Infrared Spectroscopy of Alanine and Its Water Clusters



- Slides: 17

Infrared Spectroscopy of Alanine and Its Water Clusters Isolated in Solid Parahydrogen Brendan Moore Shin Yi Toh, Ying-Tung Angel Wong, Pavle Djuricanin, Takamasa Momose University of British Columbia ISMS 2018

Matrix Isolation Host materials : Chemically inert cryogenic crystals (Ar, Ne) Compatible with many spectroscopic tools: IR, UV, VIS, ESR, NMR, etc. Uses in Spectroscopy (1) reactive / unstable molecules (2) different conformers (3) low temperature chemistry 1

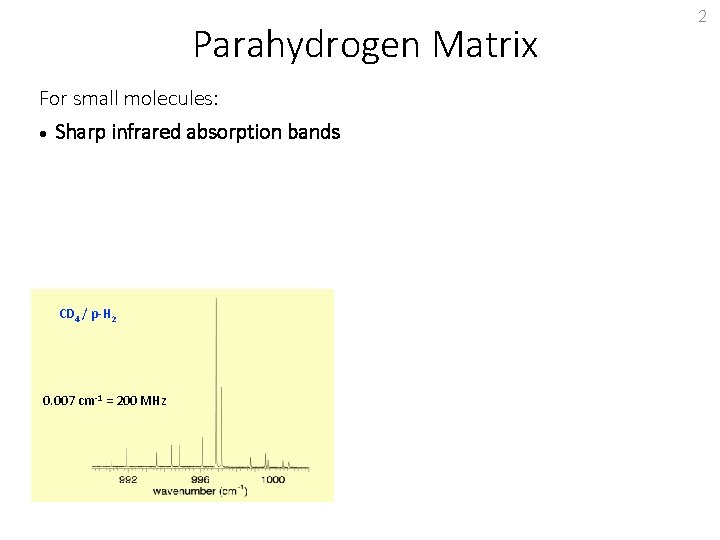
Parahydrogen Matrix For small molecules: Sharp infrared absorption bands CD 4 / p-H 2 0. 007 cm-1 = 200 MHz 2

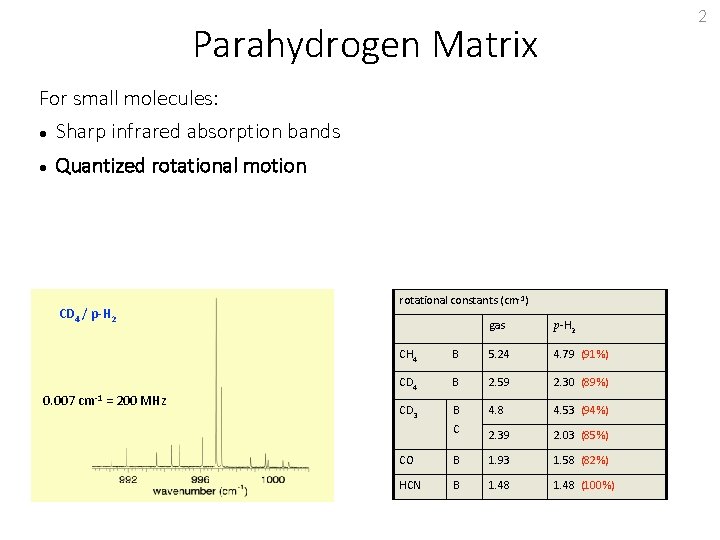
2 Parahydrogen Matrix For small molecules: Sharp infrared absorption bands Quantized rotational motion CD 4 / p-H 2 0. 007 cm-1 = 200 MHz rotational constants (cm-1) gas p-H 2 CH 4 B 5. 24 4. 79 (91%) CD 4 B 2. 59 2. 30 (89%) CD 3 B 4. 8 4. 53 (94%) 2. 39 2. 03 (85%) C CO B 1. 93 1. 58 (82%) HCN B 1. 48 (100%)

