Inorganic Chemistry Chemical Periodicity PART II Chemical and




- Slides: 45

Inorganic Chemistry Chemical Periodicity PART II: Chemical and Physical Properties of Elements, Oxides and Chlorides from Period 3 CONTINUE EXIT

Before you embark… In this Inorganic Chemistry Part II, you will learn the chemical and physical properties of elements from Period 3. For a more effective learning experience, do make use of the "MAIN" to navigate. MAIN EXIT

Assessment Objectives Candidates should, for the third Period (sodium to argon), be able to: a. b. c. d. e. f. describe the reactions, if any, of the elements with oxygen (to give Na 2 O; Mg. O; Al 2 O 3; P 4 O 10; SO 2; SO 3), and chlorine (to give Na. Cl; Mg. Cl 2; Al. Cl 3; Si. Cl 4; PCl 5) state and explain the variation in oxidation number of the oxides and chlorides describe the reactions of the oxides with water [treatment of peroxides and superoxides is not required] describe and explain the acid/base behaviour of oxides and hydroxides, including, where relevant, amphoteric behaviour in reaction with sodium hydroxide (only) and acids describe and explain the reactions of the chlorides with water interpret the variations and trends in (b), (c), (d), and (e) in terms of bonding and electronegativity Cont’d MAIN

Assessment Objectives Candidates should, for the third Period (sodium to argon), be able to: g. h. i. suggest the types of chemical bonding present in chlorides and oxides from observations of their chemical and physical properties predict the characteristic properties of an element in a given Group by using knowledge of chemical periodicity deduce the nature, possible position in the Periodic Table, and identity of unknown elements from given information of physical and chemical properties Prev MAIN

Oxidation number of elements in Oxides and Chlorides MAIN COVER Chemical Properties of Elements (Reaction with O 2, H 2 O and Cl 2) Physical Properties of Oxides ASSESSMENT OBJECTIVES Reaction of Oxides with Water Acid-base reaction of Oxides Physical Properties of Chlorides Reaction of Chlorides with Water ASSIGNMENT END

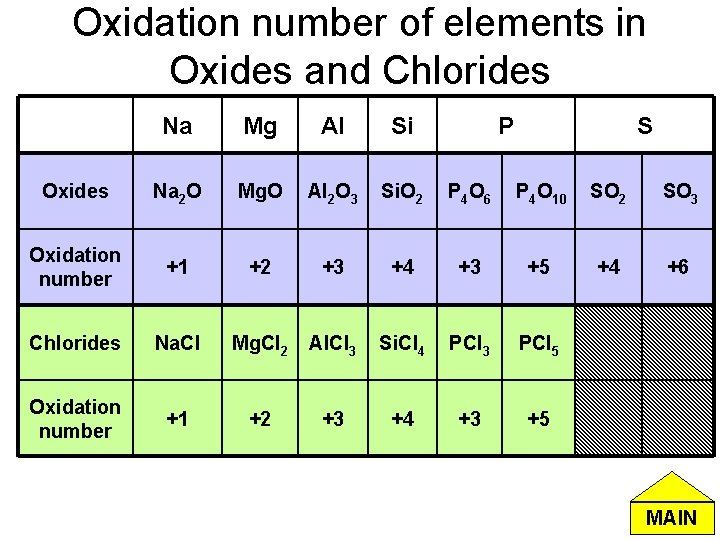
Oxidation number of elements in Oxides and Chlorides Na Mg Al Si P S Oxides Na 2 O Mg. O Al 2 O 3 Si. O 2 P 4 O 6 P 4 O 10 SO 2 SO 3 Oxidation number +1 +2 +3 +4 +3 +5 +4 +6 Chlorides Na. Cl Mg. Cl 2 Al. Cl 3 Si. Cl 4 PCl 3 PCl 5 Oxidation number +1 +2 +3 +4 +3 +5 MAIN

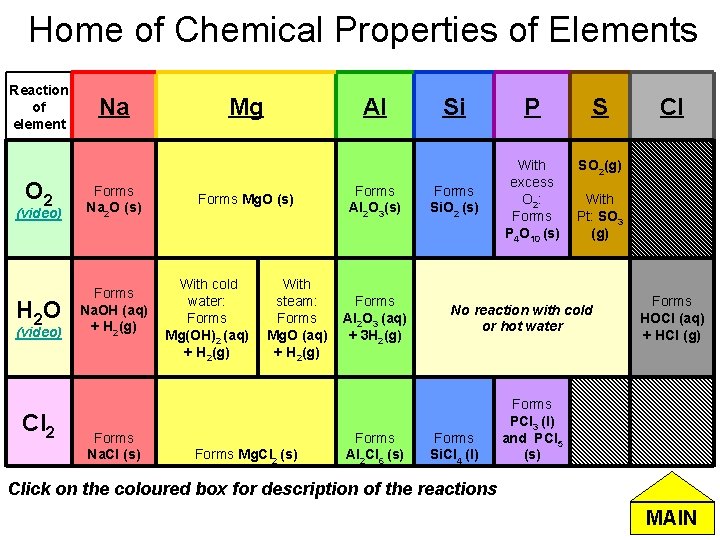
Home of Chemical Properties of Elements Reaction of element O 2 (video) H 2 O (video) Cl 2 Na Forms Na 2 O (s) Forms Na. OH (aq) + H 2(g) Forms Na. Cl (s) Mg Al Forms Mg. O (s) With cold water: Forms Mg(OH)2 (aq) + H 2(g) With steam: Forms Mg. O (aq) + H 2(g) Forms Mg. Cl 2 (s) Forms Al 2 O 3 (aq) + 3 H 2(g) Forms Al 2 Cl 6 (s) Si P S SO 2(g) Forms Si. O 2 (s) With excess O 2: Forms P 4 O 10 (s) With Pt: SO 3 (g) No reaction with cold or hot water Forms Si. Cl 4 (l) Cl Forms HOCl (aq) + HCl (g) Forms PCl 3 (l) and PCl 5 (s) Click on the coloured box for description of the reactions MAIN

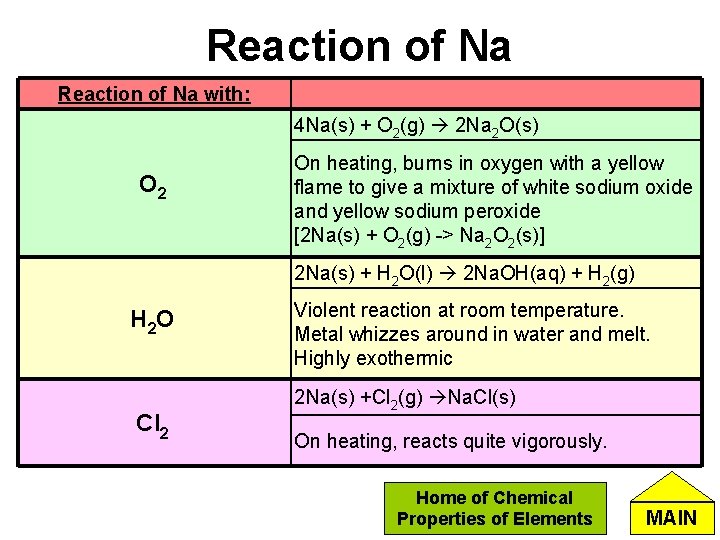
Reaction of Na with: 4 Na(s) + O 2(g) 2 Na 2 O(s) O 2 On heating, burns in oxygen with a yellow flame to give a mixture of white sodium oxide and yellow sodium peroxide [2 Na(s) + O 2(g) -> Na 2 O 2(s)] 2 Na(s) + H 2 O(l) 2 Na. OH(aq) + H 2(g) H 2 O Cl 2 Violent reaction at room temperature. Metal whizzes around in water and melt. Highly exothermic 2 Na(s) +Cl 2(g) Na. Cl(s) On heating, reacts quite vigorously. Home of Chemical Properties of Elements MAIN

