Inorganic chemistry q Electrochemistry q Oxidation reduction concepts









- Slides: 11

Inorganic chemistry q Electrochemistry. q Oxidation –reduction concepts. q Standard reduction potentials. Assistance Lecturer Amjad Ahmed Jumaa www. soran. edu. iq 1

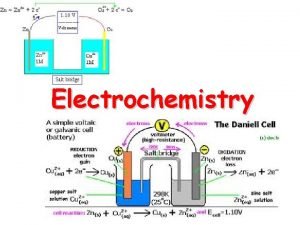
Electrochemistry Is the study of the relationship between chemical change and electrical work, it is typically investigated through the use of electrochemical cells, systems that incorporate a redox reaction to produce or utilize electrical energy. Oxidation –reduction concepts: In the reaction between zinc (Zn), and hydrogen ion (H+), zinc is oxidized hydrogen ion is reduced: Zn(s) + 2 H+ (aq) → Zn+2(aq) + H 2 (g) Oxidation: 1 -one reactant loses electrons. Zinc loses electron. www. soran. edu. iq

2 - Reducing agent is oxidized. Zinc is the reducing agent and become oxidized. 3 - Oxidation number increases. The oxidation number of (Zn) increases from (0) to (+2). Reduction: 1 - Other reactant gains electrons. Hydrogen gains electrons. 2 - Oxidizing agent is reduced. Hydrogen ion is the oxidizing agent and becomes reduced. 3 - Oxidation number decreases. The oxidation number of (H+) ion decrease from (+1) to (0). www. soran. edu. iq

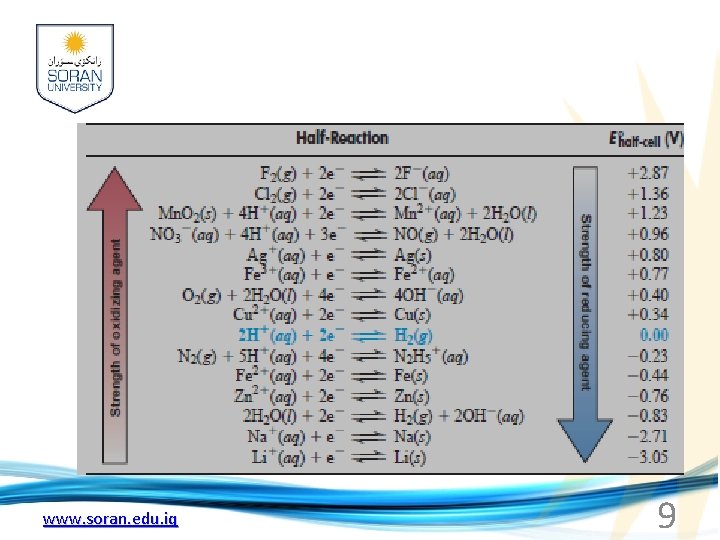
Standard reduction potentials: For a reduction reaction at an electrode when all solutes are (1 M) and all gases are at ( 1 atm) the voltage is called the standard reduction potential ( E°). Comparing strengths of oxidizing agents: The more positive the value of the standard reduction potential (E°), the greater the tendency for the substance to be reduced, and therefore the stronger its tendency to act as an oxidizing agent. www. soran. edu. iq

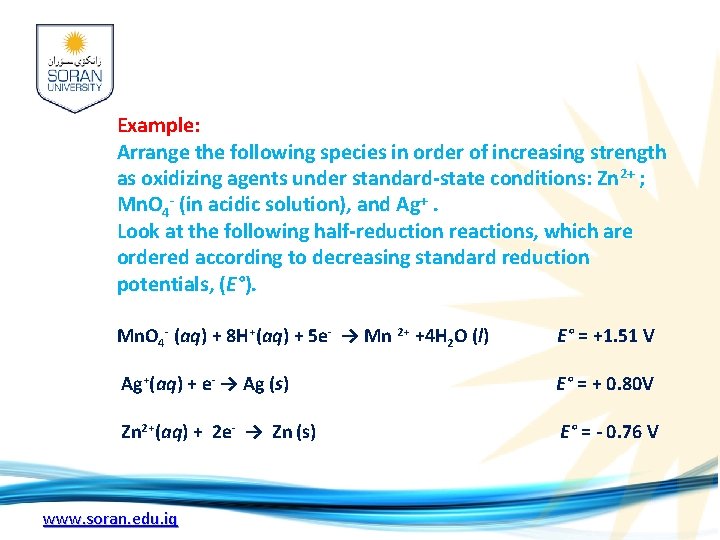
Example: Arrange the following species in order of increasing strength as oxidizing agents under standard-state conditions: Zn 2+ ; Mn. O 4 - (in acidic solution), and Ag+. Look at the following half-reduction reactions, which are ordered according to decreasing standard reduction potentials, (E°). Mn. O 4 - (aq) + 8 H+(aq) + 5 e- → Mn 2+ +4 H 2 O (l) E° = +1. 51 V Ag+(aq) + e- → Ag (s) E° = + 0. 80 V Zn 2+(aq) + 2 e- → Zn (s) E° = - 0. 76 V www. soran. edu. iq

Solution: Zn 2+ < Ag+ < Mn. O 4 -. The large positive reduction potential for (Mn. O 4 - ) indicates the strong tendency for permanganate ion to be reduced, and therefore the stronger its tendency to act as an oxidizing agent. www. soran. edu. iq

q One of the things we can learn from measuring potentials of voltaic cells is the relative strengths of the oxidizing and reducing agents involved. Three oxidizing agents present in the voltaic cell just discussed are Cu+2, H+, and Zn+2. We can rank their relative oxidizing strengths by writing each halfreaction as a gain of electrons (reduction), with its corresponding standard electrode potential: www. soran. edu. iq 7

www. soran. edu. iq 8

www. soran. edu. iq 9

www. soran. edu. iq 10

www. soran. edu. iq 11
 Organic vs inorganic chemistry
Organic vs inorganic chemistry Dry oxidation wet oxidation
Dry oxidation wet oxidation Is electrochemistry
Is electrochemistry Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Ap chemistry ced
Ap chemistry ced Ap chemistry electrochemistry
Ap chemistry electrochemistry What is electrochemistry in chemistry
What is electrochemistry in chemistry Oxidation number rukes
Oxidation number rukes Oxidation-reduction quiz
Oxidation-reduction quiz Titration mechanism
Titration mechanism Principles of corrosion
Principles of corrosion Reduction vs oxidation
Reduction vs oxidation