Infrared IR Spectroscopy Used to investigate specific functional





- Slides: 21

Infrared (IR) Spectroscopy • Used to investigate specific functional groups • No information on total structure, connectivity • Direct absorption spectroscopy; involves excitation of a vibrational mode with an IR photon hν O C

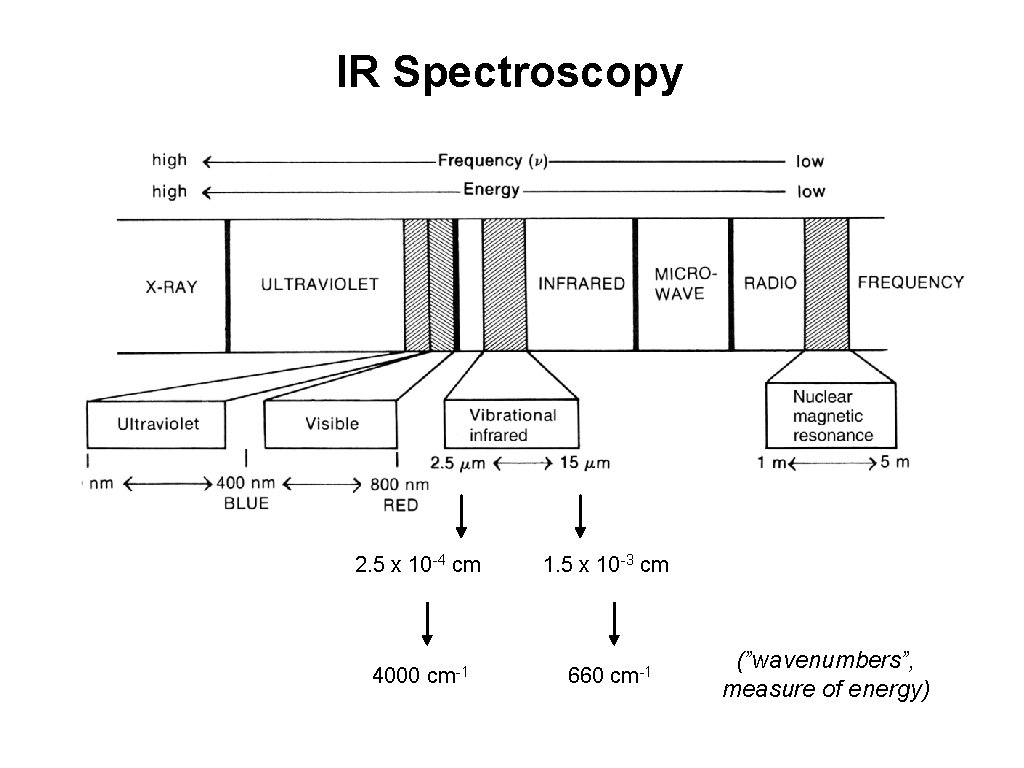
IR Spectroscopy 2. 5 x 10 -4 cm 1. 5 x 10 -3 cm 4000 cm-1 660 cm-1 (”wavenumbers”, measure of energy)

