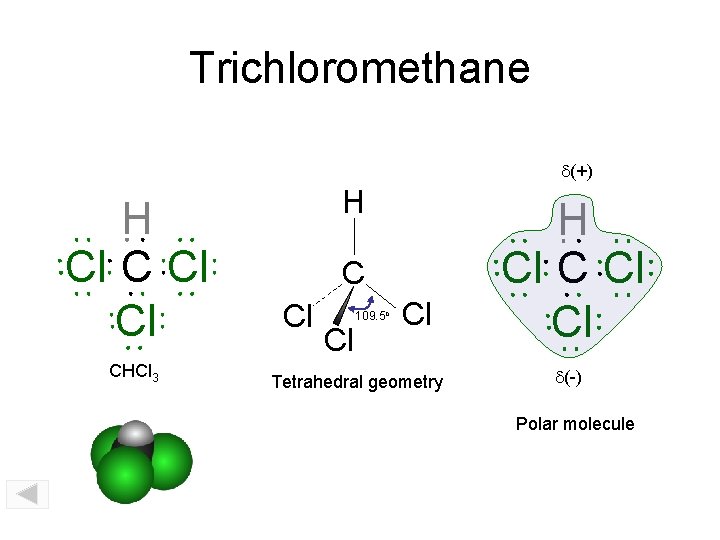
Trichloromethane d(+) H Cl Cl CHCl 3 H C Cl Cl 109. 5 o Cl Tetrahedral geometry H Cl Cl d(-) Polar molecule

Urea NOT “di-urea” H H N O C N H H CO(NH 2)2 Urea – The first organic compound to be synthesized (Wohler, 1828).

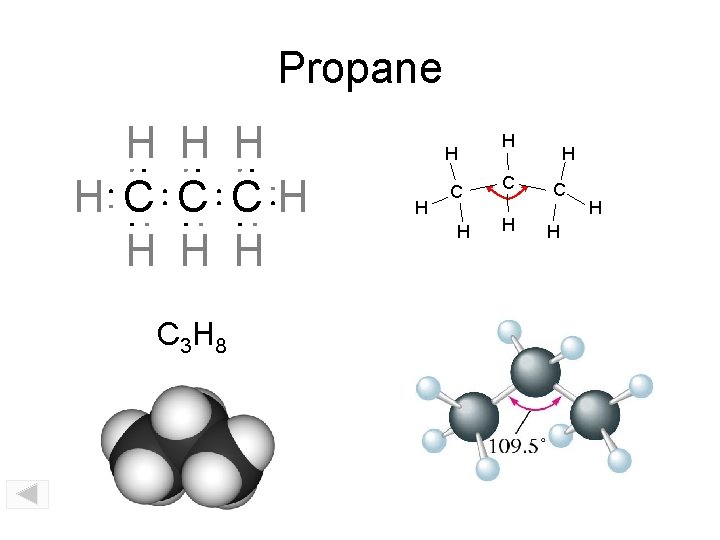
Propane H H C C CH H C 3 H 8 H H C H H

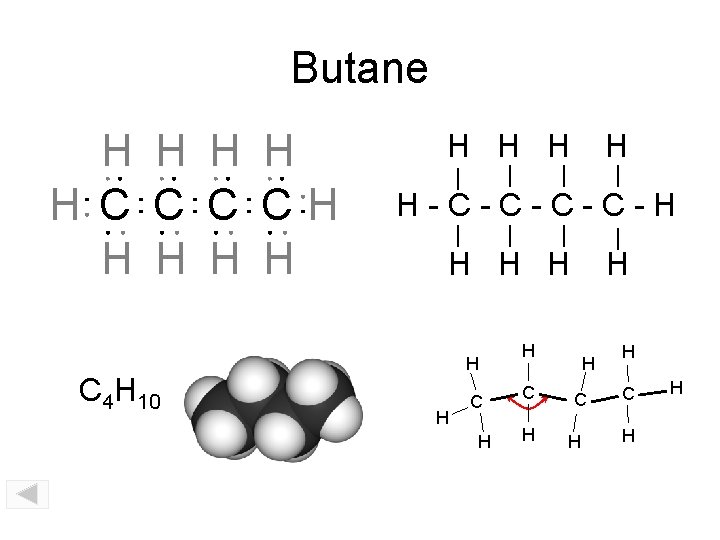
Butane H H H C CH H H C 4 H 10 H H H-C-C-H H H C H H C H H

