Initiation Chain steps Chain Reaction Mechanism Weak ClCl
- Slides: 28

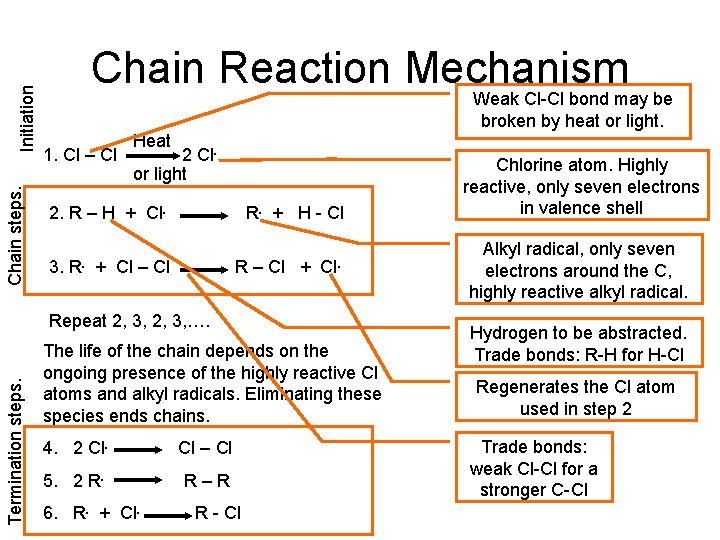
Initiation Chain steps. Chain Reaction Mechanism Weak Cl-Cl bond may be broken by heat or light. 1. Cl – Cl Heat 2 Cl. or light 2. R – H + Cl. 3. R. + H - Cl + Cl – Cl R – Cl + Cl. Termination steps. Repeat 2, 3, …. The life of the chain depends on the ongoing presence of the highly reactive Cl atoms and alkyl radicals. Eliminating these species ends chains. 4. 2 Cl. Cl – Cl 5. 2 R. R–R 6. R. + Cl. R - Cl Chlorine atom. Highly reactive, only seven electrons in valence shell Alkyl radical, only seven electrons around the C, highly reactive alkyl radical. Hydrogen to be abstracted. Trade bonds: R-H for H-Cl Regenerates the Cl atom used in step 2 Trade bonds: weak Cl-Cl for a stronger C-Cl

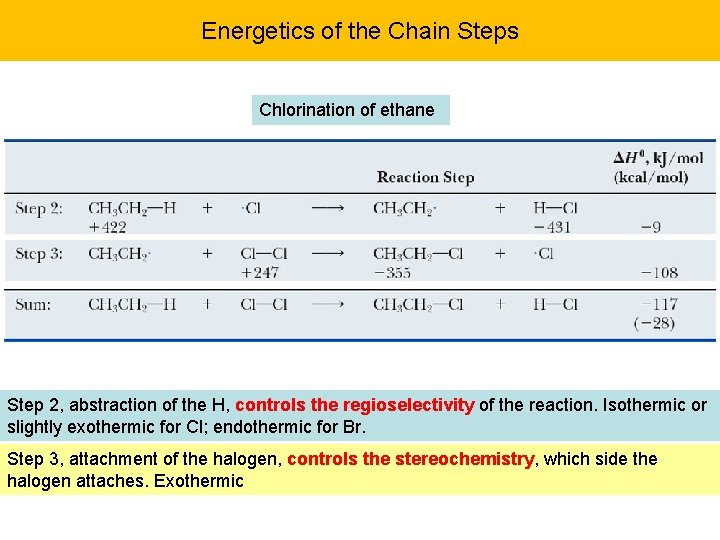
Energetics of the Chain Steps Chlorination of ethane Step 2, abstraction of the H, controls the regioselectivity of the reaction. Isothermic or slightly exothermic for Cl; endothermic for Br. Step 3, attachment of the halogen, controls the stereochemistry, which side the halogen attaches. Exothermic

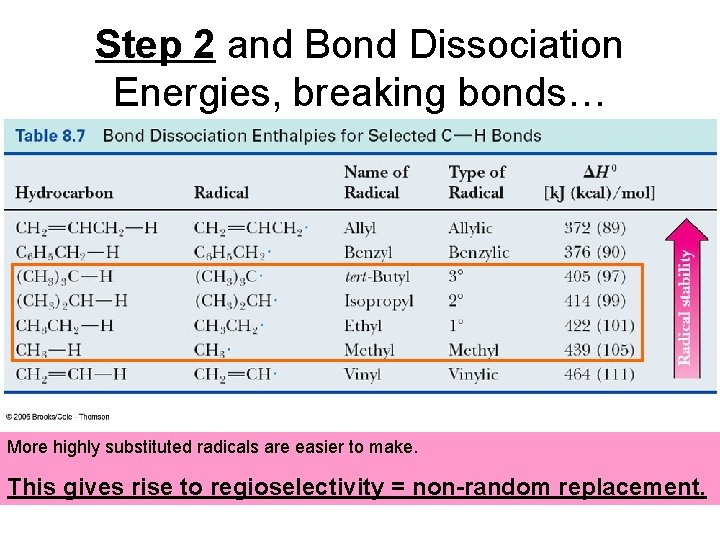
Step 2 and Bond Dissociation Energies, breaking bonds… More highly substituted radicals are easier to make. This gives rise to regioselectivity = non-random replacement.

