INORGANIC COMPOUNDS OXIDES Classification of the chemical compounds








- Slides: 10

INORGANIC COMPOUNDS OXIDES

Classification of the chemical compounds Chemical Compounds: Organic Inorganic: • OXIDS • ACIDS • HYDROXIDES AND BASES • SALTS • COMPLEX COMPOUNDS

OXIDS Binary oxides represent compounds of oxygen (oxygen is bound to a metal, non-metal, semimetal) in which Oxidation number of oxygen is -2. Separation of oxides - according to properties: • Acids, • Bases, • Amphoteric, • Indifferent (neutral)

OXIDS Acid oxides are oxides that react with hydroxide (bases) or base oxides and thus form salts and water form acids. Example: (CO 2, SO 2, NO, P 2 O 5) CO 2 + Ca(OH)2 → Ca. CO 3 + H 2 O CO 2 + Ca. O → Ca. CO 3 SO 3 + H 2 O → H 2 SO 4 CO 2 + H 2 O →H 2 CO 3 Acid oxides can arise with deprivation of water from acids, therefore still called anhydride acid (acid without water) Example: CO 2 anhydride carbonic acid H 2 CO 3 SO 3 anhydrous sulfuric acid H 2 SO 4

Oxides Base oxides are oxides that react with acids or acid form and oxides thereof and water form bases. For example: (Ca. O, Cu 2 O, Cu. O, Fe 2 O 3, ) Cu. O + H 2 SO 4 → Cu. SO 4 + H 2 O Ca. O + CO 2 → Ca. CO 3 Ca. O + H 2 O → Ca (OH) 2 Na 2 O + H 2 O → 2 Na. OH

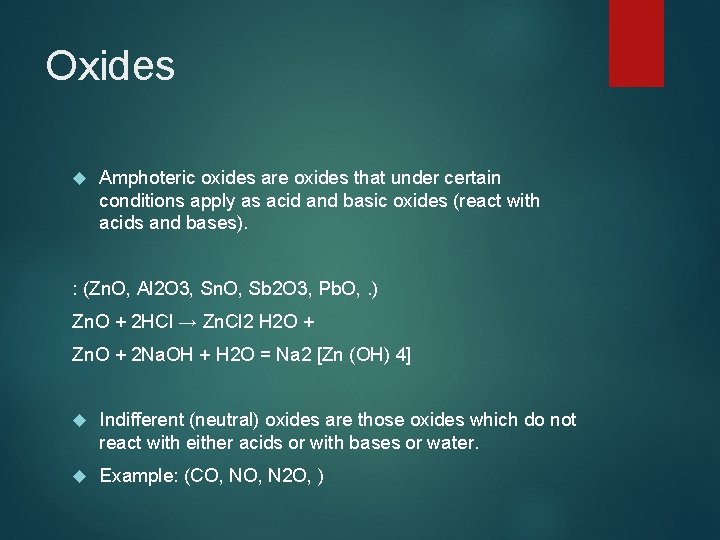
Oxides Amphoteric oxides are oxides that under certain conditions apply as acid and basic oxides (react with acids and bases). : (Zn. O, Al 2 O 3, Sn. O, Sb 2 O 3, Pb. O, . ) Zn. O + 2 HCl → Zn. Cl 2 H 2 O + Zn. O + 2 Na. OH + H 2 O = Na 2 [Zn (OH) 4] Indifferent (neutral) oxides are those oxides which do not react with either acids or with bases or water. Example: (CO, N 2 O, )

Oxides -Nomenclature of metal (base) oxide If metal built only one type oxide or metal if there is only one possible oxidation state, then the name of the oxide form of the name of the metal by adding the word "oxide". Example: Sodium oxide Na 2 O Calcium oxide Ca. O Aluminum oxide Al 2 O 3 If the metal has more possible oxidation states (variable valence), that builds more kinds oxides, then between the name of the metal oxide and the word write the oxidation state of the metal with Roman number placed in parenthesis, merged with the name of the metal.

OXIDES examples: Cu 2 O copper (I) oxide Cu. O copper (II) oxide Fe. O iron (II) oxide Fe 2 O 3 iron (III) oxide Look out! The name of the metal and the Roman numbers are merged write the name of the metal (with or without roman number) and write the word oxide separated. Nomenclature of non-metallic (acid) oxide Name of non-metal oxide in addition to the previous method, can be formed from the name of the metal and the word "oxide". Before the name of nonmetal before the word oxide are added prefixes that mark the appropriate stoichiometric index named in Greek

OXIDES examples: N 2 O 5 dinitrogen pentoxide Sulfur trioxide SO 3 P 4 O 10 tetraphosphoric dekaoksid

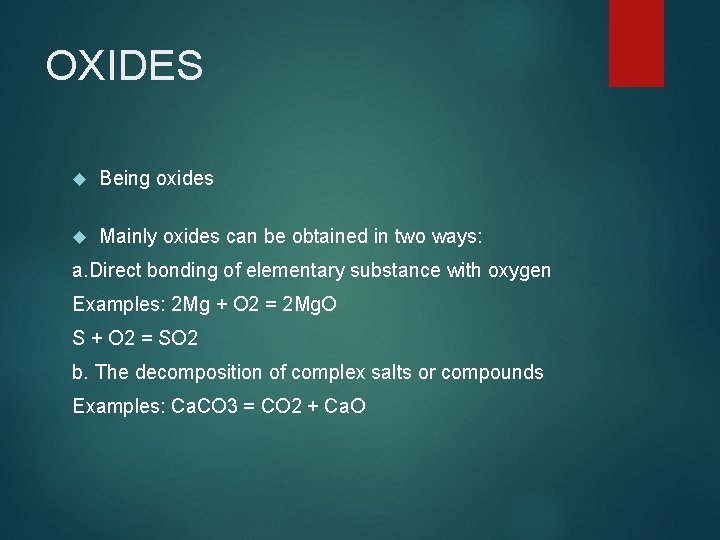
OXIDES Being oxides Mainly oxides can be obtained in two ways: a. Direct bonding of elementary substance with oxygen Examples: 2 Mg + O 2 = 2 Mg. O S + O 2 = SO 2 b. The decomposition of complex salts or compounds Examples: Ca. CO 3 = CO 2 + Ca. O