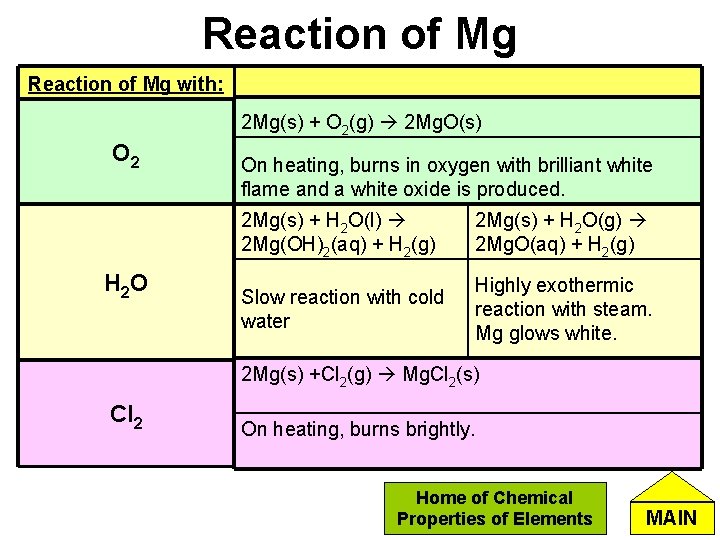
Reaction of Mg with: 2 Mg(s) + O 2(g) 2 Mg. O(s) O 2 H 2 O On heating, burns in oxygen with brilliant white flame and a white oxide is produced. 2 Mg(s) + H 2 O(l) 2 Mg(OH)2(aq) + H 2(g) 2 Mg(s) + H 2 O(g) 2 Mg. O(aq) + H 2(g) Slow reaction with cold water Highly exothermic reaction with steam. Mg glows white. 2 Mg(s) +Cl 2(g) Mg. Cl 2(s) Cl 2 On heating, burns brightly. Home of Chemical Properties of Elements MAIN

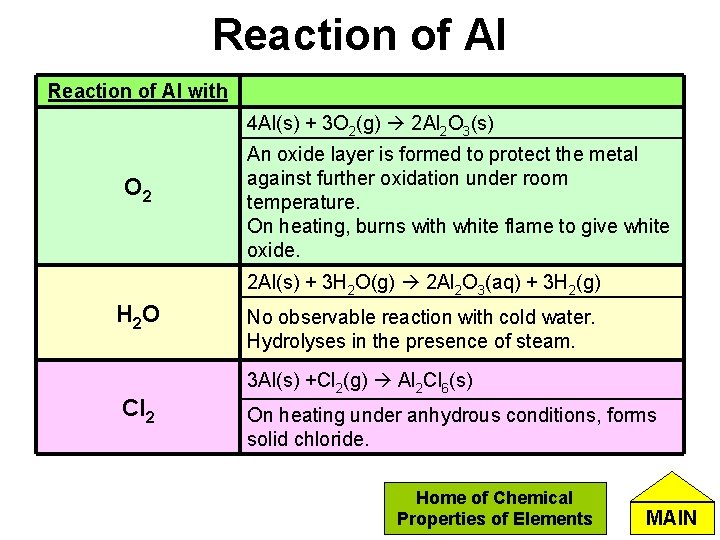
Reaction of Al with 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) O 2 An oxide layer is formed to protect the metal against further oxidation under room temperature. On heating, burns with white flame to give white oxide. 2 Al(s) + 3 H 2 O(g) 2 Al 2 O 3(aq) + 3 H 2(g) H 2 O Cl 2 No observable reaction with cold water. Hydrolyses in the presence of steam. 3 Al(s) +Cl 2(g) Al 2 Cl 6(s) On heating under anhydrous conditions, forms solid chloride. Home of Chemical Properties of Elements MAIN

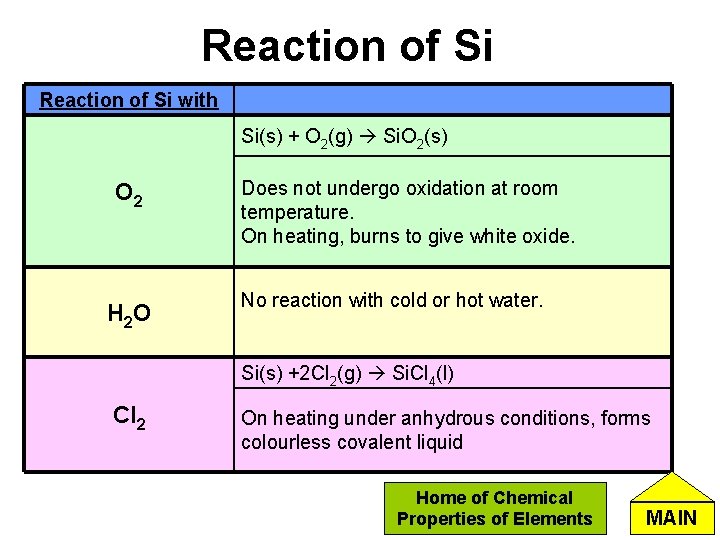
Reaction of Si with Si(s) + O 2(g) Si. O 2(s) O 2 H 2 O Does not undergo oxidation at room temperature. On heating, burns to give white oxide. No reaction with cold or hot water. Si(s) +2 Cl 2(g) Si. Cl 4(l) Cl 2 On heating under anhydrous conditions, forms colourless covalent liquid Home of Chemical Properties of Elements MAIN

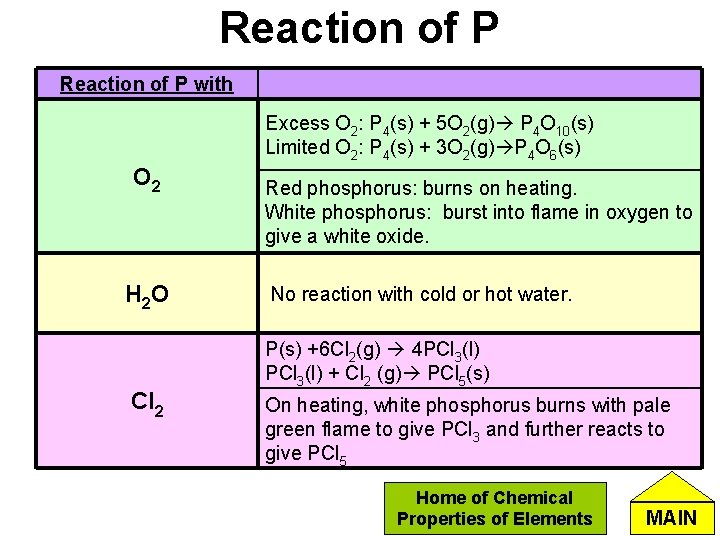
Reaction of P with Excess O 2: P 4(s) + 5 O 2(g) P 4 O 10(s) Limited O 2: P 4(s) + 3 O 2(g) P 4 O 6(s) O 2 H 2 O Cl 2 Red phosphorus: burns on heating. White phosphorus: burst into flame in oxygen to give a white oxide. No reaction with cold or hot water. P(s) +6 Cl 2(g) 4 PCl 3(l) + Cl 2 (g) PCl 5(s) On heating, white phosphorus burns with pale green flame to give PCl 3 and further reacts to give PCl 5 Home of Chemical Properties of Elements MAIN