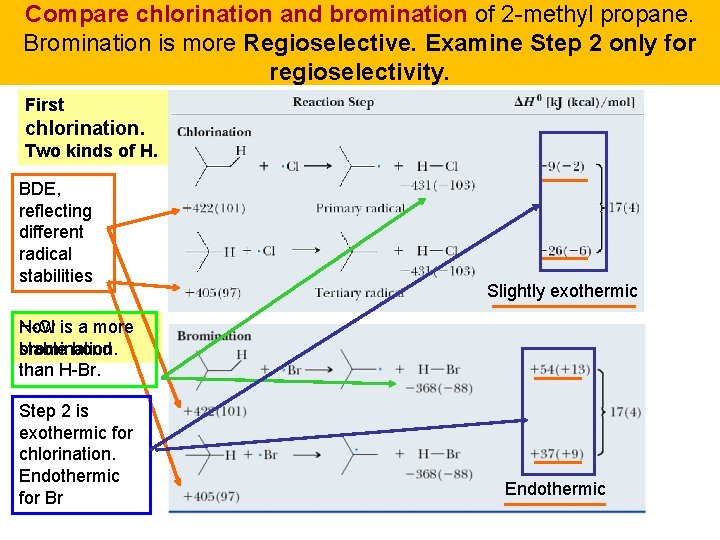
Compare chlorination and bromination of 2 -methyl propane. Bromination is more Regioselective. Examine Step 2 only for regioselectivity. First chlorination. Two kinds of H. BDE, reflecting different radical stabilities Slightly exothermic H-Cl is a more Now bromination. stable bond than H-Br. Step 2 is exothermic for chlorination. Endothermic for Br Endothermic

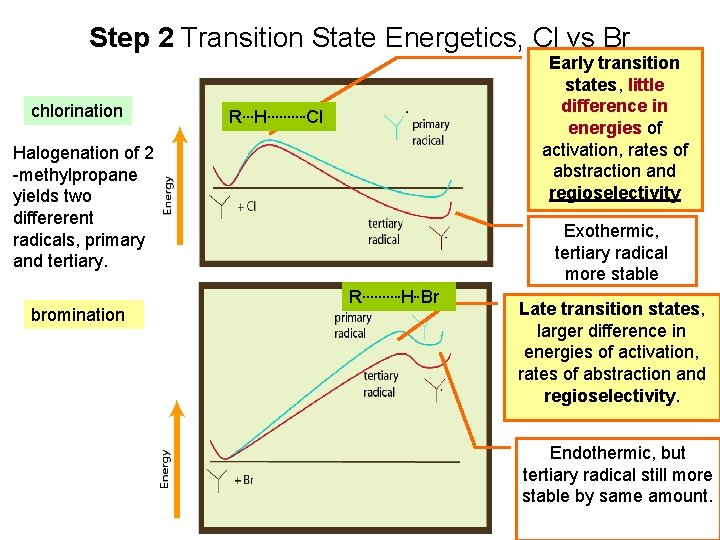
Step 2 Transition State Energetics, Cl vs Br chlorination Early transition states, little difference in energies of activation, rates of abstraction and regioselectivity R…H………. Cl Halogenation of 2 -methylpropane yields two differerent radicals, primary and tertiary. bromination Exothermic, tertiary radical more stable R………. H. . Br Late transition states, larger difference in energies of activation, rates of abstraction and regioselectivity. Endothermic, but tertiary radical still more stable by same amount.

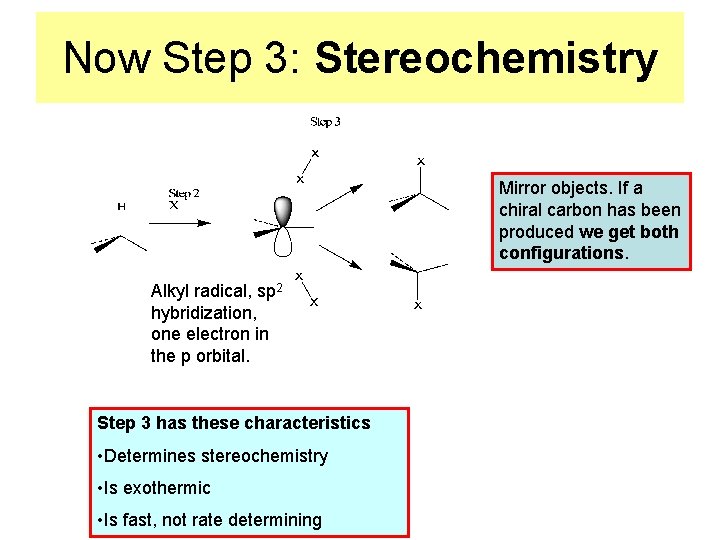
Now Step 3: Stereochemistry Mirror objects. If a chiral carbon has been produced we get both configurations. Alkyl radical, sp 2 hybridization, one electron in the p orbital. Step 3 has these characteristics • Determines stereochemistry • Is exothermic • Is fast, not rate determining

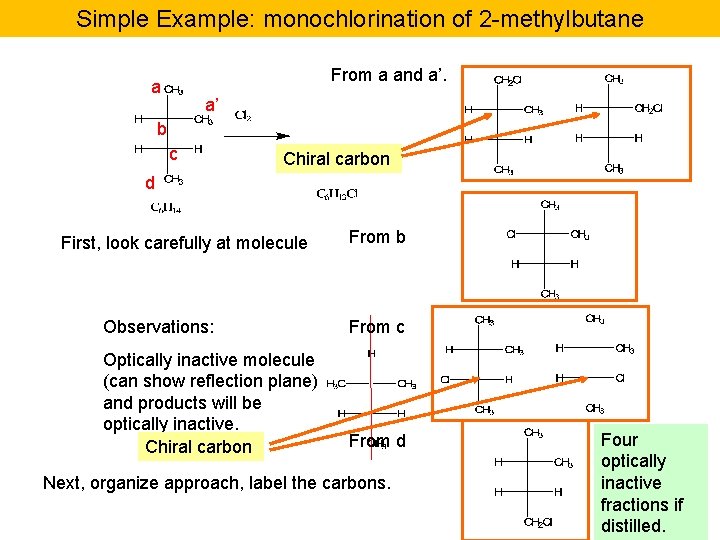
Simple Example: monochlorination of 2 -methylbutane From a and a’. a a’ b c Chiral carbon d First, look carefully at molecule From b Observations: From c Optically inactive molecule (can show reflection plane) and products will be optically inactive. Chiral carbon From d Next, organize approach, label the carbons. Four optically inactive fractions if distilled.