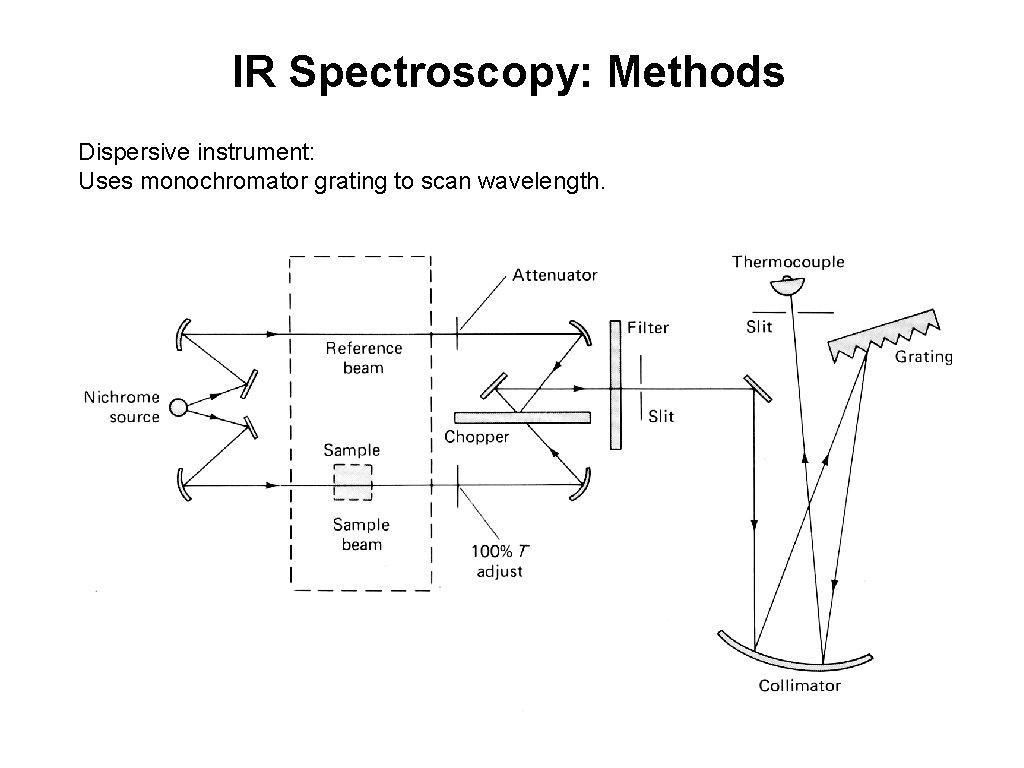
IR Spectroscopy: Methods Dispersive instrument: Uses monochromator grating to scan wavelength.

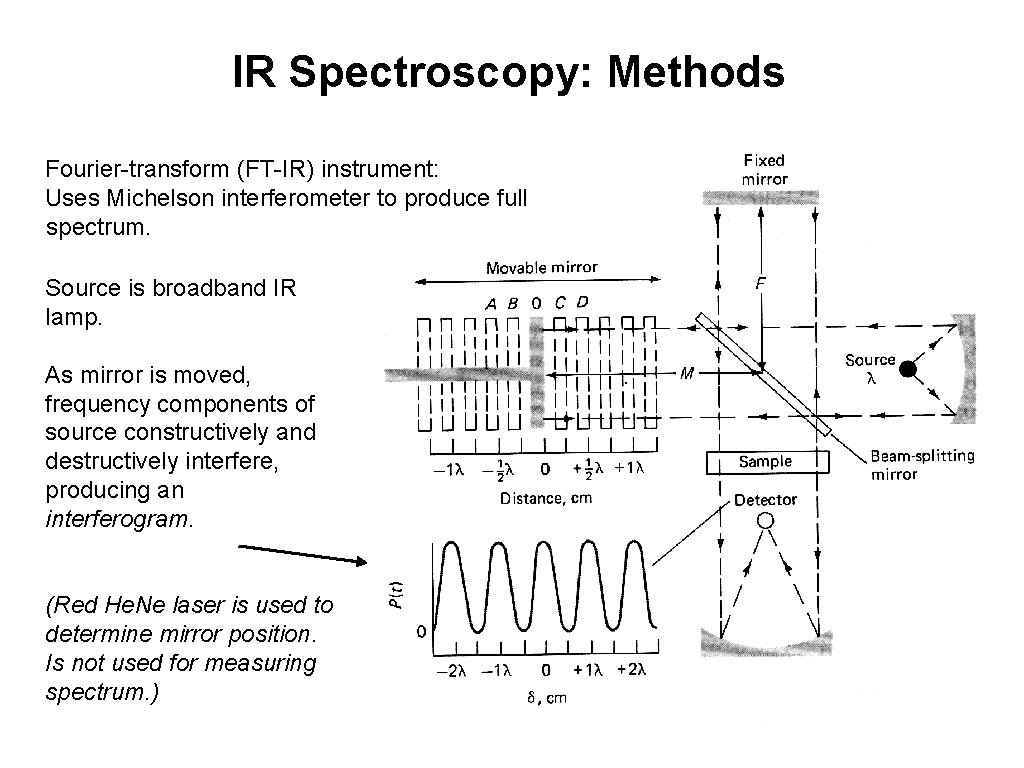
IR Spectroscopy: Methods Fourier-transform (FT-IR) instrument: Uses Michelson interferometer to produce full spectrum. Source is broadband IR lamp. As mirror is moved, frequency components of source constructively and destructively interfere, producing an interferogram. (Red He. Ne laser is used to determine mirror position. Is not used for measuring spectrum. )