Nitrogen triiodide. . N I I I NI 3 N I 107 o I I Trigonal Pyramidal geometry Video clip: (slow motion) detonation of NI 3

Supplies 15 black 8 green 1 yellow 4 blue 4 red 42 hydrogen 67 bonds (carbon) (chlorine and iodine) (sulfur) (oxygen) (nitrogen) (hydrogen) (bonds)

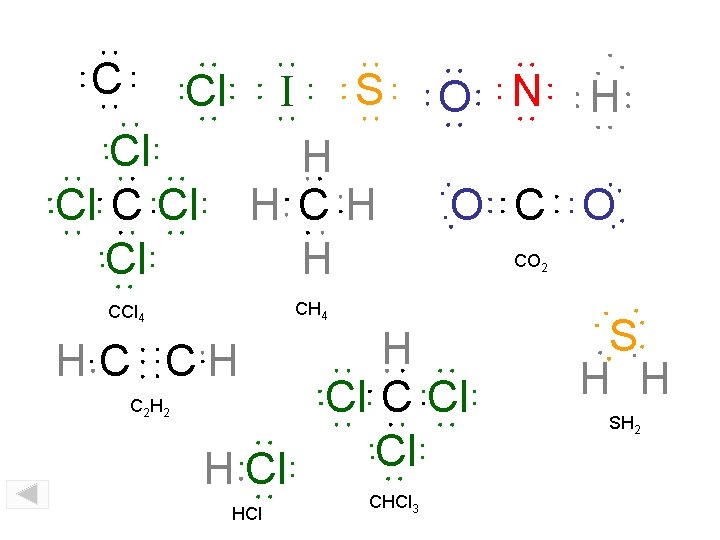
C Cl S I Cl Cl Cl H H CCl 4 CH 4 HCl CHCl 3 H O C O H Cl Cl HC CH C 2 H 2 O N CO 2 S H H SH 2
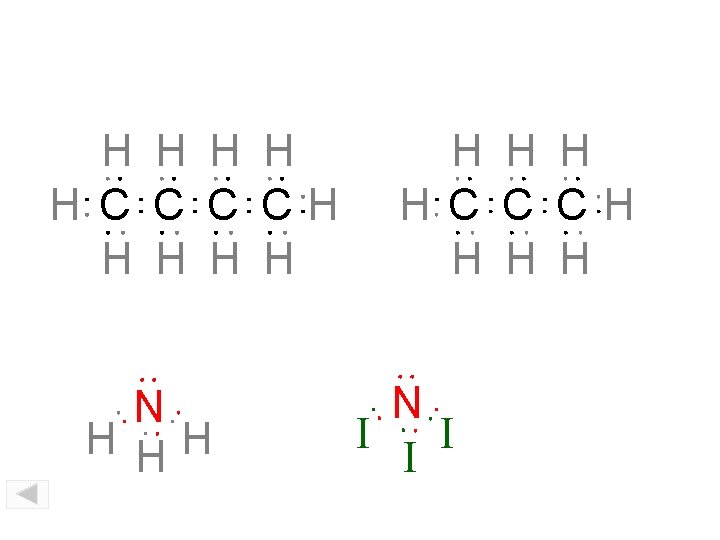
H H H C CH H H N H HH H H C C CH H N I I I

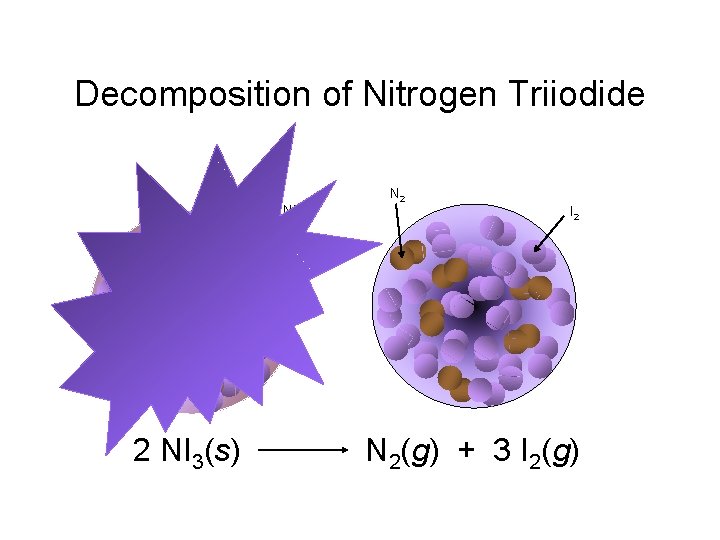
Decomposition of Nitrogen Triiodide


Decomposition of Nitrogen Triiodide NI 3 2 NI 3(s) N 2 I 2 N 2(g) + 3 I 2(g)