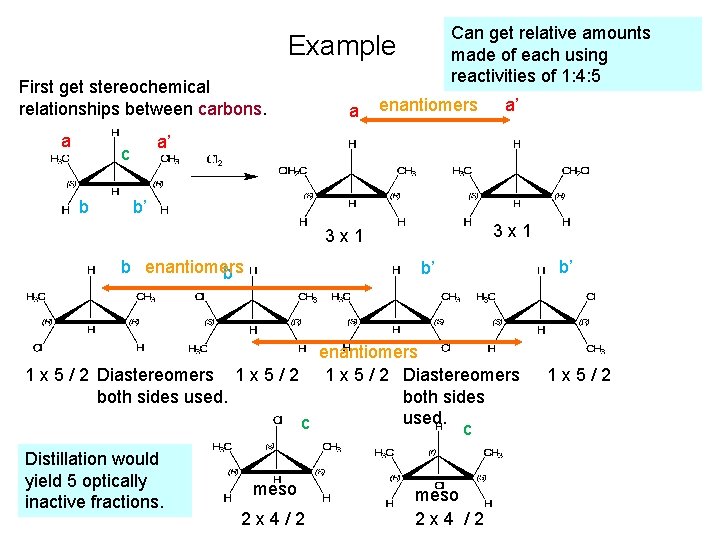
Can get relative amounts made of each using reactivities of 1: 4: 5 Example First get stereochemical relationships between carbons. a enantiomers a’ a’ c b a b’ 3 x 1 b enantiomers b b’ enantiomers 1 x 5 / 2 Diastereomers both sides used. c c Distillation would yield 5 optically inactive fractions. meso 2 x 4/2 meso 2 x 4 /2 b’ 1 x 5/2

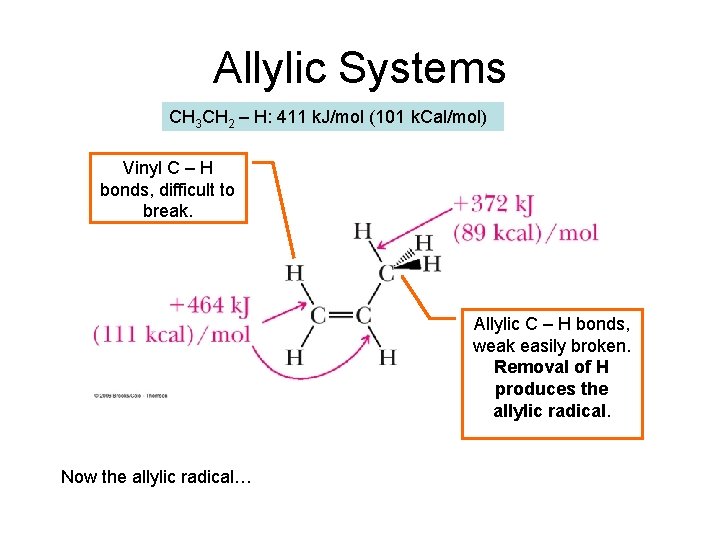
Allylic Systems CH 3 CH 2 – H: 411 k. J/mol (101 k. Cal/mol) Vinyl C – H bonds, difficult to break. Allylic C – H bonds, weak easily broken. Removal of H produces the allylic radical. Now the allylic radical…

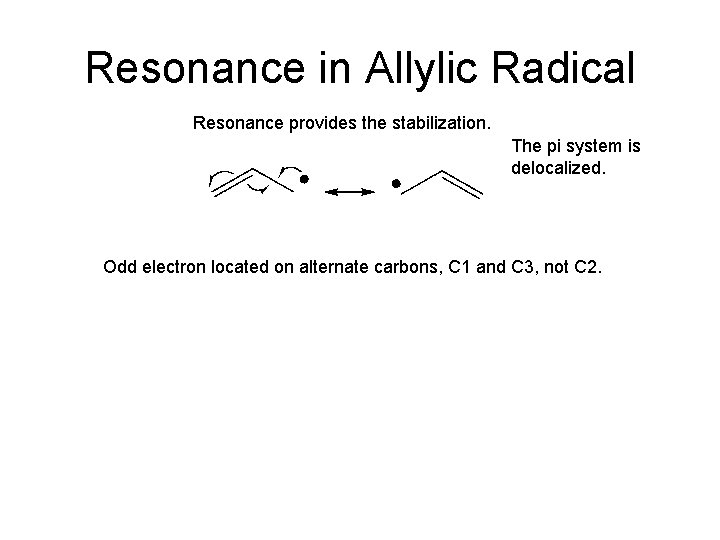
Resonance in Allylic Radical Resonance provides the stabilization. The pi system is delocalized. Odd electron located on alternate carbons, C 1 and C 3, not C 2.