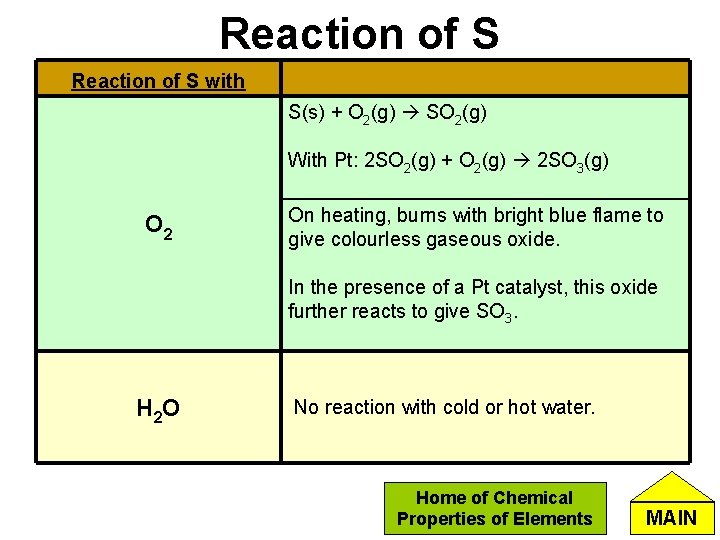
Reaction of S with S(s) + O 2(g) SO 2(g) With Pt: 2 SO 2(g) + O 2(g) 2 SO 3(g) O 2 On heating, burns with bright blue flame to give colourless gaseous oxide. In the presence of a Pt catalyst, this oxide further reacts to give SO 3. H 2 O No reaction with cold or hot water. Home of Chemical Properties of Elements MAIN

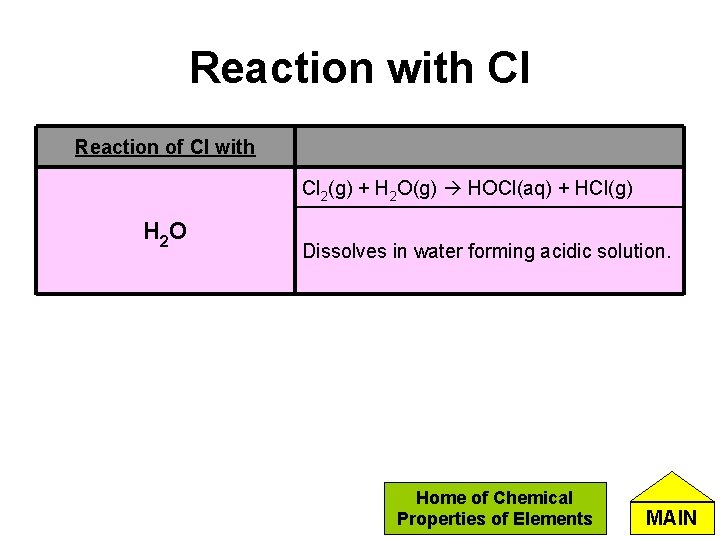
Reaction with Cl Reaction of Cl with Cl 2(g) + H 2 O(g) HOCl(aq) + HCl(g) H 2 O Dissolves in water forming acidic solution. Home of Chemical Properties of Elements MAIN

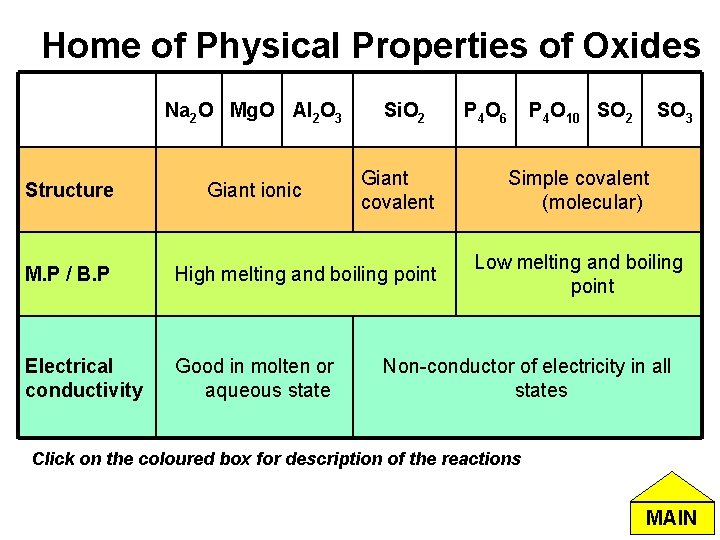
Home of Physical Properties of Oxides Na 2 O Mg. O Al 2 O 3 Structure Giant ionic Si. O 2 Giant covalent M. P / B. P High melting and boiling point Electrical conductivity Good in molten or aqueous state P 4 O 6 P 4 O 10 SO 2 SO 3 Simple covalent (molecular) Low melting and boiling point Non-conductor of electricity in all states Click on the coloured box for description of the reactions MAIN

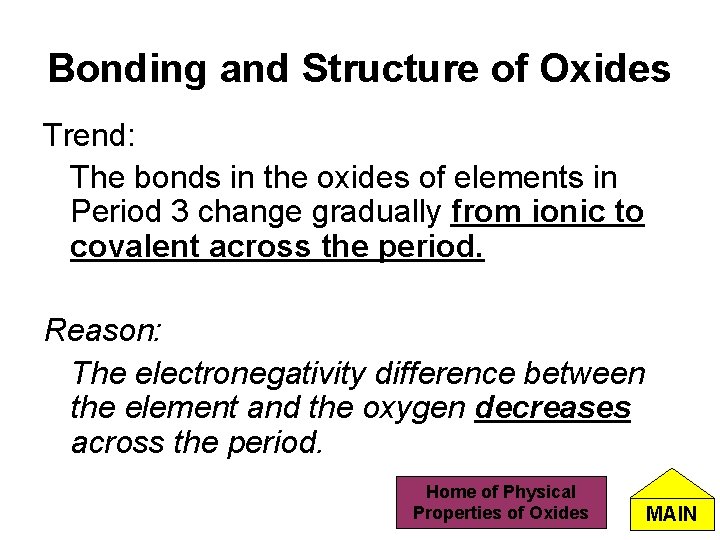
Bonding and Structure of Oxides Trend: The bonds in the oxides of elements in Period 3 change gradually from ionic to covalent across the period. Reason: The electronegativity difference between the element and the oxygen decreases across the period. Home of Physical Properties of Oxides MAIN

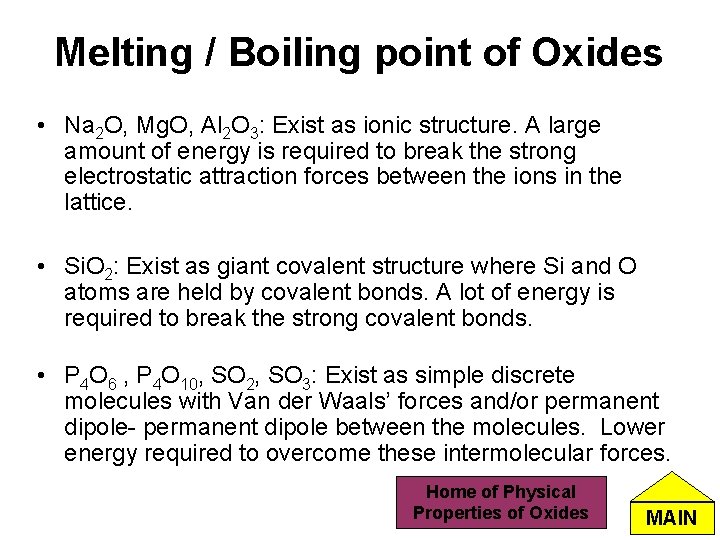
Melting / Boiling point of Oxides • Na 2 O, Mg. O, Al 2 O 3: Exist as ionic structure. A large amount of energy is required to break the strong electrostatic attraction forces between the ions in the lattice. • Si. O 2: Exist as giant covalent structure where Si and O atoms are held by covalent bonds. A lot of energy is required to break the strong covalent bonds. • P 4 O 6 , P 4 O 10, SO 2, SO 3: Exist as simple discrete molecules with Van der Waals’ forces and/or permanent dipole- permanent dipole between the molecules. Lower energy required to overcome these intermolecular forces. Home of Physical Properties of Oxides MAIN

