Inorganic chemistry Organic Compounds Cool Carbohydrates Lipids Proteins
Inorganic chemistry Organic Compounds Cool Carbohydrates Lipids Proteins & Nucleic Acids 5 pt 5 pt 10 pt 10 pt 15 pt 15 pt 20 pt 20 pt 25 pt 25 1 pt

The chemical formula for water. 2

What is H 2 O? 3

Water is inorganic because it does NOT contain this element. 4

What is Carbon? 5

What water is said to be because of its uneven distribution of charge. 6

What is polar? 7

p. H between 0 -6. 9. 8

What is an acid or acidic? 9

p. H of 13 (be specific). 10

What is a “strong” base? 11
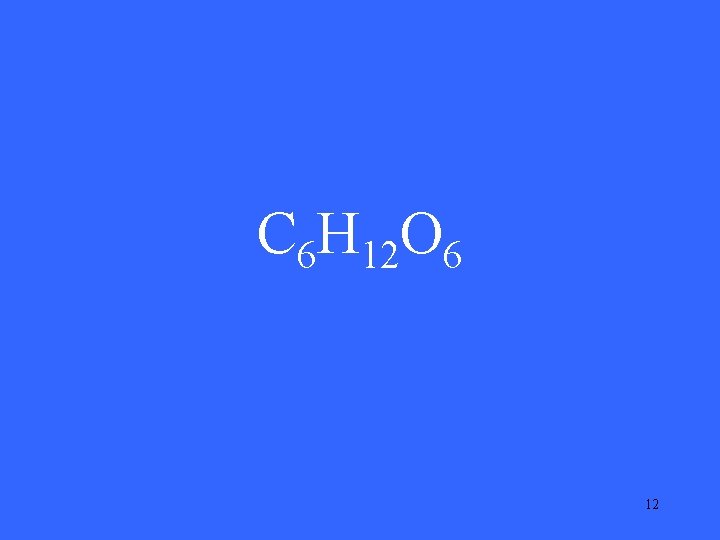
C 6 H 12 O 6 12

What is glucose? 13

The four organic compounds/ biomolecules/ biochemical compounds. 14

What are carbohydrates, lipids, proteins, & nucleic acids? 15
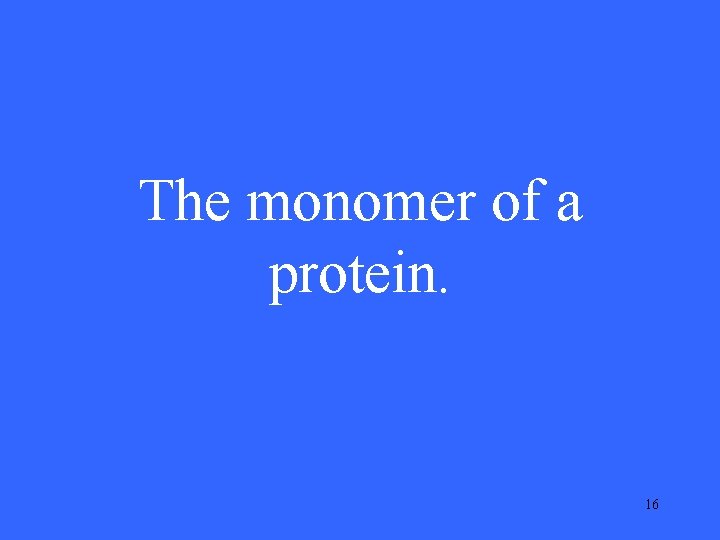
The monomer of a protein. 16

What is/are amino acid(s)? 17

Offers the most Calories per gram (9 Cal/g), unsaturated ones have some double bonds between the carbons and are liquids at room temperature. 18

What are lipids (or fats)? 19

The category of organic compound that consists of CHO in a 1: 2: 1 ratio and sacchar and –ose are word parts. 20