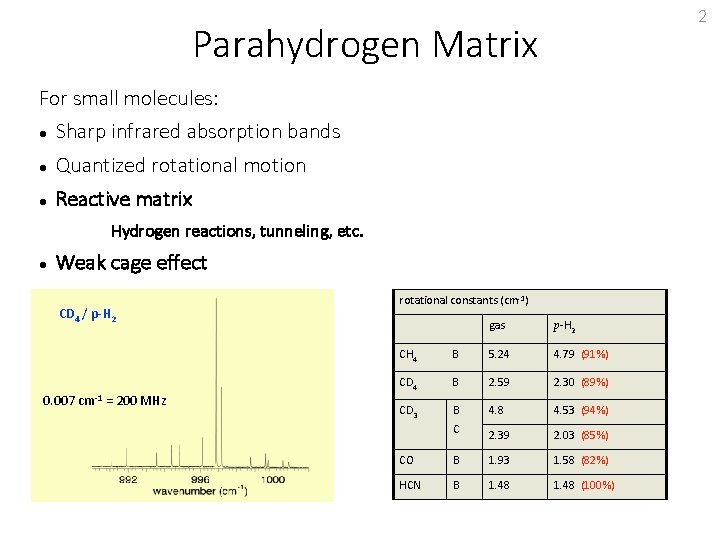
2 Parahydrogen Matrix For small molecules: Sharp infrared absorption bands Quantized rotational motion Reactive matrix Hydrogen reactions, tunneling, etc. Weak cage effect CD 4 / p-H 2 0. 007 cm-1 = 200 MHz rotational constants (cm-1) gas p-H 2 CH 4 B 5. 24 4. 79 (91%) CD 4 B 2. 59 2. 30 (89%) CD 3 B 4. 8 4. 53 (94%) 2. 39 2. 03 (85%) C CO B 1. 93 1. 58 (82%) HCN B 1. 48 (100%)

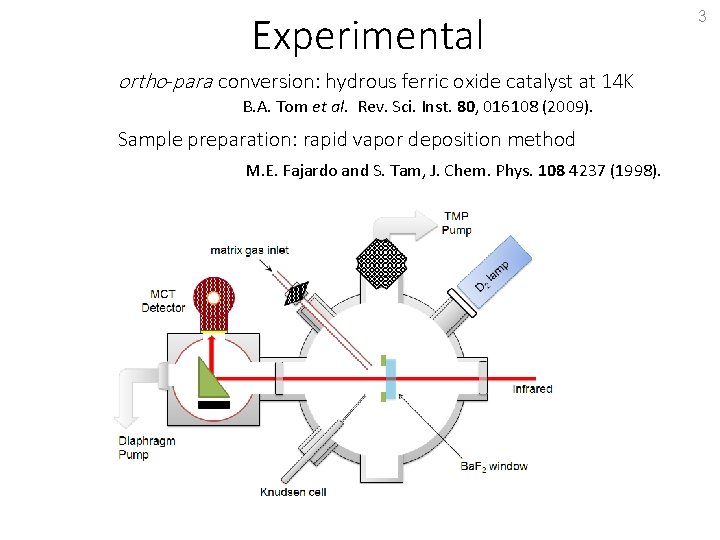
Experimental ortho-para conversion: hydrous ferric oxide catalyst at 14 K B. A. Tom et al. Rev. Sci. Inst. 80, 016108 (2009). Sample preparation: rapid vapor deposition method M. E. Fajardo and S. Tam, J. Chem. Phys. 108 4237 (1998). 3