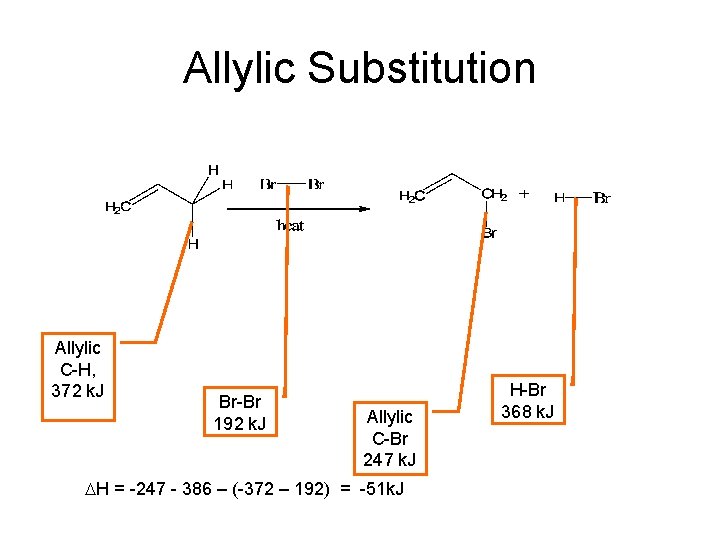
Allylic Substitution Allylic C-H, 372 k. J Br-Br 192 k. J Allylic C-Br 247 k. J DH = -247 - 386 – (-372 – 192) = -51 k. J H-Br 368 k. J

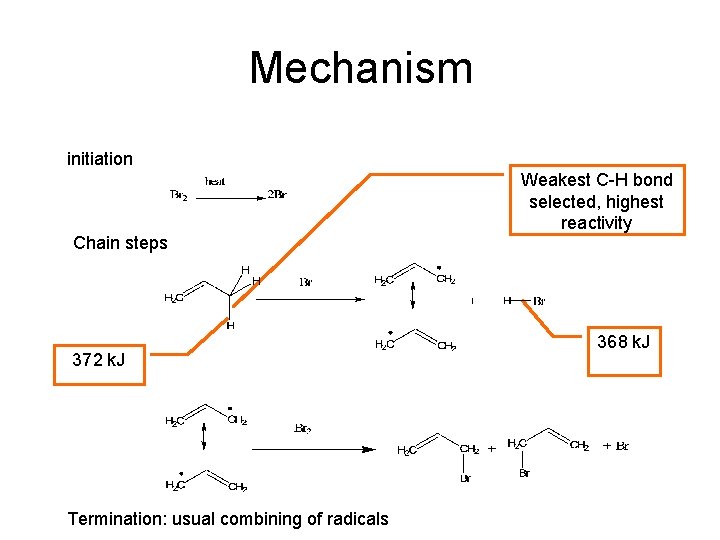
Mechanism initiation Chain steps 372 k. J Termination: usual combining of radicals Weakest C-H bond selected, highest reactivity 368 k. J

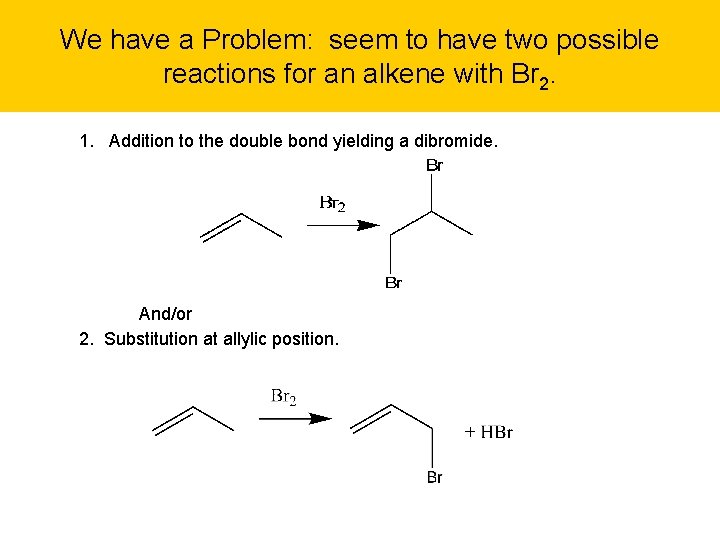
We have a Problem: seem to have two possible reactions for an alkene with Br 2. 1. Addition to the double bond yielding a dibromide. And/or 2. Substitution at allylic position.

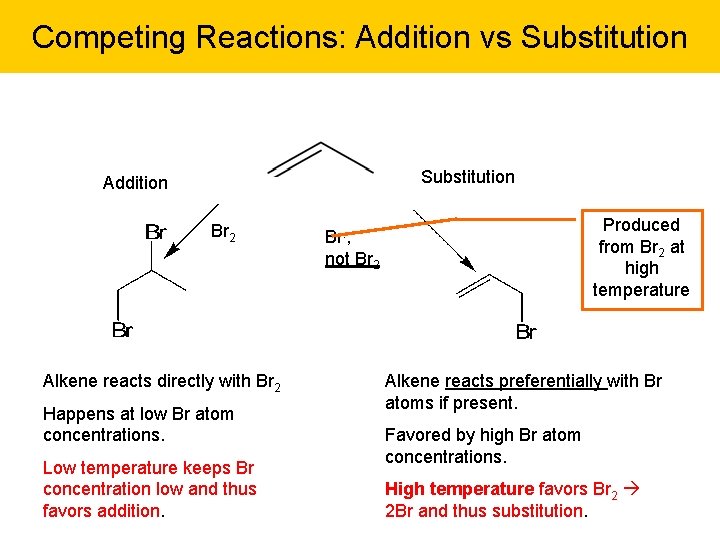
Competing Reactions: Addition vs Substitution Addition Br 2 Alkene reacts directly with Br 2 Happens at low Br atom concentrations. Low temperature keeps Br concentration low and thus favors addition. Produced from Br 2 at high temperature Br. , not Br 2 Alkene reacts preferentially with Br atoms if present. Favored by high Br atom concentrations. High temperature favors Br 2 2 Br and thus substitution.

