INORGANIC CARBON AND ACIDIFICATION DYNAMICS IN WESTERN GULF
INORGANIC CARBON AND ACIDIFICATION DYNAMICS IN WESTERN GULF OF MAINE SURFACE WATERS. J. Salisbury, D. Vandemark, C. Hunt, S. Shellito (Univ. of New Hampshire) W. R. Mc. Gillis (Columbia Univ. /LDEO) C. L. Sabine (NOAA/PMEL)

Atmospheric carbon dioxide record from Mauna Loa for 1958 -2000 by C. D. Keeling and T. P. Whorf http: //cdiac. esd. ornl. gov/trends/co 2/sio-mlo. htm Average for year 2000 = 369. 4 ppm


Outline 1. Our place and our observation kit 2. Background on CO 2 and acid variability and western GOM 3. Ideas on variability from our data set 4. Coastal acidification 5. Conclusions and questions

Our Science Questions • Is the Gulf of Maine a source or sink of atmospheric CO 2? • What are the space/time variability and mechanistic controls of surface p. CO 2 and subsequent acidification? • Can we apply CO 2 and O 2 data to support monitoring/prediction of lower trophic status via net ecosystem metabolism estimates? Climate and the Integrated Ocean Observing System ( First of the Seven IOOS Societal Goals ) 1. Improve predictions of climate change and weather and their effects on coastal communities and the nation
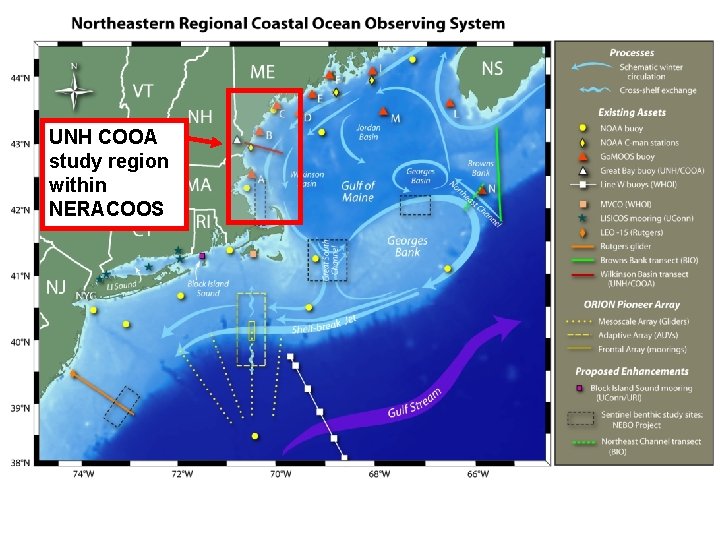
UNH COOA study region within NERACOOS
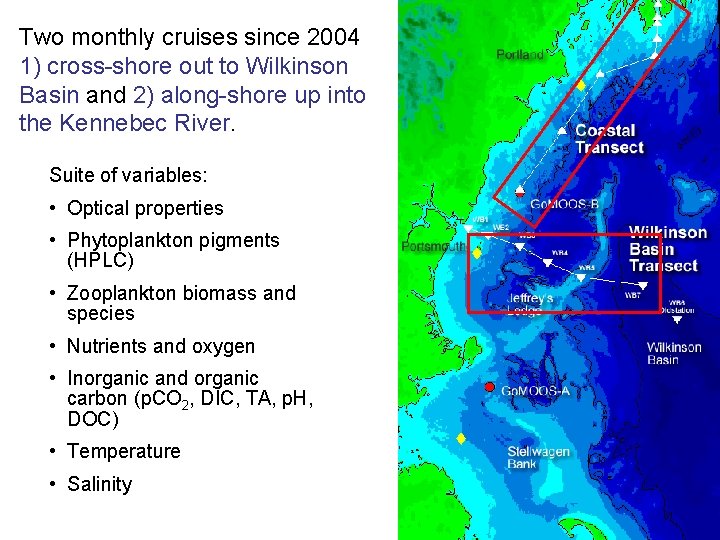