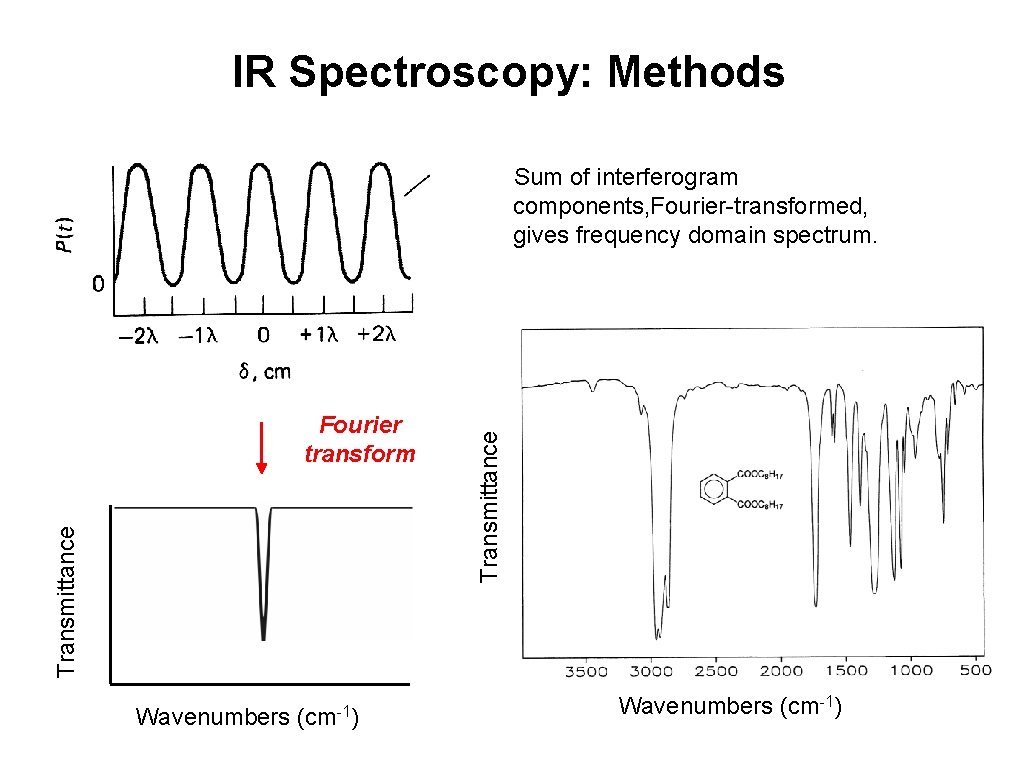
IR Spectroscopy: Methods Transmittance Fourier transform Wavenumbers (cm-1) Transmittance Sum of interferogram components, Fourier-transformed, gives frequency domain spectrum. Wavenumbers (cm-1)

IR Spectroscopy: Quantum Limitations IR-absorbing transitions are allowed only when dipole moment changes during vibrational motion. Modes can be combinations of bond vibrations. O O O C C C O O O O symmetric stretch: IR forbidden (1340 cm-1) asymmetric stretch: IR allowed (2350 cm-1) bend: IR allowed (660 cm-1) As a result, IR instruments sometimes purged with N 2 to get rid of CO 2.