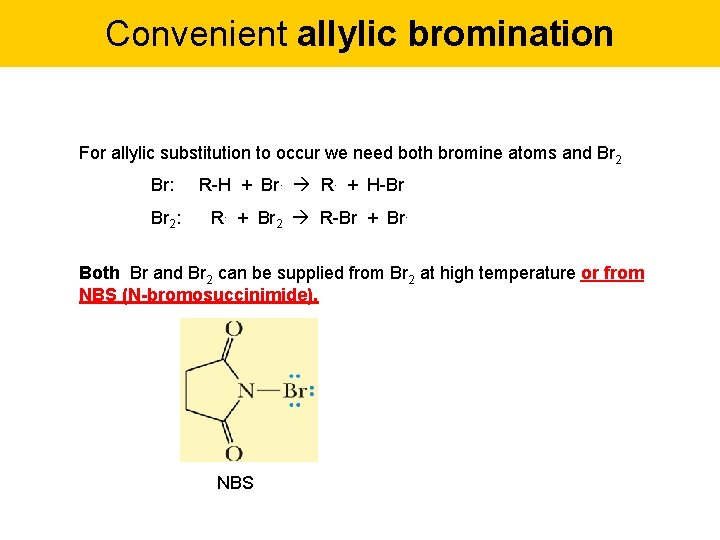
Convenient allylic bromination For allylic substitution to occur we need both bromine atoms and Br 2 Br: Br 2: R-H + Br. R. + H-Br R. + Br 2 R-Br + Br. Both Br and Br 2 can be supplied from Br 2 at high temperature or from NBS (N-bromosuccinimide). NBS

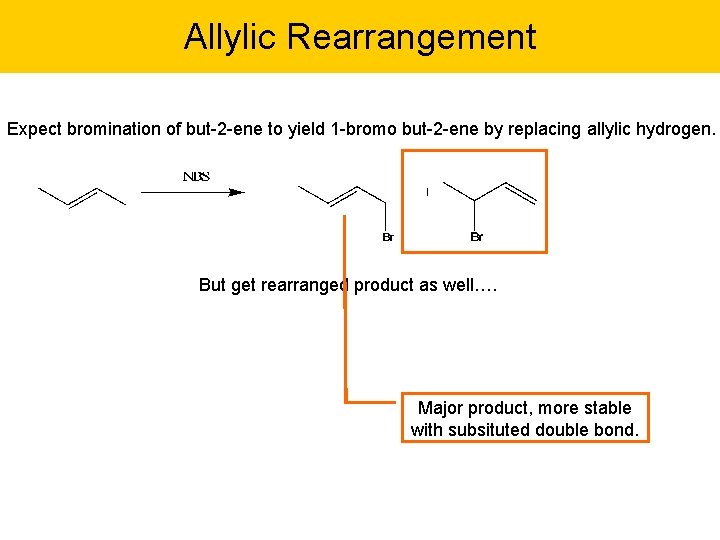
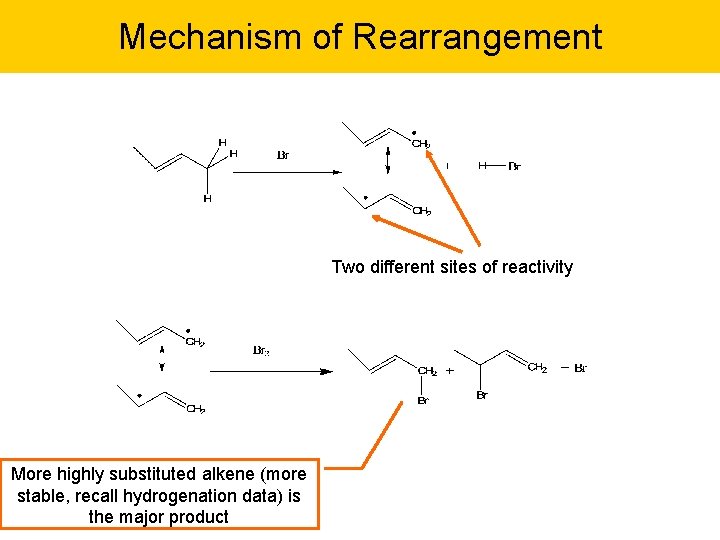
Allylic Rearrangement Expect bromination of but-2 -ene to yield 1 -bromo but-2 -ene by replacing allylic hydrogen. But get rearranged product as well…. Major product, more stable with subsituted double bond.

Mechanism of Rearrangement Two different sites of reactivity More highly substituted alkene (more stable, recall hydrogenation data) is the major product

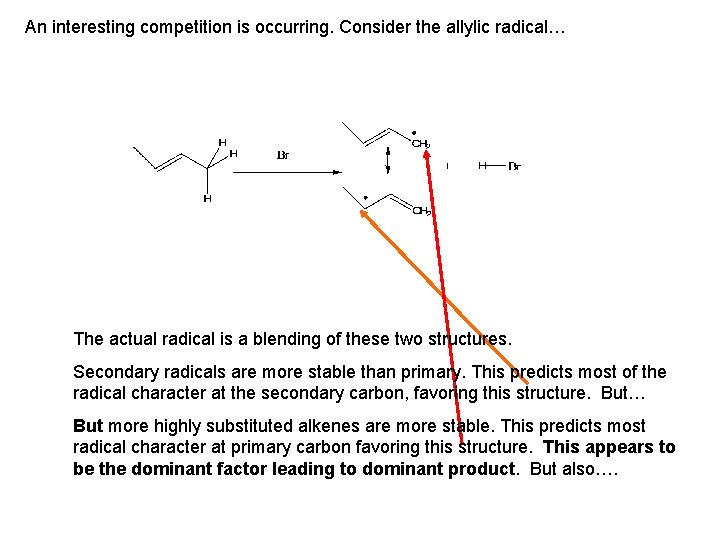
An interesting competition is occurring. Consider the allylic radical… The actual radical is a blending of these two structures. Secondary radicals are more stable than primary. This predicts most of the radical character at the secondary carbon, favoring this structure. But… But more highly substituted alkenes are more stable. This predicts most radical character at primary carbon favoring this structure. This appears to be the dominant factor leading to dominant product. But also….