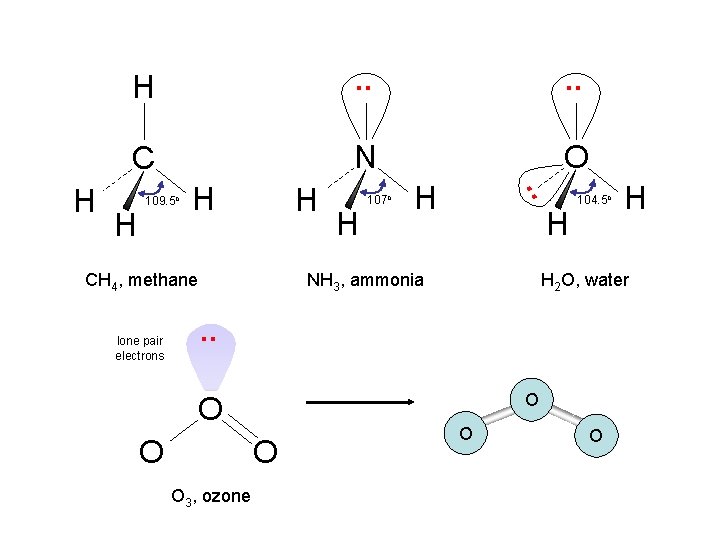
. . C N O H 109. 5 o H H CH 4, methane lone pair electrons H 107 o H . . H H H NH 3, ammonia 104. 5 o H 2 O, water . . O O O 3, ozone H O O

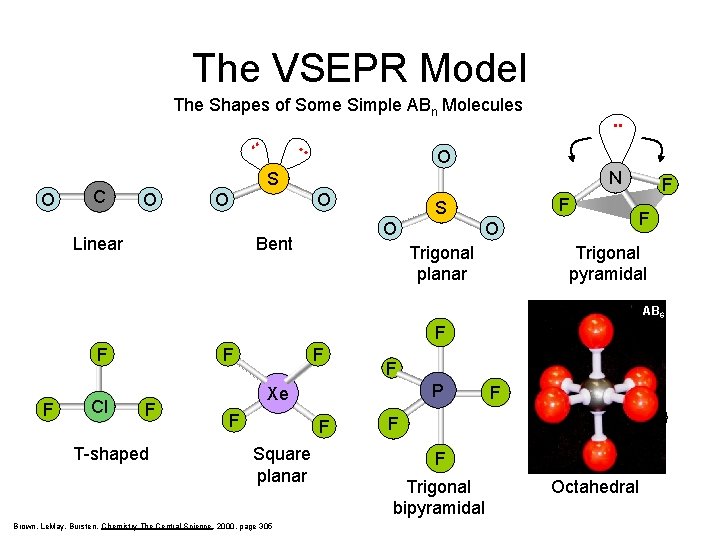
The VSEPR Model The Shapes of Some Simple ABn Molecules . . O C . . SO 2 . . O N S O O Linear O Bent F S O F F O Trigonal planar Trigonal pyramidal AB 6 F F F Cl F F T-shaped F F F Square planar Brown, Le. May, Bursten, Chemistry The Central Science, 2000, page 305 F F P Xe F F F S F F F Trigonal bipyramidal Octahedral