Two monthly cruises since 2004 1) cross-shore out to Wilkinson Basin and 2) along-shore up into the Kennebec River. Suite of variables: • Optical properties • Phytoplankton pigments (HPLC) • Zooplankton biomass and species • Nutrients and oxygen • Inorganic and organic carbon (p. CO 2, DIC, TA, p. H, DOC) • Temperature • Salinity
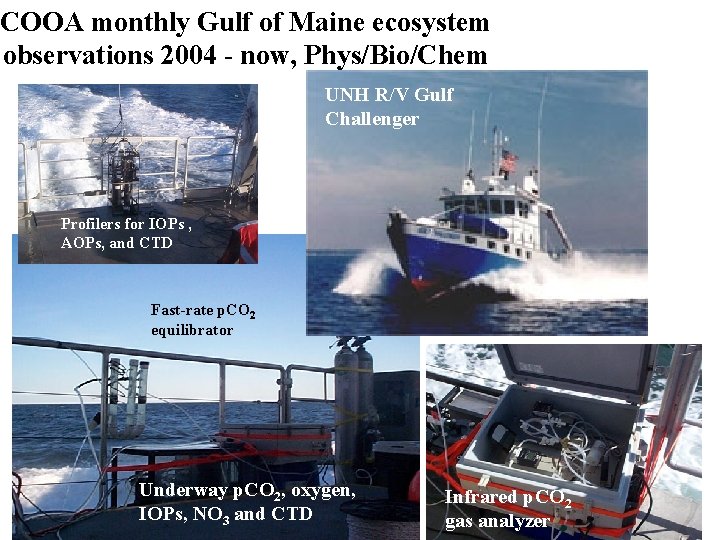
COOA monthly Gulf of Maine ecosystem observations 2004 - now, Phys/Bio/Chem UNH R/V Gulf Challenger Profilers for IOPs , AOPs, and CTD Fast-rate p. CO 2 equilibrator Underway p. CO 2, oxygen, IOPs, NO 3 and CTD Infrared p. CO 2 gas analyzer
http: //www. cooa. unh. edu
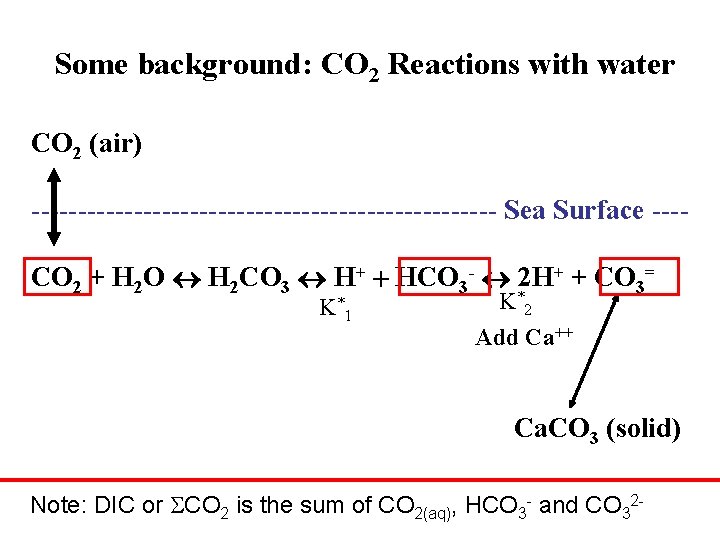
Some background: CO 2 Reactions with water CO 2 (air) ------------------------- Sea Surface ---CO 2 + H 2 O H 2 CO 3 H+ + HCO 3 - 2 H+ + CO 3= K *1 K *2 Add Ca++ Ca. CO 3 (solid) Note: DIC or CO 2 is the sum of CO 2(aq), HCO 3 - and CO 32 -
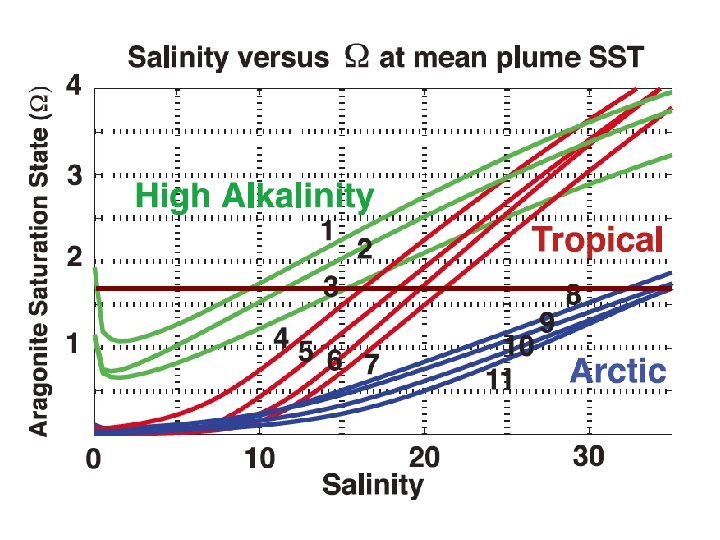
![Aragonite saturation state: Solubility a function of temperature and “salinity” agaronite ≈ [Ca++] [CO Aragonite saturation state: Solubility a function of temperature and “salinity” agaronite ≈ [Ca++] [CO](http://slidetodoc.com/presentation_image_h2/7f47c598a1e65b98ef922810e1d1f834/image-11.jpg)
Aragonite saturation state: Solubility a function of temperature and “salinity” agaronite ≈ [Ca++] [CO 3 --] Ksp In simple terms, Ω is a measure of the product of the concentrations of CO 32 - and Ca 2+ ions relative to the amount of aragonite that can be dissolved at a given temperature, salinity and pressure.