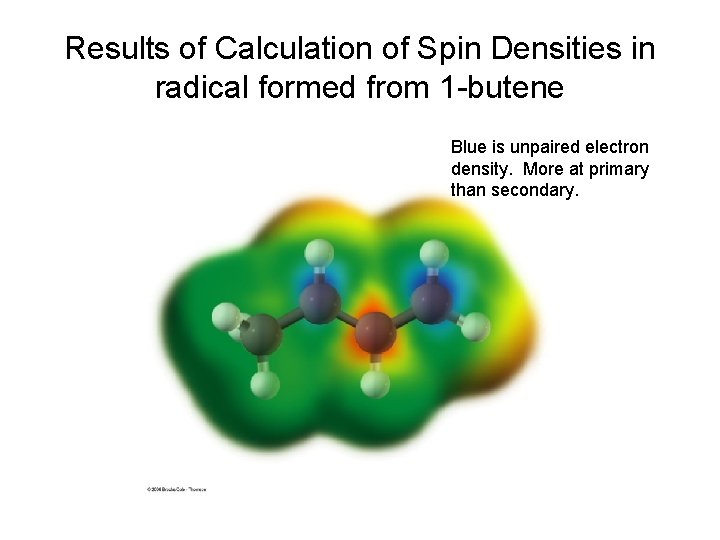
Results of Calculation of Spin Densities in radical formed from 1 -butene Blue is unpaired electron density. More at primary than secondary.

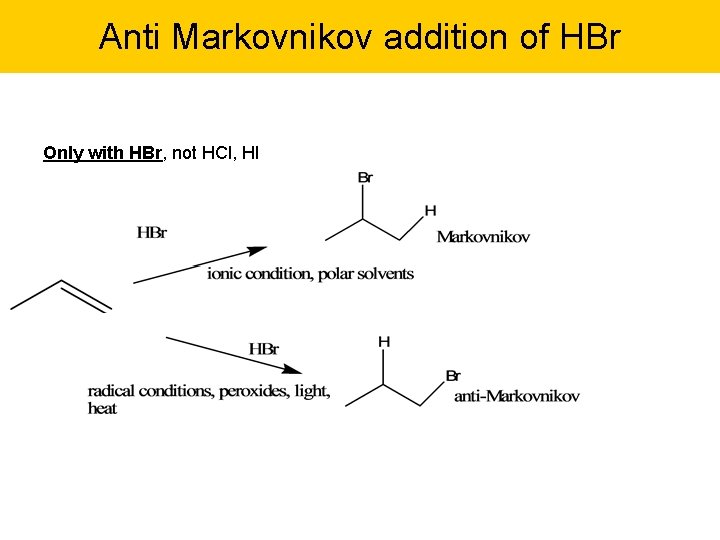
Anti Markovnikov addition of HBr Only with HBr, not HCl, HI

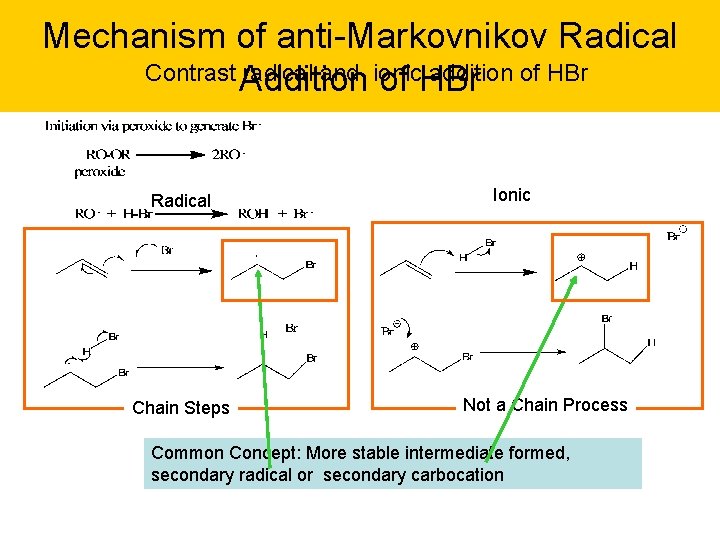
Mechanism of anti-Markovnikov Radical Contrast Addition radical and ionic addition of HBr Radical Chain Steps Ionic Not a Chain Process Common Concept: More stable intermediate formed, secondary radical or secondary carbocation