What is carbohydrate? 21

The main function of carbohydrates. 22

What is provides energy or quick energy? 23

The name for table sugar and type of that saccharide (mono, di, or poly). 24

What is sucrose a disaccharide? 25

The polysaccharide that humans CANNOT digest and plants store in their cell walls. 26

What is cellulose? 27

The chemical formula for the main product of photosynthesis and starting material of cellular respiration. 28

What is C 6 H 12 O 6? 29

The name of the polysaccharide carbohydrate that is stored in animal liver and muscles. 30

What is glycogen? 31
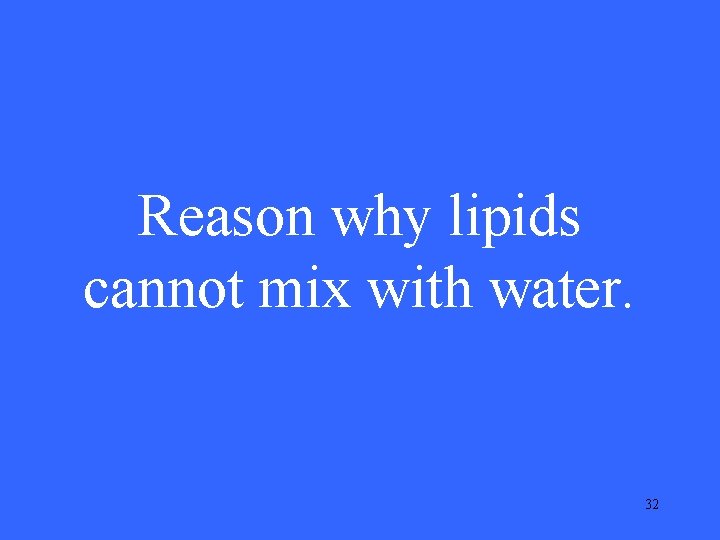
Reason why lipids cannot mix with water. 32

What is they are nonpolar? 33

The function of fats, a type of lipid. 34

What are insulators or stored energy? 35

These may be converted into lipids for long term energy storage. 36

What are carbohydrates? 37
The phospholipid structure of the cell membrane (draw on the marker board). 38
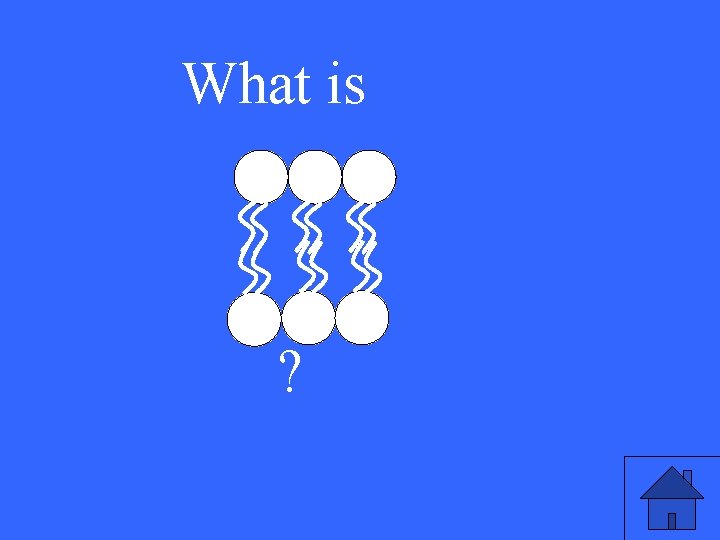
What is ? 39

Three main characteristics of saturated fats. 40

What are: 1. NO double bonds 2. from animals 3. solids at room temp? 41

Examples include enzymes, antibodies, and hemoglobin. 42
What are proteins? 43

The name of this monomer. 44

What is a nucleotide? 45

The two sugars that make up nucleic acids DNA and RNA. 46

What are deoxyribose and ribose? 47

The elements that make up proteins (at least 5). 48

What are CHNOPS Fe Cu? 49

The three parts of the monomer of a nucleic acid. 50

What are a sugar, phosphate, and nitrogen base. 51
- Slides: 51