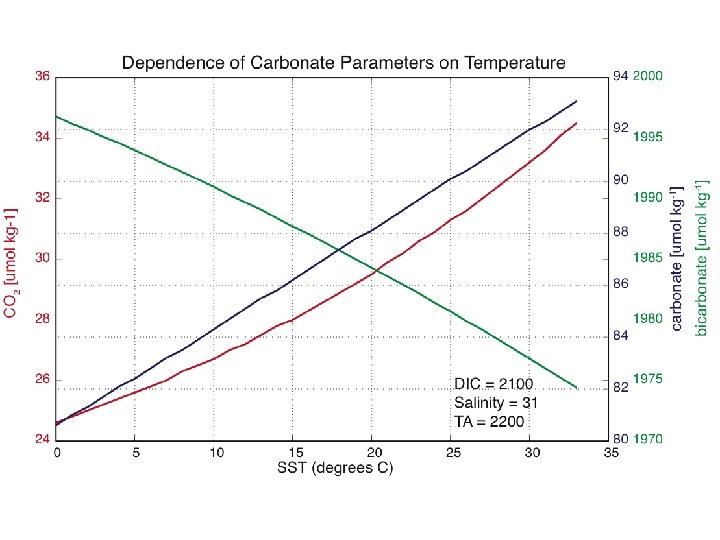
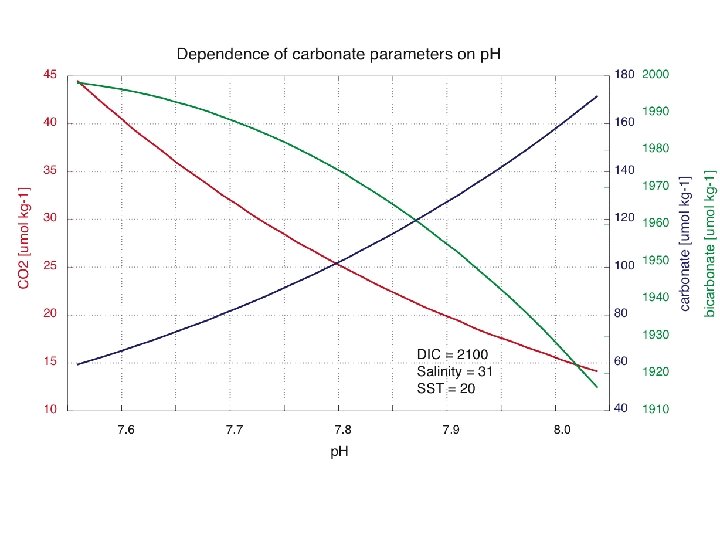
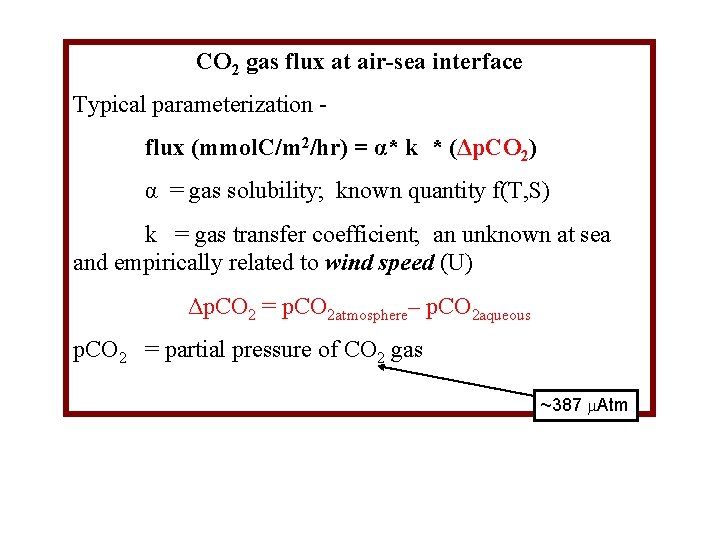
CO 2 gas flux at air-sea interface Typical parameterization flux (mmol. C/m 2/hr) = α* k * (Δp. CO 2) α = gas solubility; known quantity f(T, S) k = gas transfer coefficient; an unknown at sea and empirically related to wind speed (U) Δp. CO 2 = p. CO 2 atmosphere– p. CO 2 aqueous p. CO 2 = partial pressure of CO 2 gas ~387 Atm
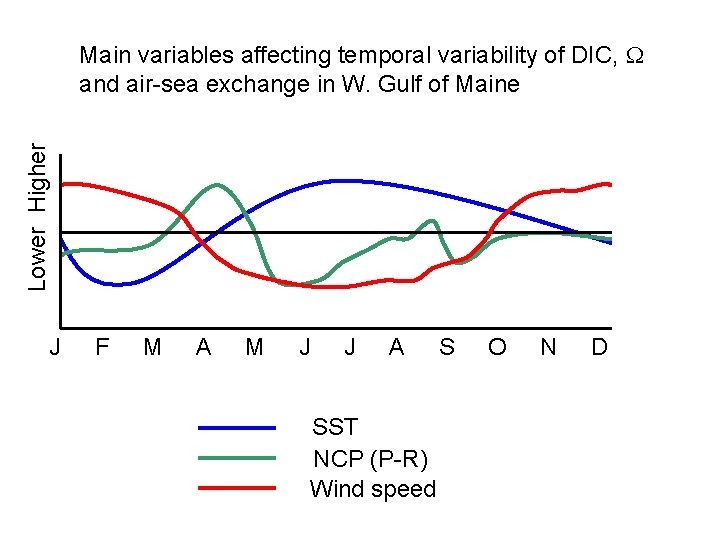
Lower Higher Main variables affecting temporal variability of DIC, and air-sea exchange in W. Gulf of Maine J F M A M J J A SST NCP (P-R) Wind speed S O N D

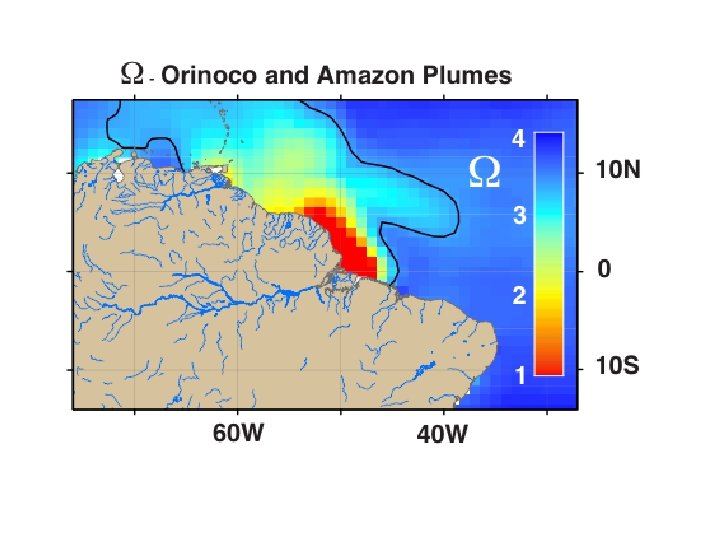
The role of river discharge? Influence spring stratification … and impart resistance to mixing …often has a different temperature signature Potential subsides of nutrients, DOC, acid … and deficits of DIC and alkalinity

Observed variability relevant to CO 2.