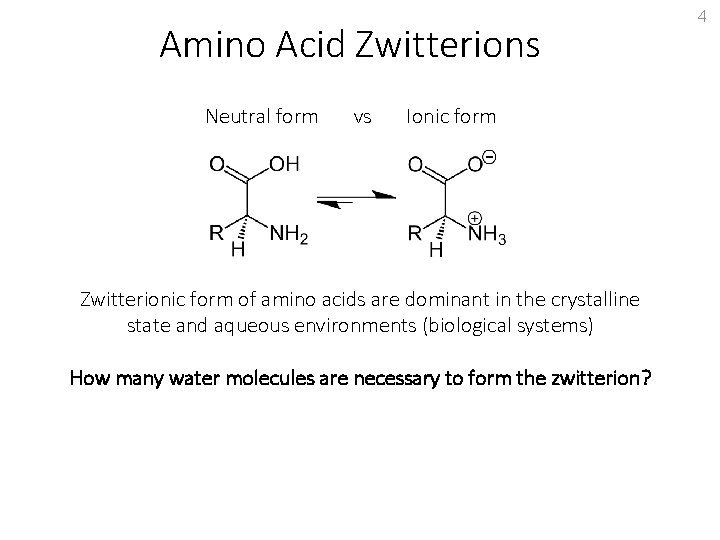
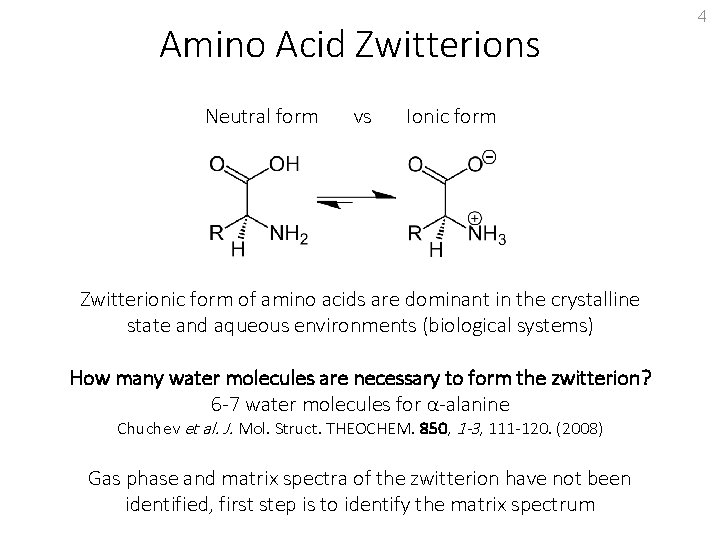
Amino Acid Zwitterions Neutral form vs Ionic form Zwitterionic form of amino acids are dominant in the crystalline state and aqueous environments (biological systems) How many water molecules are necessary to form the zwitterion? 4

Amino Acid Zwitterions Neutral form vs Ionic form Zwitterionic form of amino acids are dominant in the crystalline state and aqueous environments (biological systems) How many water molecules are necessary to form the zwitterion? 6 -7 water molecules for α-alanine Chuchev et al. J. Mol. Struct. THEOCHEM. 850, 1 -3, 111 -120. (2008) Gas phase and matrix spectra of the zwitterion have not been identified, first step is to identify the matrix spectrum 4

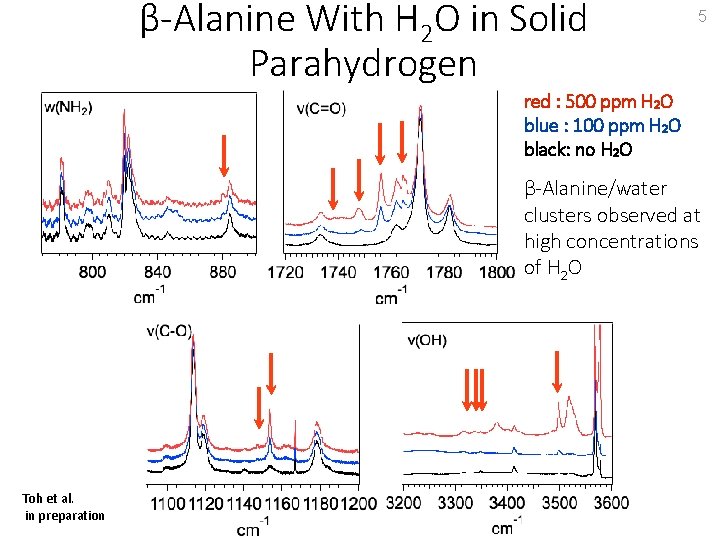
β-Alanine With H 2 O in Solid Parahydrogen 5 red : 500 ppm H 2 O blue : 100 ppm H 2 O black: no H 2 O β-Alanine/water clusters observed at high concentrations of H 2 O Toh et al. in preparation