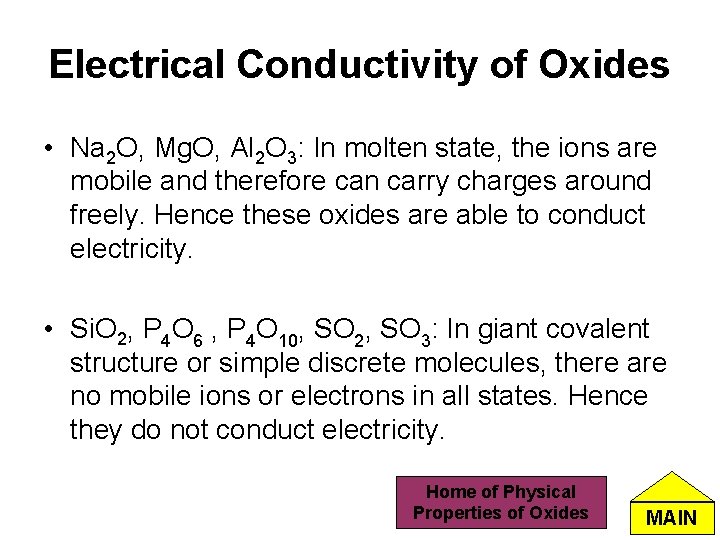
Electrical Conductivity of Oxides • Na 2 O, Mg. O, Al 2 O 3: In molten state, the ions are mobile and therefore can carry charges around freely. Hence these oxides are able to conduct electricity. • Si. O 2, P 4 O 6 , P 4 O 10, SO 2, SO 3: In giant covalent structure or simple discrete molecules, there are no mobile ions or electrons in all states. Hence they do not conduct electricity. Home of Physical Properties of Oxides MAIN

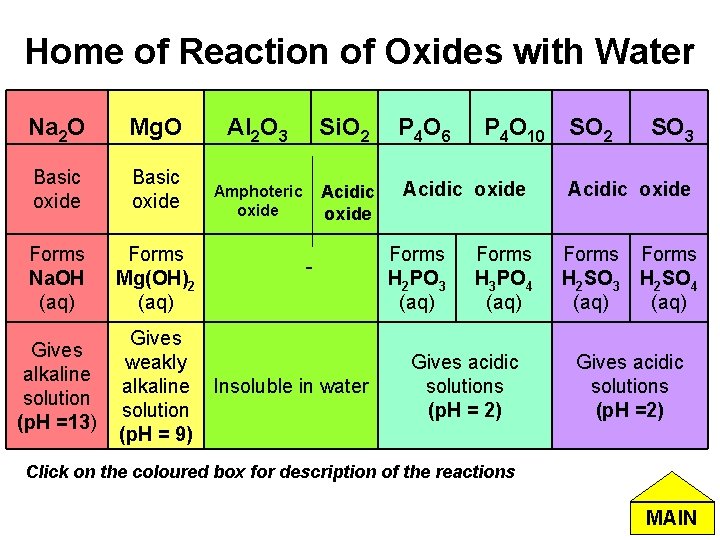
Home of Reaction of Oxides with Water Na 2 O Mg. O Al 2 O 3 Si. O 2 P 4 O 6 Basic oxide Amphoteric oxide Acidic oxide Forms Na. OH (aq) Forms Mg(OH)2 (aq) Gives alkaline solution (p. H =13) Gives weakly alkaline solution (p. H = 9) - Insoluble in water Forms H 2 PO 3 (aq) P 4 O 10 Forms H 3 PO 4 (aq) Gives acidic solutions (p. H = 2) SO 2 SO 3 Acidic oxide Forms H 2 SO 3 H 2 SO 4 (aq) Gives acidic solutions (p. H =2) Click on the coloured box for description of the reactions MAIN

Na 2 O with Water Basic oxides react with water to form alkaline solutions Na 2 O + H 2 O 2 Na. OH Home of Reaction of Oxides with Water MAIN

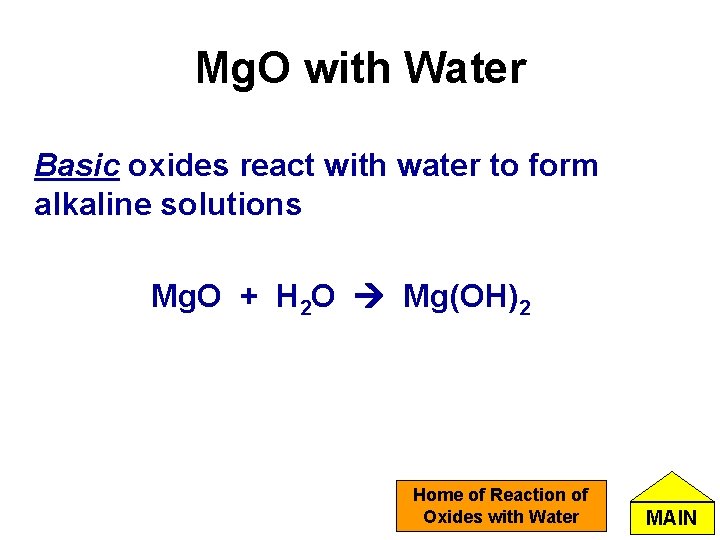
Mg. O with Water Basic oxides react with water to form alkaline solutions Mg. O + H 2 O Mg(OH)2 Home of Reaction of Oxides with Water MAIN

Al 2 O 3 and Si. O 2 with Water Al 2 O 3 does not react with water due to high lattice energy. Si. O 2 is insoluble in water due to its giant molecular structure Home of Reaction of Oxides with Water MAIN

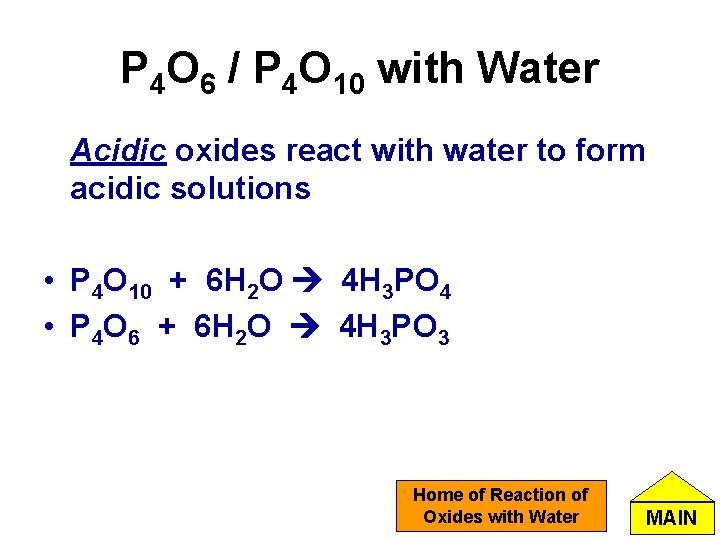
P 4 O 6 / P 4 O 10 with Water Acidic oxides react with water to form acidic solutions • P 4 O 10 + 6 H 2 O 4 H 3 PO 4 • P 4 O 6 + 6 H 2 O 4 H 3 PO 3 Home of Reaction of Oxides with Water MAIN

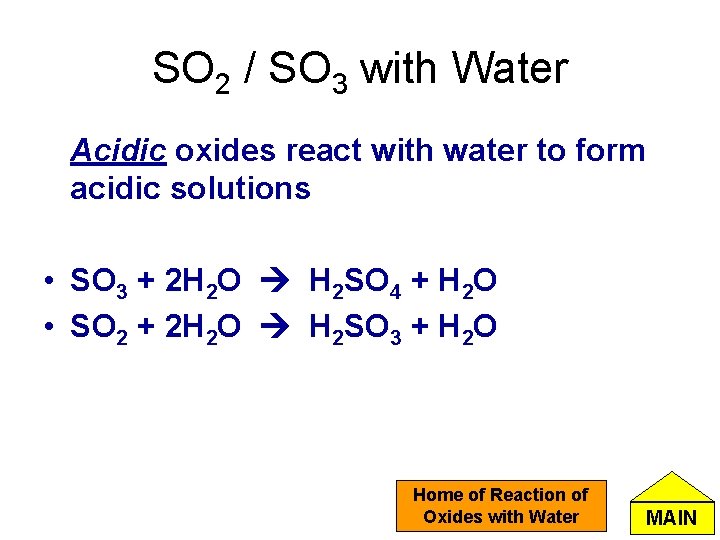
SO 2 / SO 3 with Water Acidic oxides react with water to form acidic solutions • SO 3 + 2 H 2 O H 2 SO 4 + H 2 O • SO 2 + 2 H 2 O H 2 SO 3 + H 2 O Home of Reaction of Oxides with Water MAIN