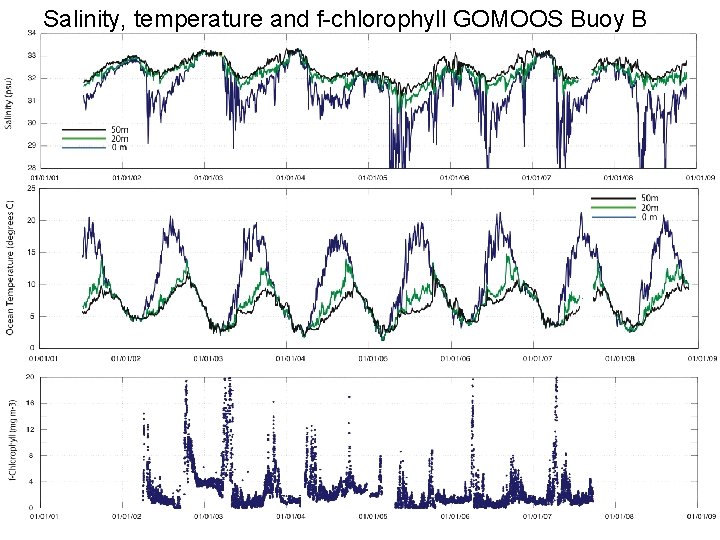
Salinity, temperature and f-chlorophyll GOMOOS Buoy B
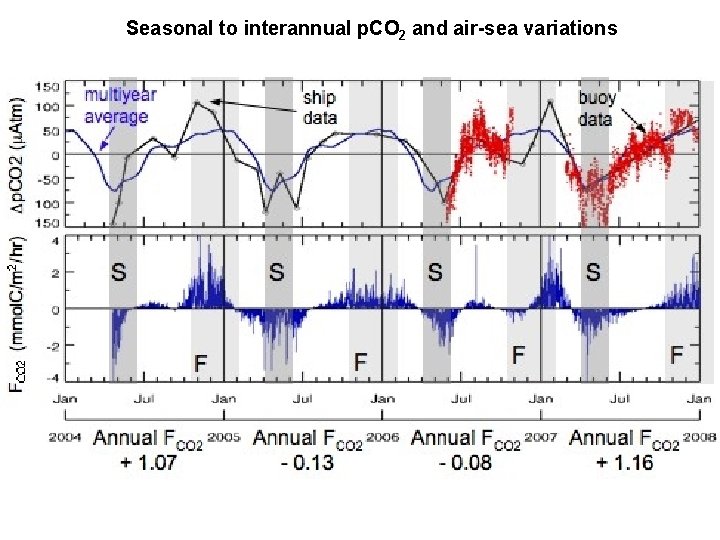
Seasonal to interannual p. CO 2 and air-sea variations
Interesting cases: Do they affect the annual budget? 1. Effect of discharge on the onset and persistence of two spring blooms? (2004 and 2005) 2. Turbulent interruption of a spring bloom (2006) 3. Timing of the fall turnover could be a significant factor influencing the annual CO 2 flux (2007).