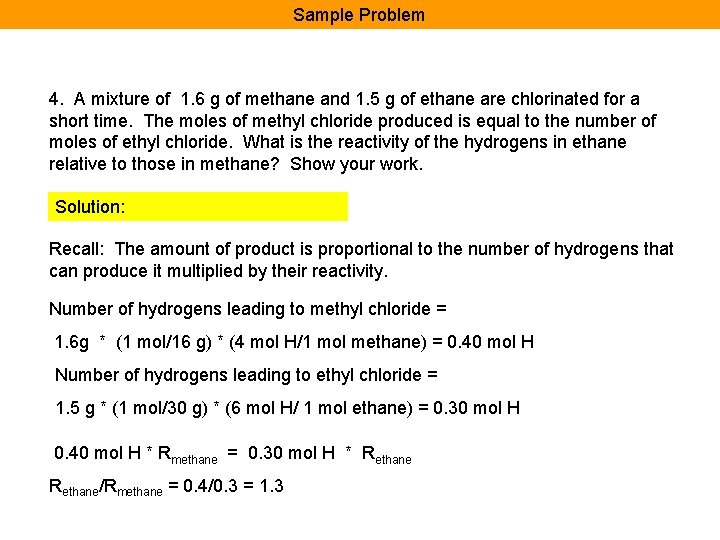
Sample Problem 4. A mixture of 1. 6 g of methane and 1. 5 g of ethane are chlorinated for a short time. The moles of methyl chloride produced is equal to the number of moles of ethyl chloride. What is the reactivity of the hydrogens in ethane relative to those in methane? Show your work. Solution: Recall: The amount of product is proportional to the number of hydrogens that can produce it multiplied by their reactivity. Number of hydrogens leading to methyl chloride = 1. 6 g * (1 mol/16 g) * (4 mol H/1 mol methane) = 0. 40 mol H Number of hydrogens leading to ethyl chloride = 1. 5 g * (1 mol/30 g) * (6 mol H/ 1 mol ethane) = 0. 30 mol H 0. 40 mol H * Rmethane = 0. 30 mol H * Rethane/Rmethane = 0. 4/0. 3 = 1. 3

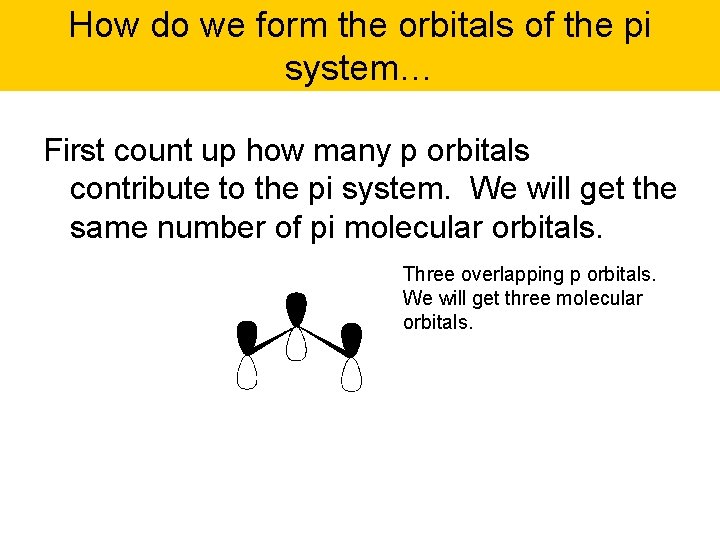
How do we form the orbitals of the pi system… First count up how many p orbitals contribute to the pi system. We will get the same number of pi molecular orbitals. Three overlapping p orbitals. We will get three molecular orbitals.

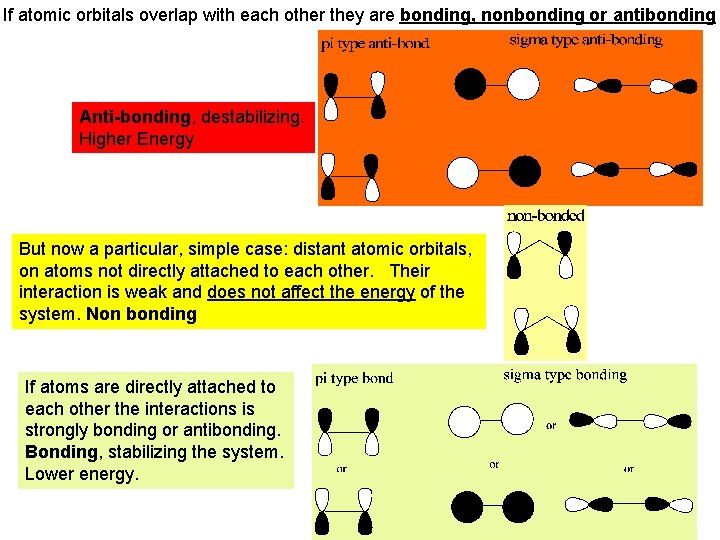
If atomic orbitals overlap with each other they are bonding, nonbonding or antibonding Anti-bonding, destabilizing. Higher Energy But now a particular, simple case: distant atomic orbitals, on atoms not directly attached to each other. Their interaction is weak and does not affect the energy of the system. Non bonding If atoms are directly attached to each other the interactions is strongly bonding or antibonding. Bonding, stabilizing the system. Lower energy.

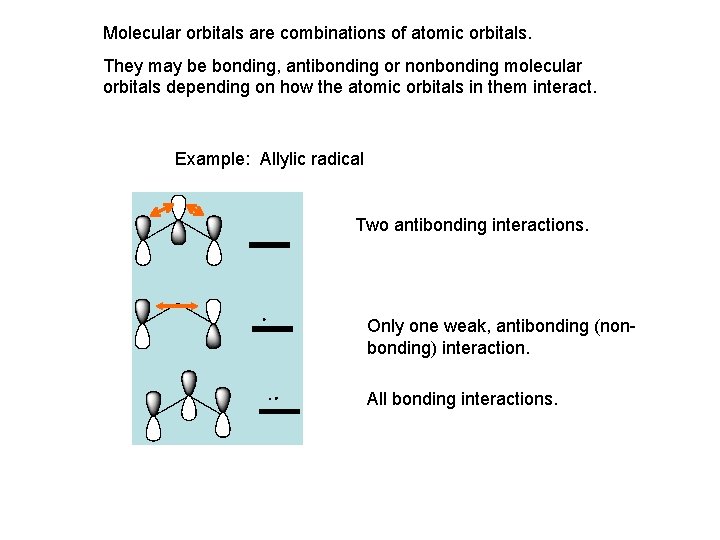
Molecular orbitals are combinations of atomic orbitals. They may be bonding, antibonding or nonbonding molecular orbitals depending on how the atomic orbitals in them interact. Example: Allylic radical Two antibonding interactions. Only one weak, antibonding (nonbonding) interaction. All bonding interactions.