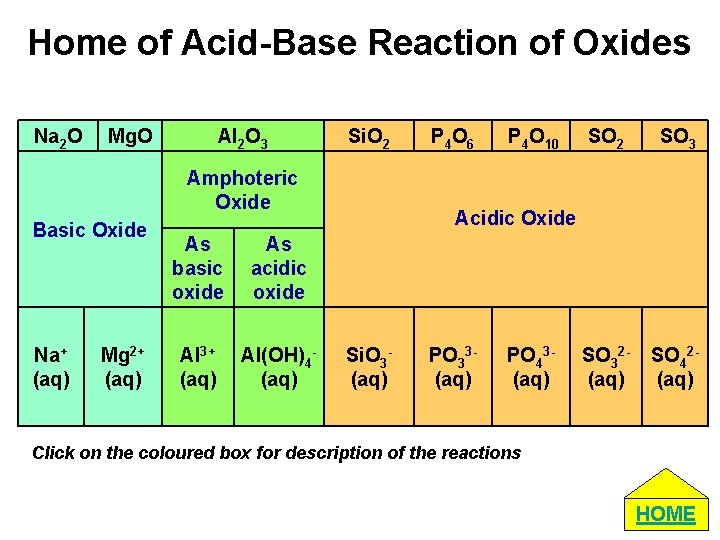
Home of Acid-Base Reaction of Oxides Na 2 O Mg. O Al 2 O 3 Si. O 2 Amphoteric Oxide Basic Oxide Na+ (aq) Mg 2+ (aq) As basic oxide As acidic oxide Al 3+ (aq) Al(OH)4(aq) P 4 O 6 P 4 O 10 SO 2 SO 32(aq) SO 42(aq) Acidic Oxide Si. O 3(aq) PO 33(aq) PO 43(aq) Click on the coloured box for description of the reactions HOME

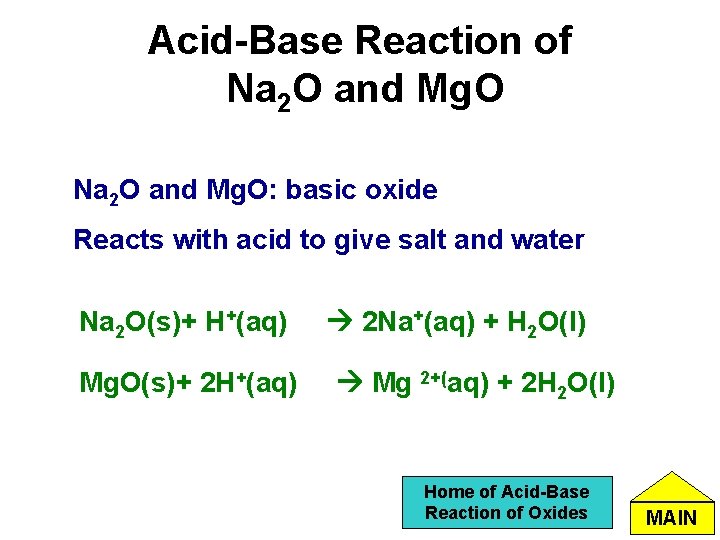
Acid-Base Reaction of Na 2 O and Mg. O: basic oxide Reacts with acid to give salt and water Na 2 O(s)+ H+(aq) Mg. O(s)+ 2 H+(aq) 2 Na+(aq) + H 2 O(l) Mg 2+(aq) + 2 H 2 O(l) Home of Acid-Base Reaction of Oxides MAIN

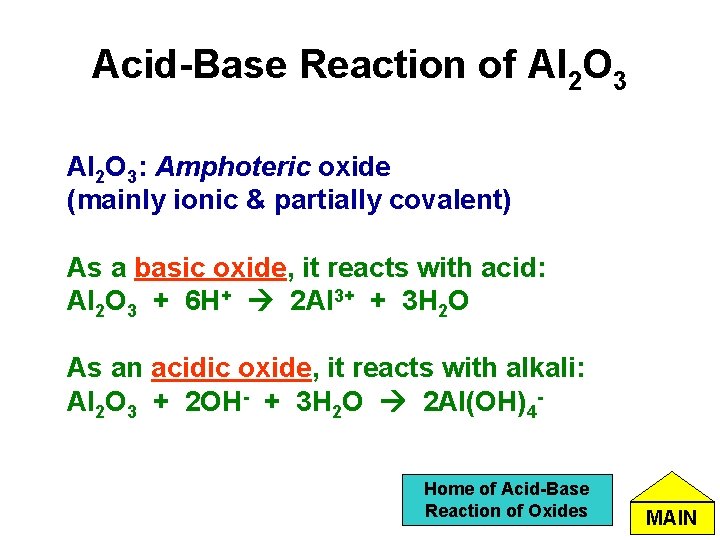
Acid-Base Reaction of Al 2 O 3: Amphoteric oxide (mainly ionic & partially covalent) As a basic oxide, it reacts with acid: Al 2 O 3 + 6 H+ 2 Al 3+ + 3 H 2 O As an acidic oxide, it reacts with alkali: Al 2 O 3 + 2 OH- + 3 H 2 O 2 Al(OH)4 Home of Acid-Base Reaction of Oxides MAIN

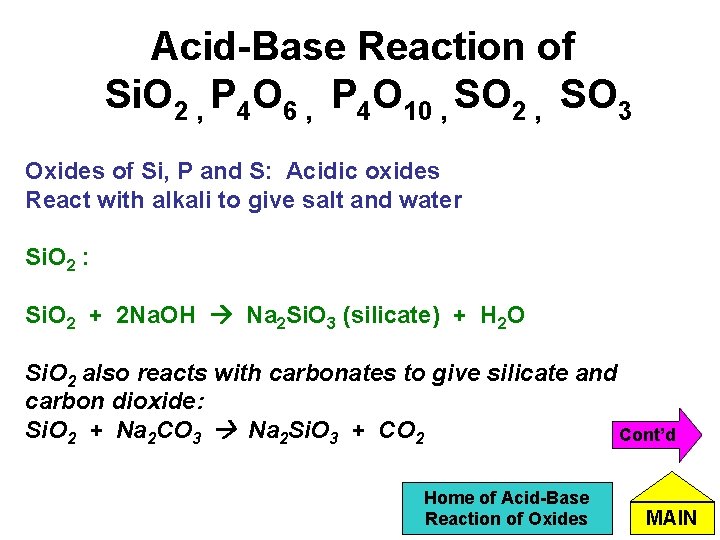
Acid-Base Reaction of Si. O 2 , P 4 O 6 , P 4 O 10 , SO 2 , SO 3 Oxides of Si, P and S: Acidic oxides React with alkali to give salt and water Si. O 2 : Si. O 2 + 2 Na. OH Na 2 Si. O 3 (silicate) + H 2 O Si. O 2 also reacts with carbonates to give silicate and carbon dioxide: Si. O 2 + Na 2 CO 3 Na 2 Si. O 3 + CO 2 Cont’d Home of Acid-Base Reaction of Oxides MAIN