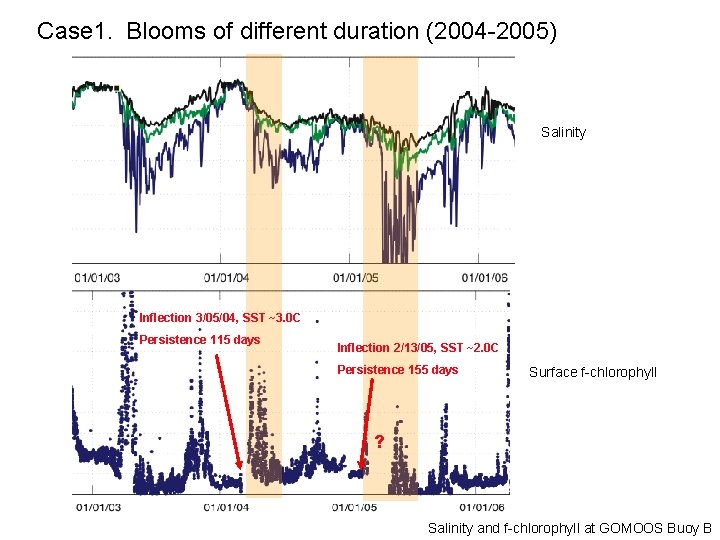
Case 1. Blooms of different duration (2004 -2005) Salinity Inflection 3/05/04, SST ~3. 0 C Persistence 115 days Inflection 2/13/05, SST ~2. 0 C Persistence 155 days Surface f-chlorophyll ? Salinity and f-chlorophyll at GOMOOS Buoy B
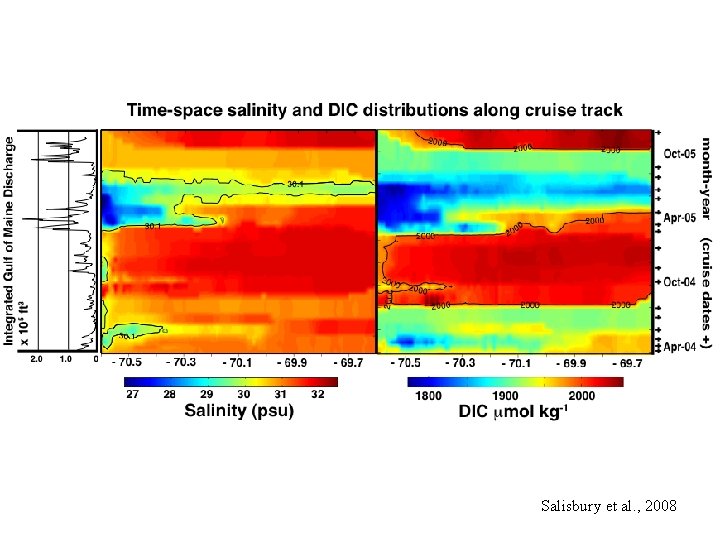
Salisbury et al. , 2008
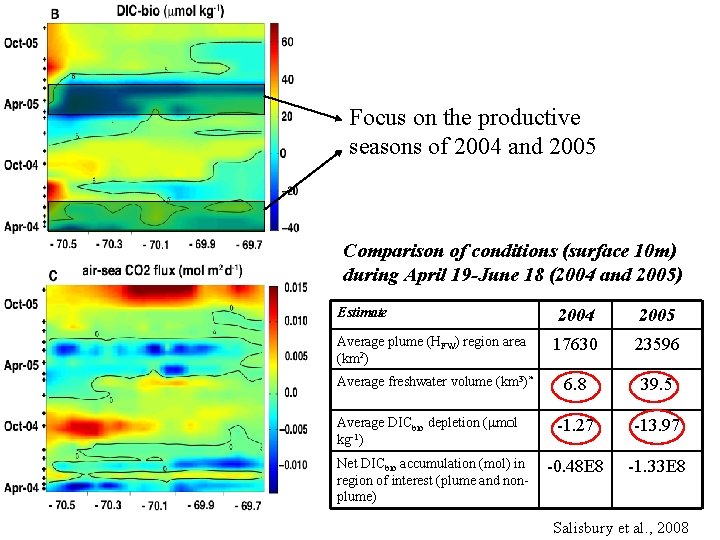
Biologically mediated DIC and air-sea flux Focus on the productive seasons of 2004 and 2005 Comparison of conditions (surface 10 m) during April 19 -June 18 (2004 and 2005) Estimate 2004 2005 Average plume (HFW) region area (km 2) 17630 23596 Average freshwater volume (km 3)* 6. 8 39. 5 -1. 27 -13. 97 -0. 48 E 8 -1. 33 E 8 Average DICbio depletion ( m l kg-1) Net DICbio accumulation (mol) in region of interest (plume and nonplume) Salisbury et al. , 2008
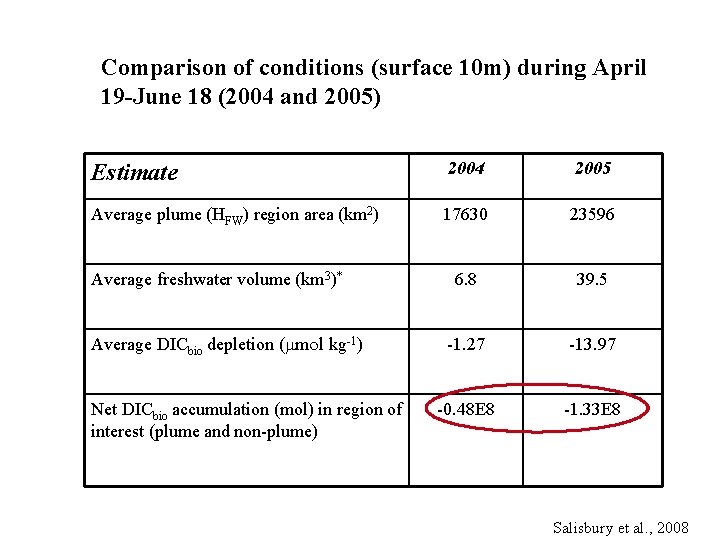