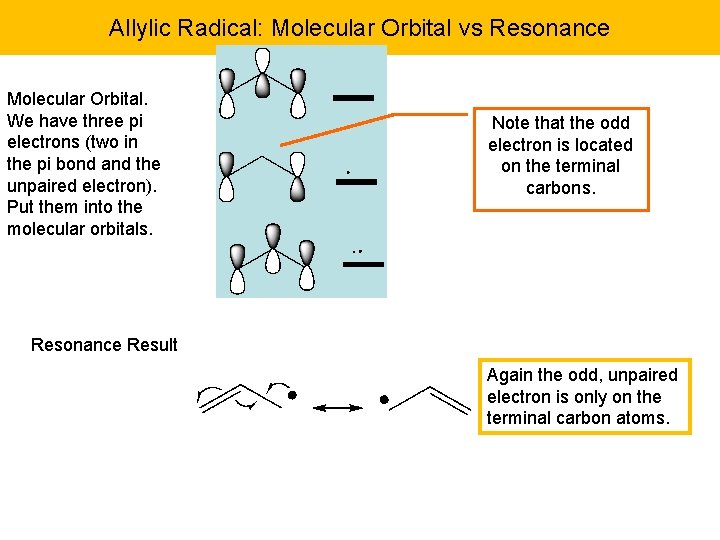
Allylic Radical: Molecular Orbital vs Resonance Molecular Orbital. We have three pi electrons (two in the pi bond and the unpaired electron). Put them into the molecular orbitals. Note that the odd electron is located on the terminal carbons. Resonance Result Again the odd, unpaired electron is only on the terminal carbon atoms.

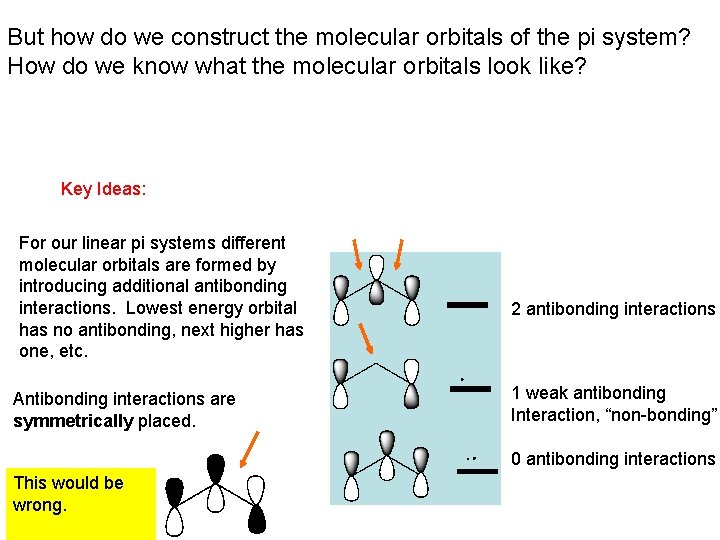
But how do we construct the molecular orbitals of the pi system? How do we know what the molecular orbitals look like? Key Ideas: For our linear pi systems different molecular orbitals are formed by introducing additional antibonding interactions. Lowest energy orbital has no antibonding, next higher has one, etc. Antibonding interactions are symmetrically placed. 2 antibonding interactions 1 weak antibonding Interaction, “non-bonding” 0 antibonding interactions This would be wrong.

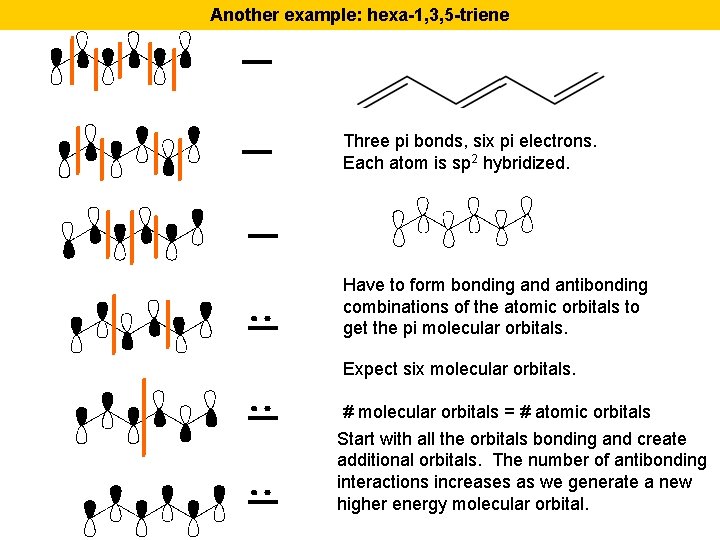
Another example: hexa-1, 3, 5 -triene Three pi bonds, six pi electrons. Each atom is sp 2 hybridized. Have to form bonding and antibonding combinations of the atomic orbitals to get the pi molecular orbitals. Expect six molecular orbitals. # molecular orbitals = # atomic orbitals Start with all the orbitals bonding and create additional orbitals. The number of antibonding interactions increases as we generate a new higher energy molecular orbital.