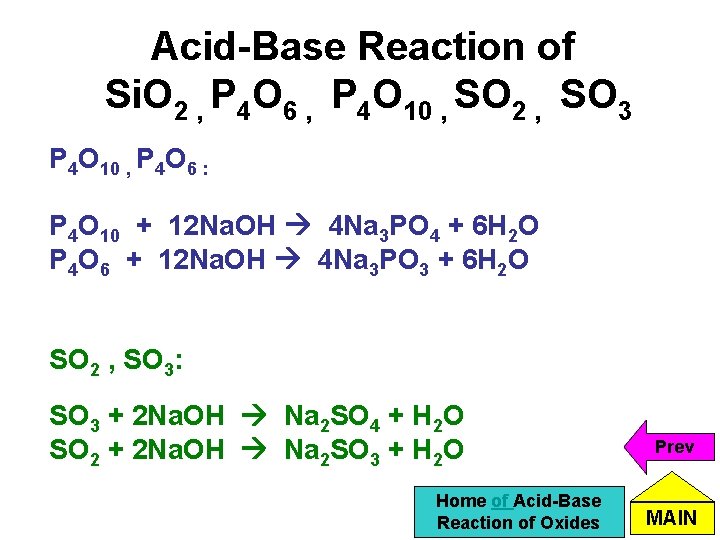
Acid-Base Reaction of Si. O 2 , P 4 O 6 , P 4 O 10 , SO 2 , SO 3 P 4 O 10 , P 4 O 6 : P 4 O 10 + 12 Na. OH 4 Na 3 PO 4 + 6 H 2 O P 4 O 6 + 12 Na. OH 4 Na 3 PO 3 + 6 H 2 O SO 2 , SO 3: SO 3 + 2 Na. OH Na 2 SO 4 + H 2 O SO 2 + 2 Na. OH Na 2 SO 3 + H 2 O Home of Acid-Base Reaction of Oxides Prev MAIN

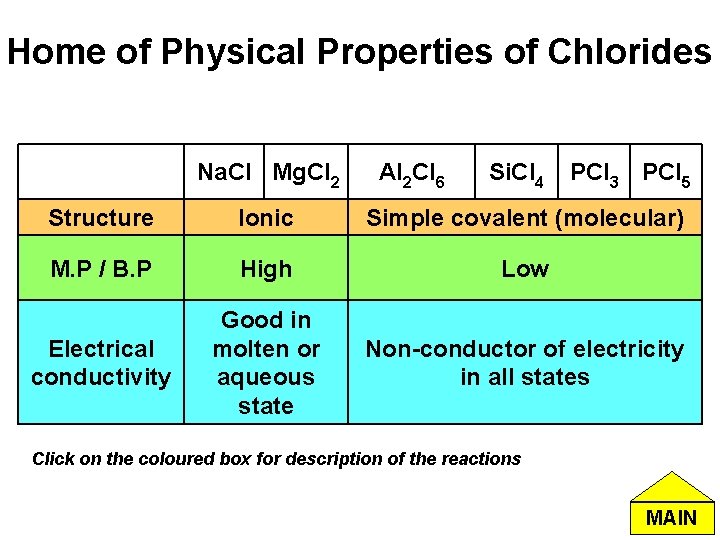
Home of Physical Properties of Chlorides Na. Cl Mg. Cl 2 Al 2 Cl 6 Si. Cl 4 PCl 3 PCl 5 Structure Ionic Simple covalent (molecular) M. P / B. P High Low Electrical conductivity Good in molten or aqueous state Non-conductor of electricity in all states Click on the coloured box for description of the reactions MAIN

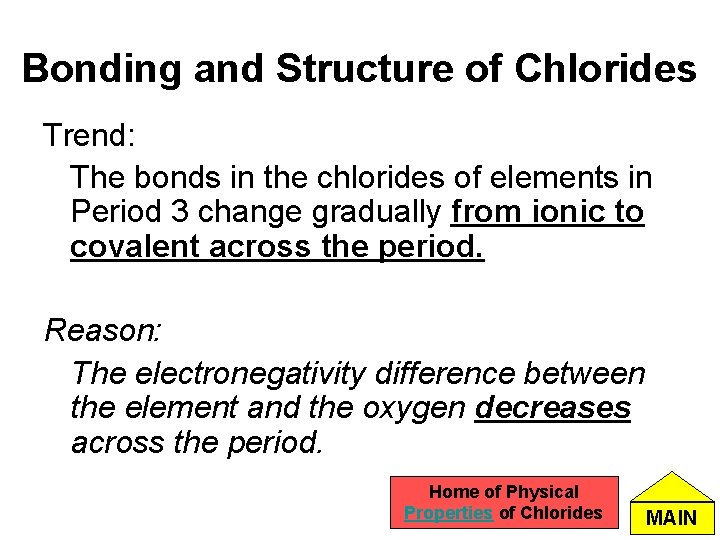
Bonding and Structure of Chlorides Trend: The bonds in the chlorides of elements in Period 3 change gradually from ionic to covalent across the period. Reason: The electronegativity difference between the element and the oxygen decreases across the period. Home of Physical Properties of Chlorides MAIN

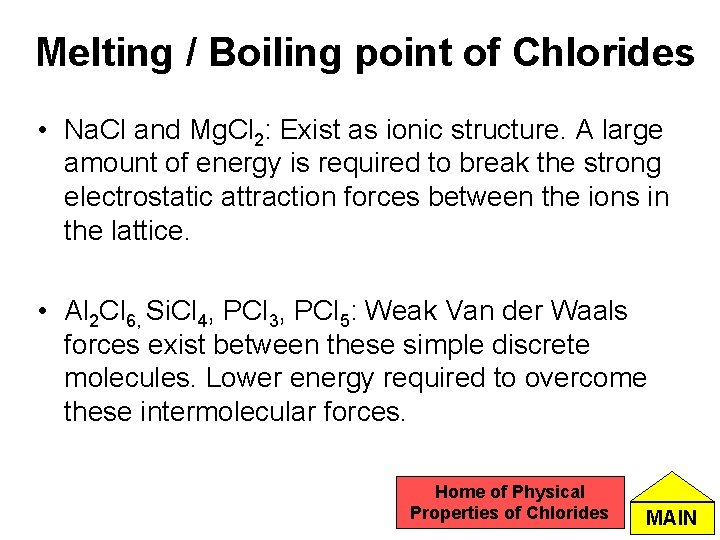
Melting / Boiling point of Chlorides • Na. Cl and Mg. Cl 2: Exist as ionic structure. A large amount of energy is required to break the strong electrostatic attraction forces between the ions in the lattice. • Al 2 Cl 6, Si. Cl 4, PCl 3, PCl 5: Weak Van der Waals forces exist between these simple discrete molecules. Lower energy required to overcome these intermolecular forces. Home of Physical Properties of Chlorides MAIN

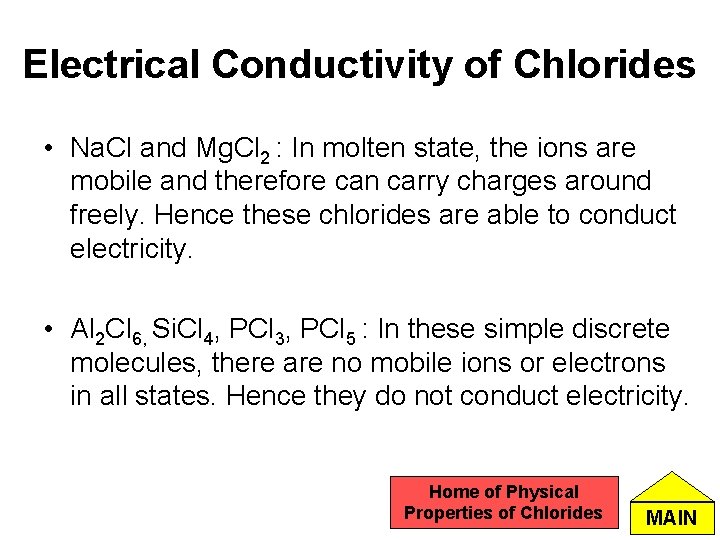
Electrical Conductivity of Chlorides • Na. Cl and Mg. Cl 2 : In molten state, the ions are mobile and therefore can carry charges around freely. Hence these chlorides are able to conduct electricity. • Al 2 Cl 6, Si. Cl 4, PCl 3, PCl 5 : In these simple discrete molecules, there are no mobile ions or electrons in all states. Hence they do not conduct electricity. Home of Physical Properties of Chlorides MAIN