Comparison of conditions (surface 10 m) during April 19 -June 18 (2004 and 2005) Estimate 2004 2005 Average plume (HFW) region area (km 2) 17630 23596 6. 8 39. 5 -1. 27 -13. 97 -0. 48 E 8 -1. 33 E 8 Average freshwater volume (km 3)* Average DICbio depletion ( m l kg-1) Net DICbio accumulation (mol) in region of interest (plume and non-plume) Salisbury et al. , 2008

Case 2: Patriot’s Day Storm (2006) A serious interruption of the spring bloom 10 m waves
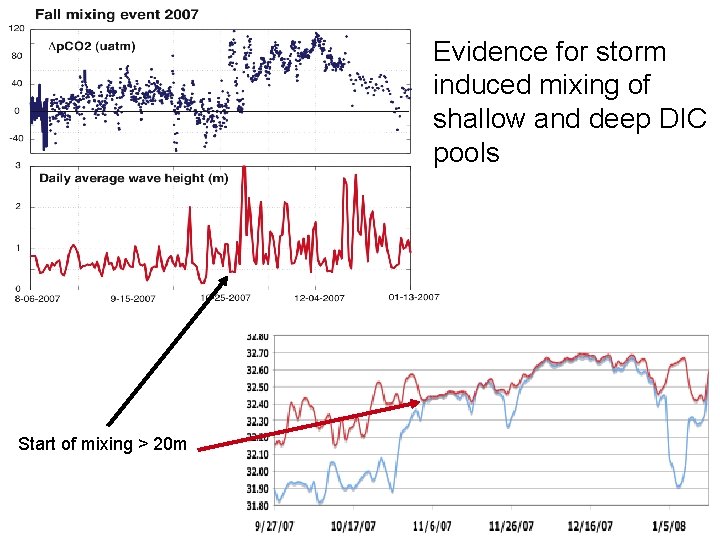
Evidence for storm induced mixing of shallow and deep DIC pools Start of mixing > 20 m

Coastal acid dynamics It’s not just and air-sea issue Joe Salisbury, Mark Green, Doug Vandemark, Chris Hunt
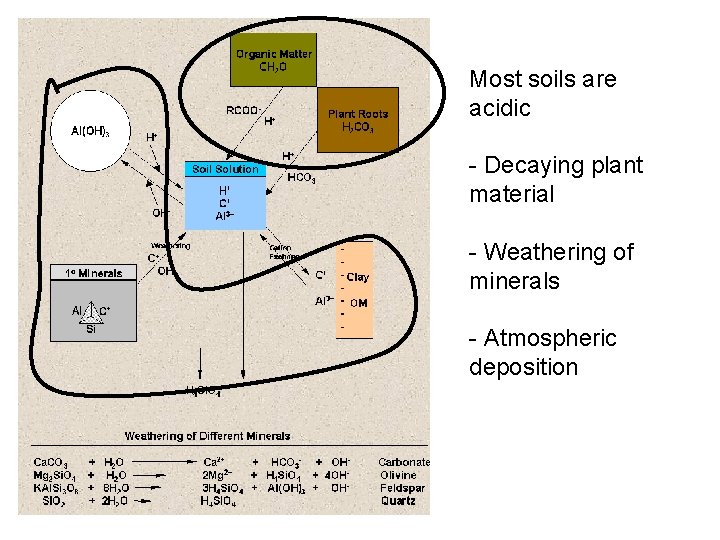
Most soils are acidic - Decaying plant material - Weathering of minerals - Atmospheric deposition

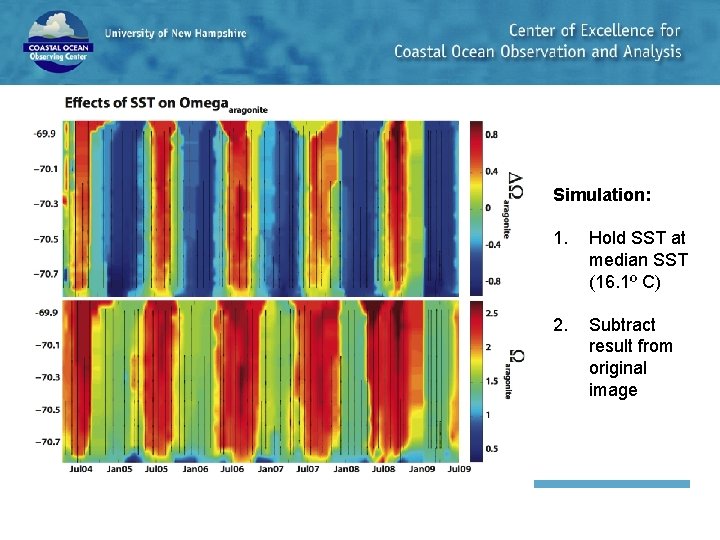
Simulation: 1. Hold SST at median SST (16. 1º C) 2. Subtract result from original image