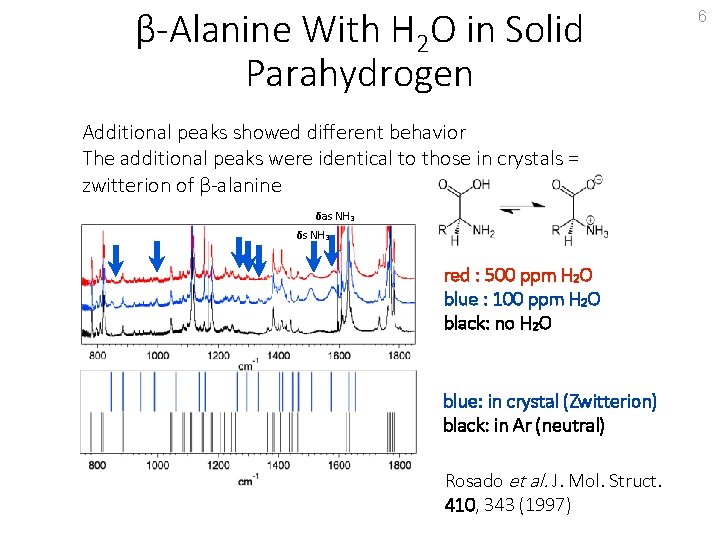
β-Alanine With H 2 O in Solid Parahydrogen Additional peaks showed different behavior The additional peaks were identical to those in crystals = zwitterion of β-alanine δas NH 3 δs NH 3 red : 500 ppm H 2 O blue : 100 ppm H 2 O black: no H 2 O blue: in crystal (Zwitterion) black: in Ar (neutral) Rosado et al. J. Mol. Struct. 410, 343 (1997) 6

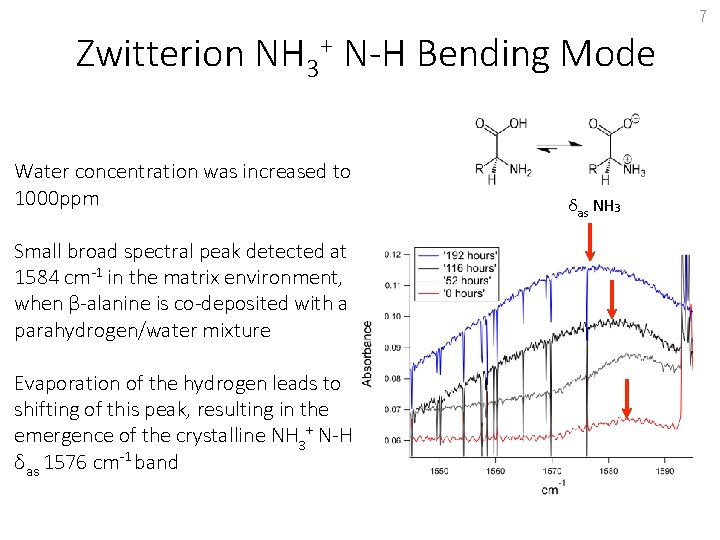
7 Zwitterion NH 3+ N-H Bending Mode Water concentration was increased to 1000 ppm Small broad spectral peak detected at 1584 cm-1 in the matrix environment, when β-alanine is co-deposited with a parahydrogen/water mixture Evaporation of the hydrogen leads to shifting of this peak, resulting in the emergence of the crystalline NH 3+ N-H δas 1576 cm-1 band δas NH 3

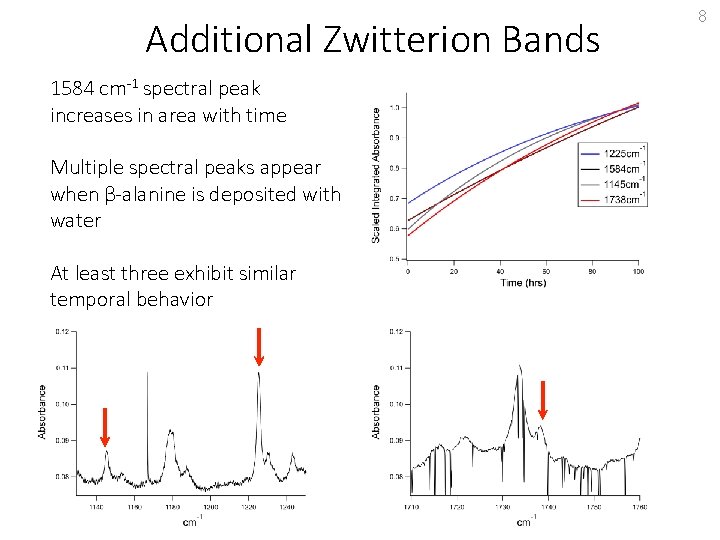
Additional Zwitterion Bands 1584 cm-1 spectral peak increases in area with time Multiple spectral peaks appear when β-alanine is deposited with water At least three exhibit similar temporal behavior 8