Resources - Nomenclature Objectives General Chemistry PP Worksheet - binary cmpds: single charge cation Worksheet - binary compounds Worksheet - ions in chemical formulas Worksheet - ions in chemical compounds Worksheet - ionic cmpds: polyatomic ions w multiple-charge cation Worksheet - ionic formulas (binary, polyatomic, transition) Worksheet - traditional system of nomenclature Worksheet - covalent binary cmpds: non-metal - non-metal Worksheet - empirical and molecular Worksheet - vocab (bonding) Worksheet - ionic cmpds: polyatomic ions Worksheet - ionic binary cmpds: multiple charge cation Worksheet - errors in chemical formulas and nomenclature Worksheet - oxidation numbers and ionic cmpds Activity - bonding pieces Activity - molecular models Activity - mole pattern Textbook - questions Worksheet - names and formulas of cmpds Outline (general)
 Humor vítreo e humor aquoso
Humor vítreo e humor aquoso Inorganic nomenclature flow chart
Inorganic nomenclature flow chart Inorganic nomenclature
Inorganic nomenclature Inorganic nomenclature worksheet
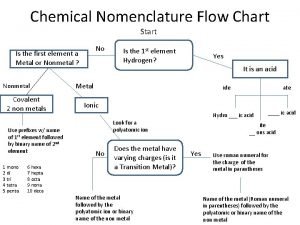
Inorganic nomenclature worksheet Chemical nomenclature flow chart
Chemical nomenclature flow chart Harrison bergeron anticipation guide
Harrison bergeron anticipation guide Sophomoric humor meaning
Sophomoric humor meaning Humor u nastavi
Humor u nastavi Postmodernism authors
Postmodernism authors Misanthrope adjective
Misanthrope adjective Muscae volitantes
Muscae volitantes Island motto
Island motto What type of literature uses humor to point
What type of literature uses humor to point Humor docente
Humor docente Tan corta la vida
Tan corta la vida Homeoptico
Homeoptico Jupiter y el garzon de ida
Jupiter y el garzon de ida Rhyming triplets
Rhyming triplets Sheep eye dissection
Sheep eye dissection Humor techniques
Humor techniques Kolder humor
Kolder humor Reinforced robot humor suppression pump
Reinforced robot humor suppression pump A catchword or phrase loaded with emotion
A catchword or phrase loaded with emotion Cow eye labeled
Cow eye labeled Comic situation
Comic situation Big exaggeration usually with humor
Big exaggeration usually with humor Self deprecating humor
Self deprecating humor Humor
Humor Contos de humor
Contos de humor Satire in huck finn
Satire in huck finn Humor
Humor Dry sense of humor
Dry sense of humor Big exaggeration usually with humor
Big exaggeration usually with humor Un humor entre perlas destilado
Un humor entre perlas destilado Aqueous humor
Aqueous humor Types of irony in pride and prejudice
Types of irony in pride and prejudice Database humor
Database humor Thermopylae a soldier's humor
Thermopylae a soldier's humor Types of humor
Types of humor Types of comedy
Types of comedy Contos narrativos
Contos narrativos Propaganda techniques
Propaganda techniques Dicionrio
Dicionrio Sardonic
Sardonic Humor in classical music
Humor in classical music Broad physical humor and stereotypical characters
Broad physical humor and stereotypical characters Association for applied and therapeutic humor
Association for applied and therapeutic humor Afrikaans mondeling onderwerpe graad 5
Afrikaans mondeling onderwerpe graad 5 Examples of limericks
Examples of limericks Romeo and juliet act 1 scene 2 vocabulary
Romeo and juliet act 1 scene 2 vocabulary Aqueous humor
Aqueous humor Humor in product design
Humor in product design Elements of humor
Elements of humor Flashback foreshadowing humor irony
Flashback foreshadowing humor irony Schwarzes humor
Schwarzes humor Form of intelligent humor
Form of intelligent humor Humor device
Humor device Humour introduction
Humour introduction Vestibular membrane
Vestibular membrane Humor column example
Humor column example Chinese humor
Chinese humor Analyserende artikel
Analyserende artikel Een relatie is hard werken
Een relatie is hard werken Ouderen humor
Ouderen humor Observational humor
Observational humor Bem voce conseguiu ferir meus sentimentos
Bem voce conseguiu ferir meus sentimentos Breza slavko kolar
Breza slavko kolar Verbal and nonverbal communication in china
Verbal and nonverbal communication in china Context jokes
Context jokes Hyperbole in the lowest animal
Hyperbole in the lowest animal Comedy in waiting for godot
Comedy in waiting for godot Humor aköz drenajı
Humor aköz drenajı Choroid of cow eye
Choroid of cow eye Virtual sheep eye dissection
Virtual sheep eye dissection Anemia conclusion
Anemia conclusion Difference between occlusion and mixed-crystal formation