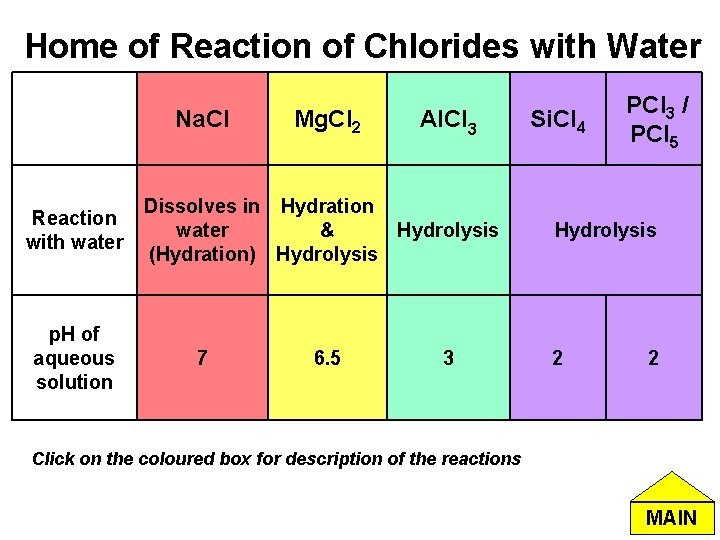
Home of Reaction of Chlorides with Water Na. Cl Reaction with water p. H of aqueous solution Mg. Cl 2 Al. Cl 3 Dissolves in Hydration water & Hydrolysis (Hydration) Hydrolysis 7 6. 5 3 Si. Cl 4 PCl 3 / PCl 5 Hydrolysis 2 2 Click on the coloured box for description of the reactions MAIN

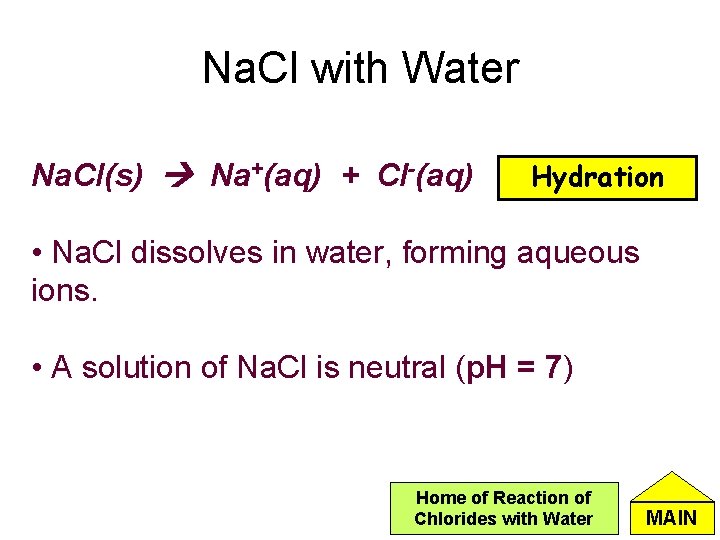
Na. Cl with Water Na. Cl(s) Na+(aq) + Cl-(aq) Hydration • Na. Cl dissolves in water, forming aqueous ions. • A solution of Na. Cl is neutral (p. H = 7) Home of Reaction of Chlorides with Water MAIN

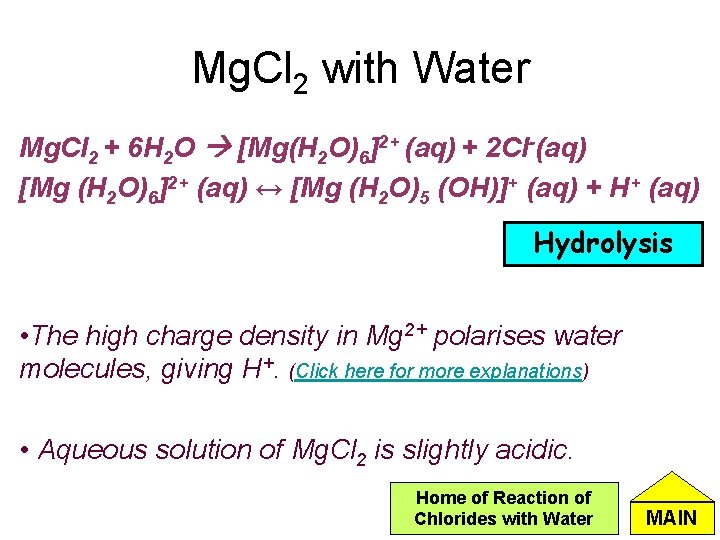
Mg. Cl 2 with Water Mg. Cl 2 + 6 H 2 O [Mg(H 2 O)6]2+ (aq) + 2 Cl-(aq) [Mg (H 2 O)6]2+ (aq) ↔ [Mg (H 2 O)5 (OH)]+ (aq) + H+ (aq) Hydrolysis • The high charge density in Mg 2+ polarises water molecules, giving H+. (Click here for more explanations) • Aqueous solution of Mg. Cl 2 is slightly acidic. Home of Reaction of Chlorides with Water MAIN

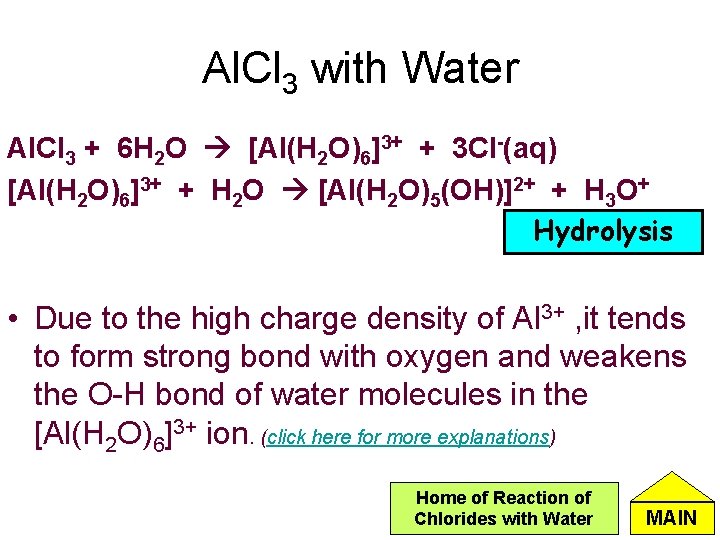
Al. Cl 3 with Water Al. Cl 3 + 6 H 2 O [Al(H 2 O)6]3+ + 3 Cl-(aq) [Al(H 2 O)6]3+ + H 2 O [Al(H 2 O)5(OH)]2+ + H 3 O+ Hydrolysis • Due to the high charge density of Al 3+ , it tends to form strong bond with oxygen and weakens the O-H bond of water molecules in the [Al(H 2 O)6]3+ ion. (click here for more explanations) Home of Reaction of Chlorides with Water MAIN

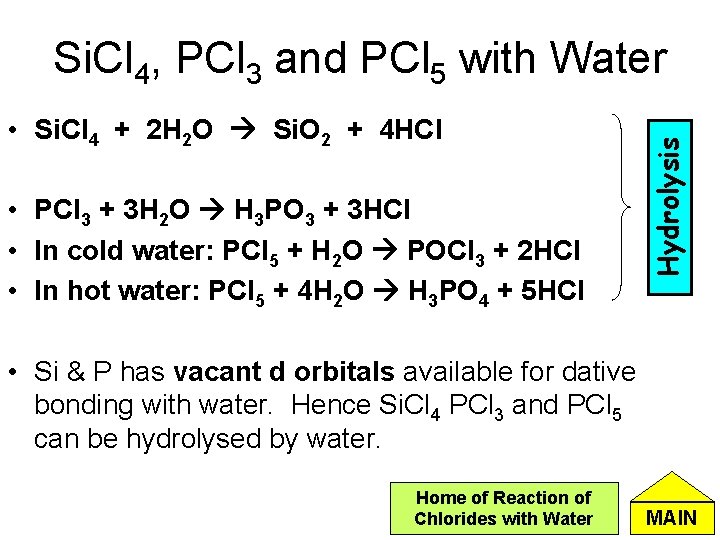
• Si. Cl 4 + 2 H 2 O Si. O 2 + 4 HCl • PCl 3 + 3 H 2 O H 3 PO 3 + 3 HCl • In cold water: PCl 5 + H 2 O POCl 3 + 2 HCl • In hot water: PCl 5 + 4 H 2 O H 3 PO 4 + 5 HCl Hydrolysis Si. Cl 4, PCl 3 and PCl 5 with Water • Si & P has vacant d orbitals available for dative bonding with water. Hence Si. Cl 4 PCl 3 and PCl 5 can be hydrolysed by water. Home of Reaction of Chlorides with Water MAIN