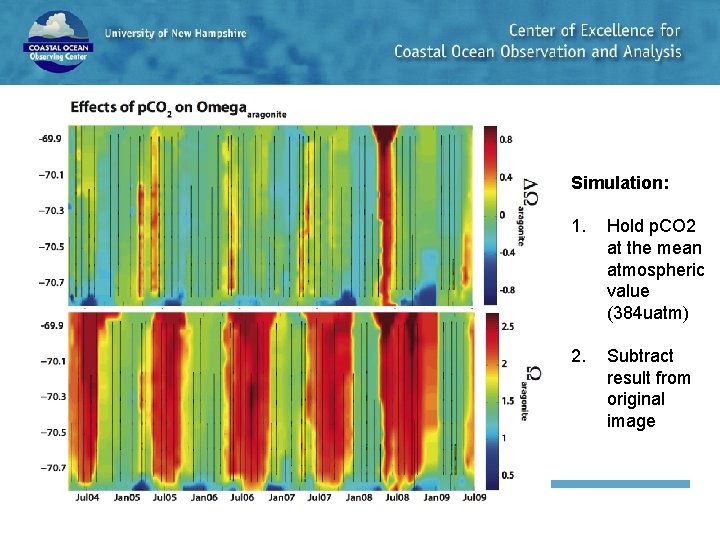
Simulation: 1. Hold p. CO 2 at the mean atmospheric value (384 uatm) 2. Subtract result from original image
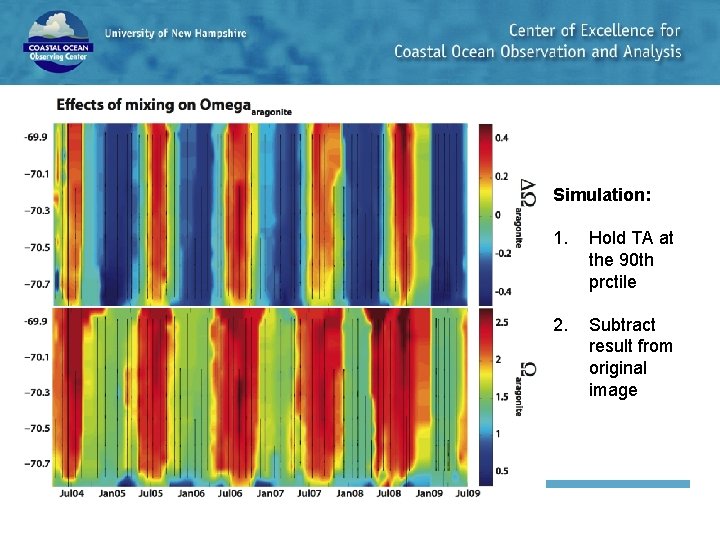
Simulation: 1. Hold TA at the 90 th prctile 2. Subtract result from original image
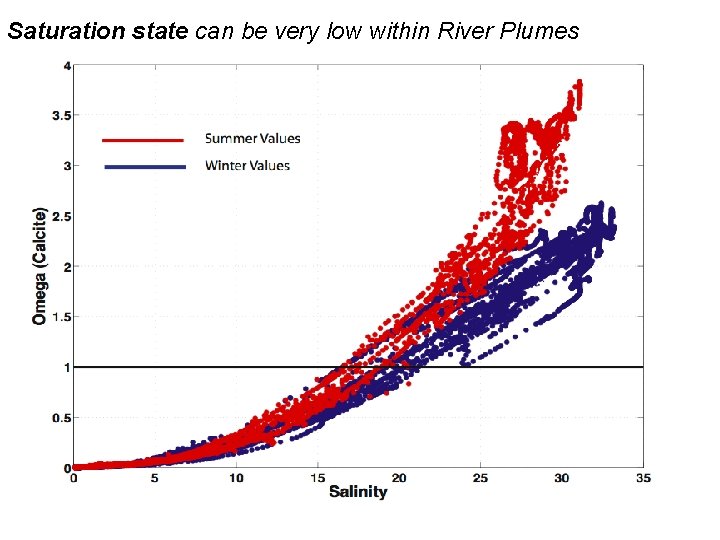
Saturation state can be very low within River Plumes
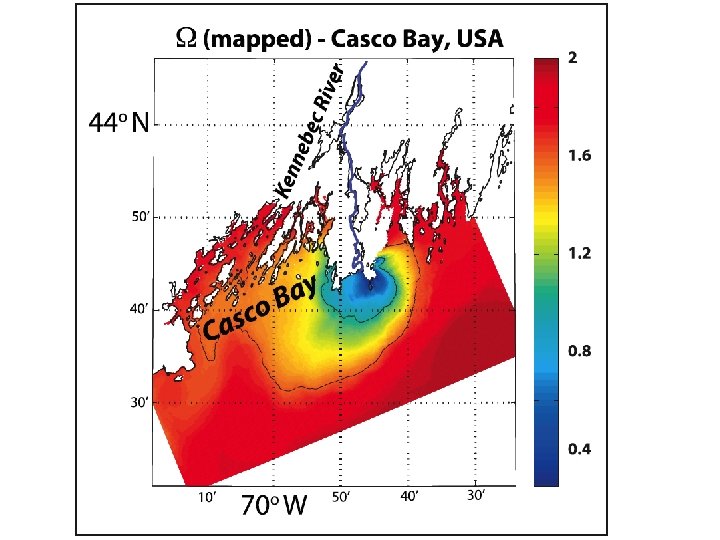

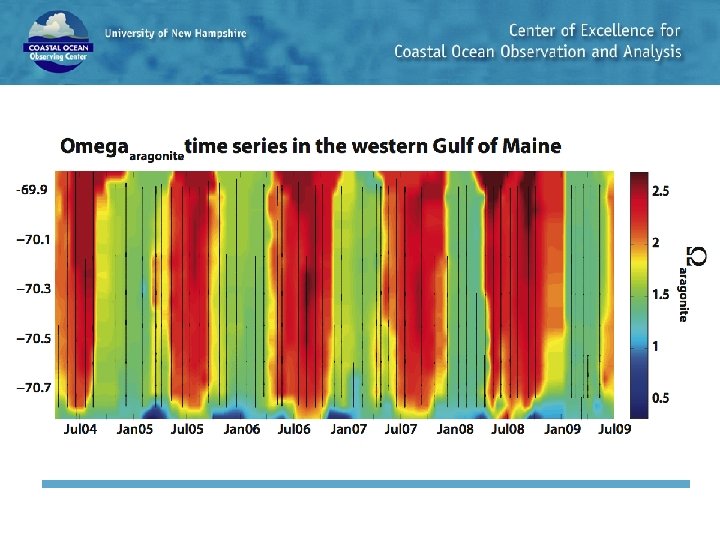
Periodic acidification in the Gulf of Maine:
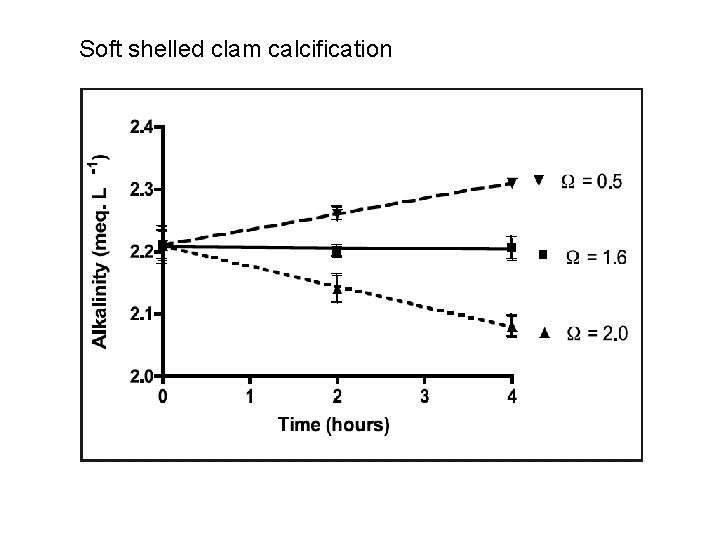
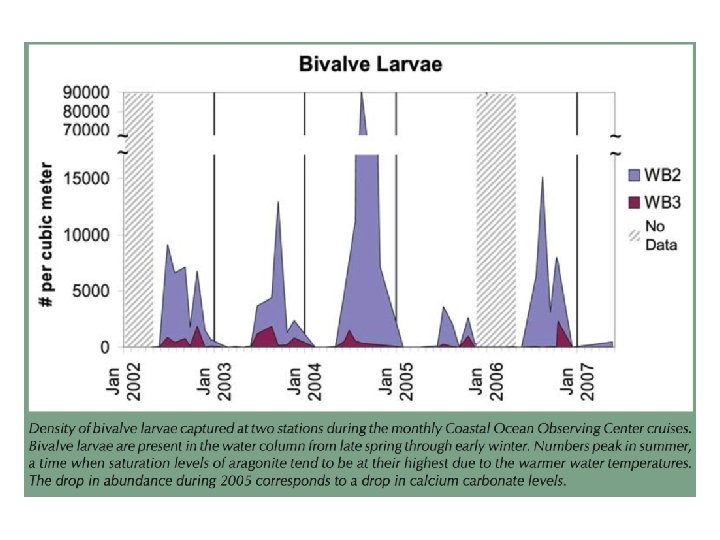
Soft shelled clam calcification


Closing remarks Operational Salinity model Huijie Xue (UMO)

Important stuff I didn’t cover very well 1. PAR (effect of clouds) 2. Distribution of PAR in the water (effects of attenuation) 3. Community dynamics e. g. Phaeocystis has a C: N of 30 (Mykelstad, 1988)
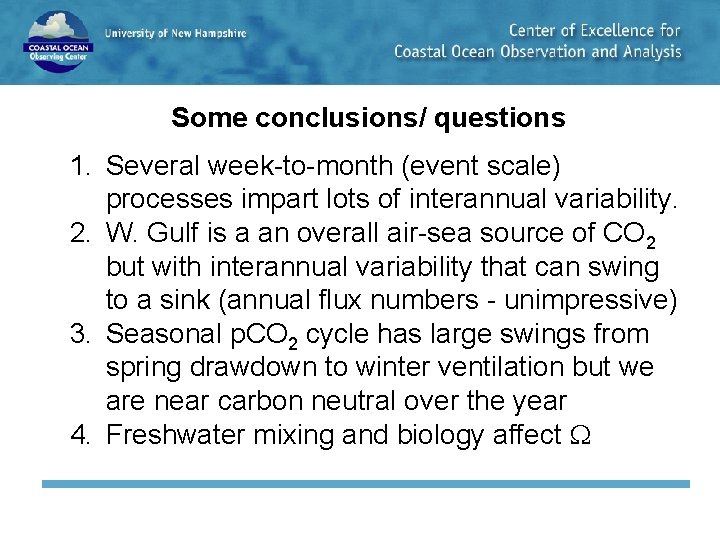
Some conclusions/ questions 1. Several week-to-month (event scale) processes impart lots of interannual variability. 2. W. Gulf is a an overall air-sea source of CO 2 but with interannual variability that can swing to a sink (annual flux numbers - unimpressive) 3. Seasonal p. CO 2 cycle has large swings from spring drawdown to winter ventilation but we are near carbon neutral over the year 4. Freshwater mixing and biology affect

Some questions 1. Why aren’t we a big sink for atmospheric CO 2? 2. What role does freshwater play in carbon and acid dynamics? 3. What role do storms play?
data collection acknowledgments - a regional and local effort UNH/COOA measurement crew: P. Pelletier, B. Soares, D. Brewitt C. Manning R. Jones C. Hunt T. Gregory M. Novak UNH WB transect PIs: S. Shellito J. Campbell and many more R. Morrison J. Salisbury D. Vandemark J. Runge (GMRI) A. Bucklin (Uconn)
- Slides: 46