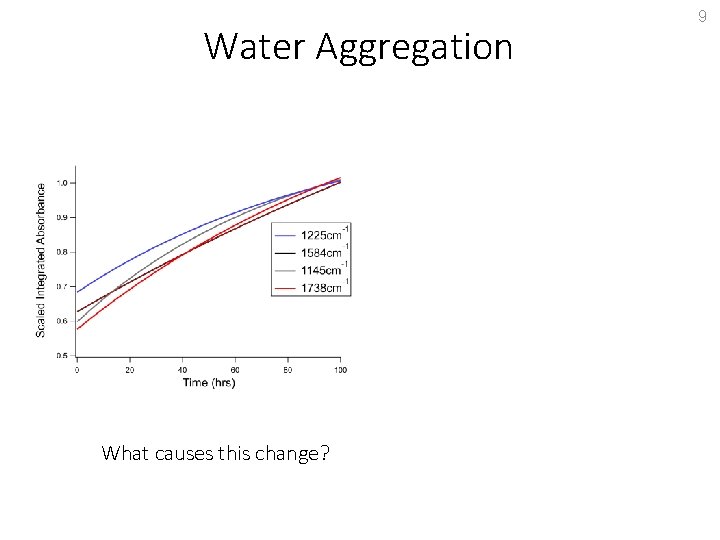
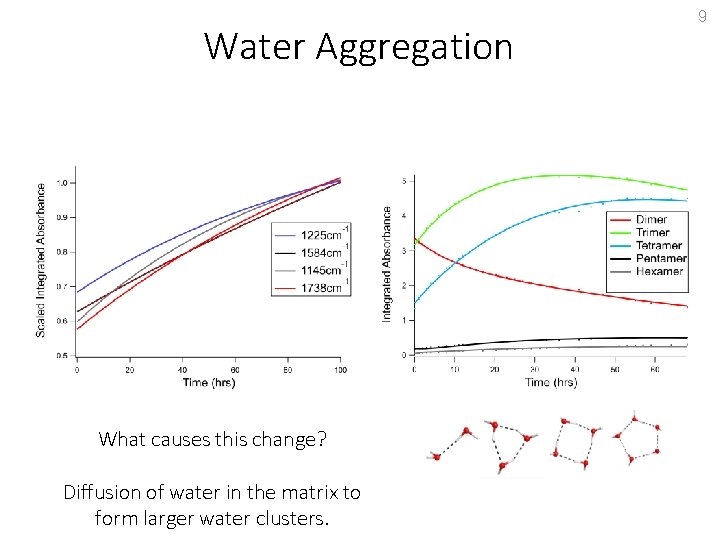
Water Aggregation What causes this change? 9

Water Aggregation What causes this change? Diffusion of water in the matrix to form larger water clusters. 9

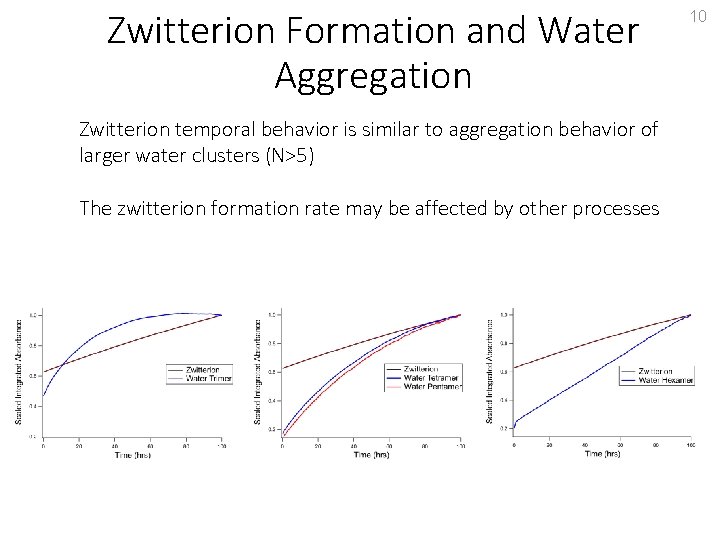
Zwitterion Formation and Water Aggregation Zwitterion temporal behavior is similar to aggregation behavior of larger water clusters (N>5) The zwitterion formation rate may be affected by other processes 10

Summary Formation of β-alanine Zwitterion was observed Multiple vibrational bands of the β-alanine Zwitterion were identified Water clusters form by water diffusion in the parahydrogen matrix β-alanine zwitterions increase in population over time due to the formation of water clusters (N>5) in the matrix 11

Acknowledgements Cindy Toh Pavle Dr. Takamasa Djuricanin Momose Angel Wong Steve Chiang 12