Difference between occlusion and mixed-crystal formation Inorganic mineral definition
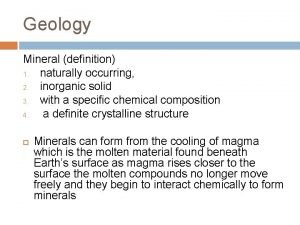
Inorganic mineral definition Inorganic mineral definition
Inorganic mineral definition Pharmaceutical inorganic chemistry chapter 1
Pharmaceutical inorganic chemistry chapter 1 Air pollution class 9
Air pollution class 9 Smear layer definizione
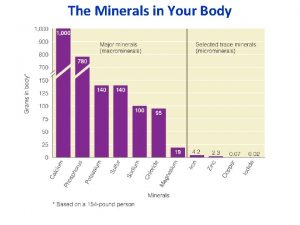
Smear layer definizione Minerals are inorganic elements that the body
Minerals are inorganic elements that the body Inorganic gases
Inorganic gases Inorganic matrix
Inorganic matrix Example of inorganic emulsifying agent
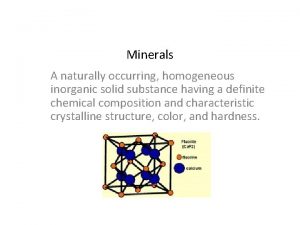
Example of inorganic emulsifying agent Naturally occurring inorganic solid
Naturally occurring inorganic solid Introduction to organic chemistry
Introduction to organic chemistry Non aqueous solvents classification
Non aqueous solvents classification Homogeneous inorganic substances
Homogeneous inorganic substances Inorganic precipitating agents
Inorganic precipitating agents C10h22 organic or inorganic
C10h22 organic or inorganic Organic and inorganic cofactors
Organic and inorganic cofactors Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Organic vs inorganic
Organic vs inorganic Organic and inorganic nutrients
Organic and inorganic nutrients Inorganic geology definition
Inorganic geology definition Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Organic and inorganic cofactors
Organic and inorganic cofactors Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Hsab chemistry
Hsab chemistry Charring test of organic and inorganic compounds
Charring test of organic and inorganic compounds Inert pair effect
Inert pair effect Difference between organic and inorganic growth
Difference between organic and inorganic growth Organic vs inorganic compounds
Organic vs inorganic compounds Organic vs inorganic compounds
Organic vs inorganic compounds Organic vs inorganic
Organic vs inorganic Is ch4o organic or inorganic
Is ch4o organic or inorganic Cons of pesticides
Cons of pesticides Oxalic acid is organic or inorganic
Oxalic acid is organic or inorganic Inorganic content of calculus
Inorganic content of calculus Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes What is inorganic matter
What is inorganic matter Heterotrophic plants
Heterotrophic plants Stickase
Stickase Inorganic non metallic materials examples
Inorganic non metallic materials examples Inorganic growth advantages and disadvantages
Inorganic growth advantages and disadvantages Silicones and phosphazenes notes
Silicones and phosphazenes notes Inorganic catalyst vs enzyme
Inorganic catalyst vs enzyme Prosthetic group example
Prosthetic group example Which compound is inorganic
Which compound is inorganic Minerals are inorganic elements that the body
Minerals are inorganic elements that the body Reduction of alcohol to alkane
Reduction of alcohol to alkane Naming acids
Naming acids Do you italicize scientific names
Do you italicize scientific names Primary amine structure
Primary amine structure Leaf type
Leaf type Nomenclature poteau incendie
Nomenclature poteau incendie Tableau de classification des bactéries
Tableau de classification des bactéries Rh nomenclature
Rh nomenclature Thioether
Thioether We
We Binomial nomenclature of cat
Binomial nomenclature of cat Pentose sugar structure in dna
Pentose sugar structure in dna Outline the binomial system of nomenclature
Outline the binomial system of nomenclature Hcl and hno3 are both strong acids
Hcl and hno3 are both strong acids Type 3 nomenclature
Type 3 nomenclature Xxx police report
Xxx police report Non essential fatty acids
Non essential fatty acids Iupac
Iupac Nomenclature
Nomenclature Ieee nomenclature
Ieee nomenclature Nomenclature of enzymes
Nomenclature of enzymes Naming alkyl halides
Naming alkyl halides Tuff kuffs handcuffs
Tuff kuffs handcuffs Binomial nomenclature examples
Binomial nomenclature examples Nomenclature arborescente
Nomenclature arborescente Alcohols nomenclature
Alcohols nomenclature General chemistry nomenclature
General chemistry nomenclature Entretoise echelle a crochet
Entretoise echelle a crochet Fisher-race nomenclature
Fisher-race nomenclature Remington 870 shotgun nomenclature
Remington 870 shotgun nomenclature Nomenclature of binary ionic compounds
Nomenclature of binary ionic compounds Properties of alkyl halides
Properties of alkyl halides Nomenclature of bolts
Nomenclature of bolts Revit nomenclature
Revit nomenclature Problems of nomenclature
Problems of nomenclature