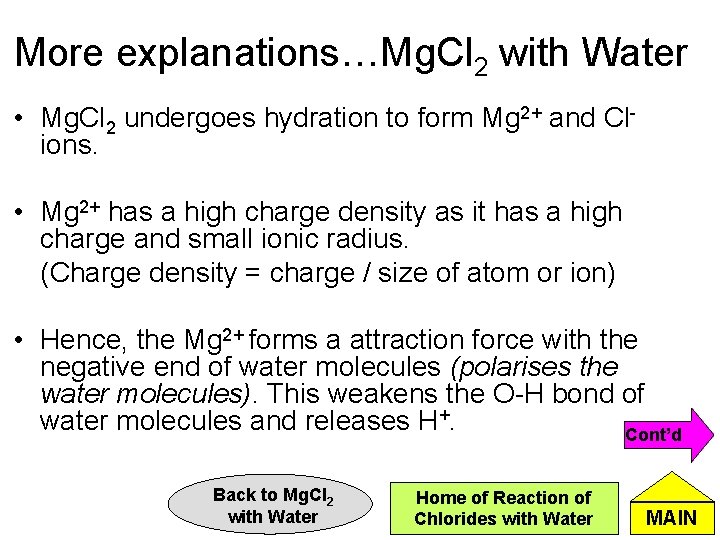
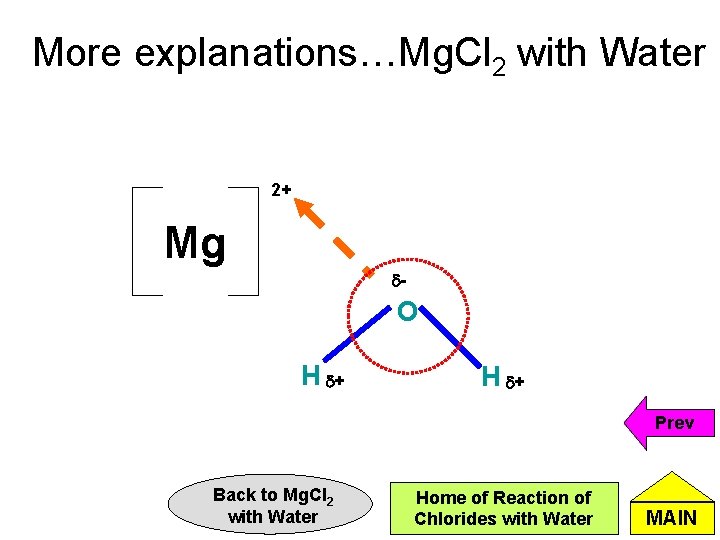
More explanations…Mg. Cl 2 with Water • Mg. Cl 2 undergoes hydration to form Mg 2+ and Clions. • Mg 2+ has a high charge density as it has a high charge and small ionic radius. (Charge density = charge / size of atom or ion) • Hence, the Mg 2+ forms a attraction force with the negative end of water molecules (polarises the water molecules). This weakens the O-H bond of water molecules and releases H+. Cont’d Back to Mg. Cl 2 with Water Home of Reaction of Chlorides with Water MAIN

More explanations…Mg. Cl 2 with Water 2+ Mg d- O H d+ Prev Back to Mg. Cl 2 with Water Home of Reaction of Chlorides with Water MAIN

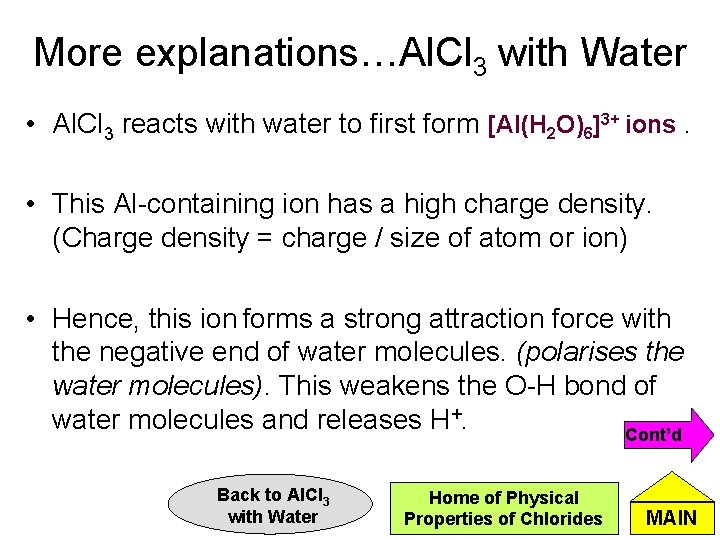
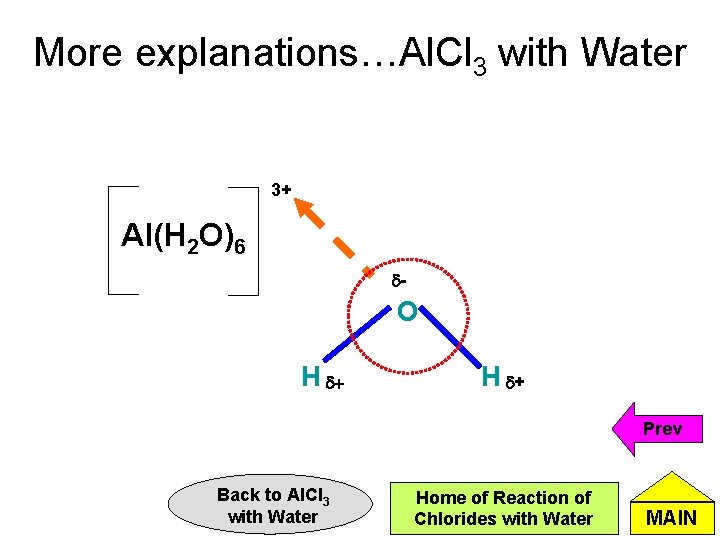
More explanations…Al. Cl 3 with Water • Al. Cl 3 reacts with water to first form [Al(H 2 O)6]3+ ions. • This Al-containing ion has a high charge density. (Charge density = charge / size of atom or ion) • Hence, this ion forms a strong attraction force with the negative end of water molecules. (polarises the water molecules). This weakens the O-H bond of water molecules and releases H+. Cont’d Back to Al. Cl 3 with Water Home of Physical Properties of Chlorides MAIN

More explanations…Al. Cl 3 with Water 3+ Al(H 2 O)6 d- O H d+ Prev Back to Al. Cl 3 with Water Home of Reaction of Chlorides with Water MAIN

Assignment Instructions: Submit your completed work by Deadline: by your chemistry tutor Via MI Link “Submit -> Tray” Cont’d MAIN

Assignment By using Powerpoint slideshow or MS Word or in other appropriate form, draw 2 concept maps / mind maps to illustrate ALL: 1. Physical and Chemical properties of Oxides and 2. Physical and Chemical properties of Chlorides 3. of elements from Period 3. You will be graded based on: -Creativity (e. g. pictures / videos / hyperlinks / flash / animations) -Clarity (information / ideas easy to understand? ) Prev - Accuracy of information MAIN

References • http: //www. chemguide. co. uk/inorganic/peri od 3/oxidesh 2 o. html • http: //www. chemguide. co. uk/inorganic/peri od 3/oxidesphys. html THE END MAIN