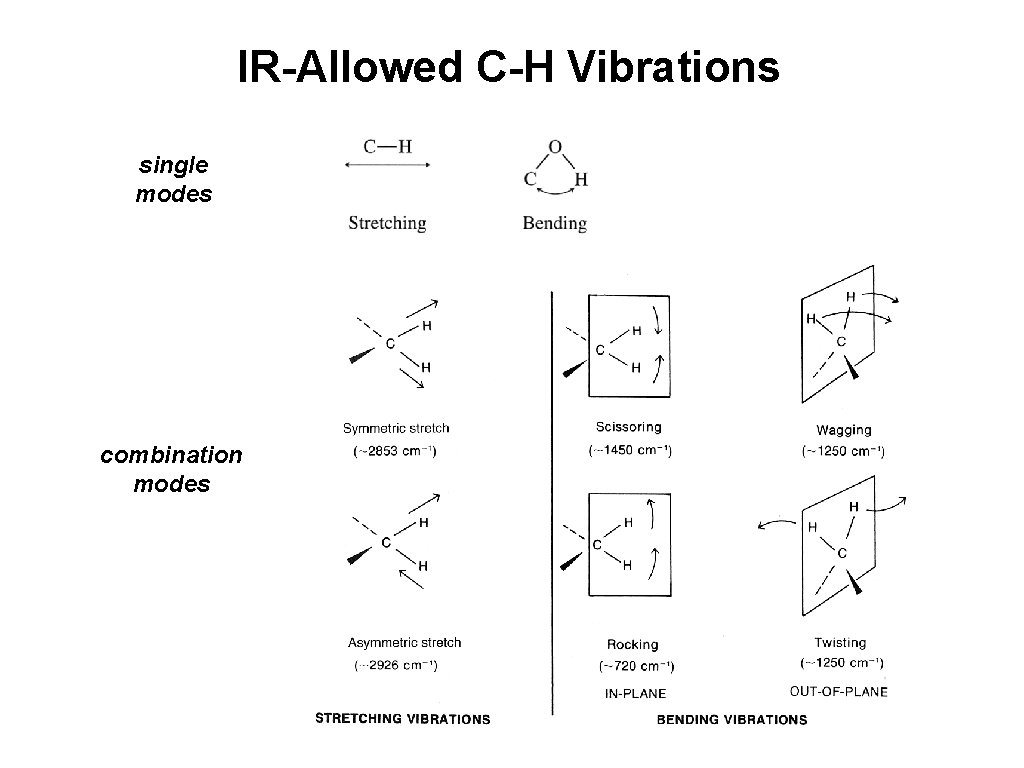
IR-Allowed C-H Vibrations single modes combination modes

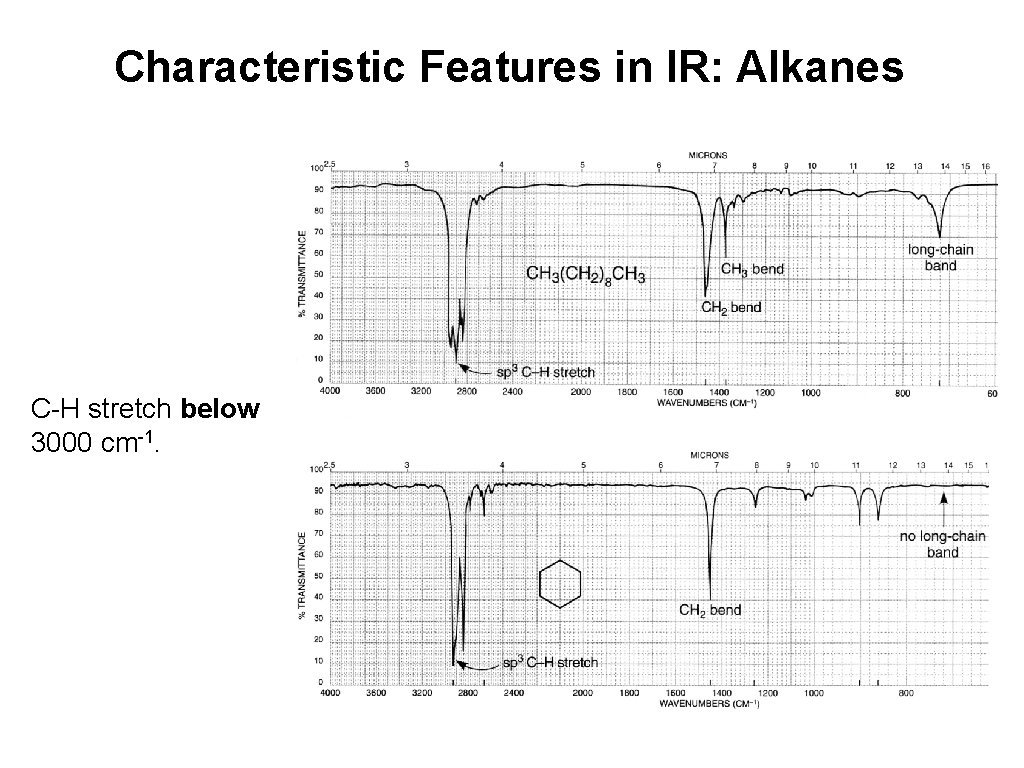
Characteristic Features in IR: Alkanes C-H stretch below 3000 cm-1.

Characteristic Features in IR: Alkenes C-H stretch above 3000 cm-1. C=C stretch ~ 1600 -1660 cm-1; affected by bond stereochemistry.

Characteristic Features in IR: Alkynes C-H stretch ~ 3300 cm-1. C≡C stretch ~ 2150 cm-1, but only when asymmetric.

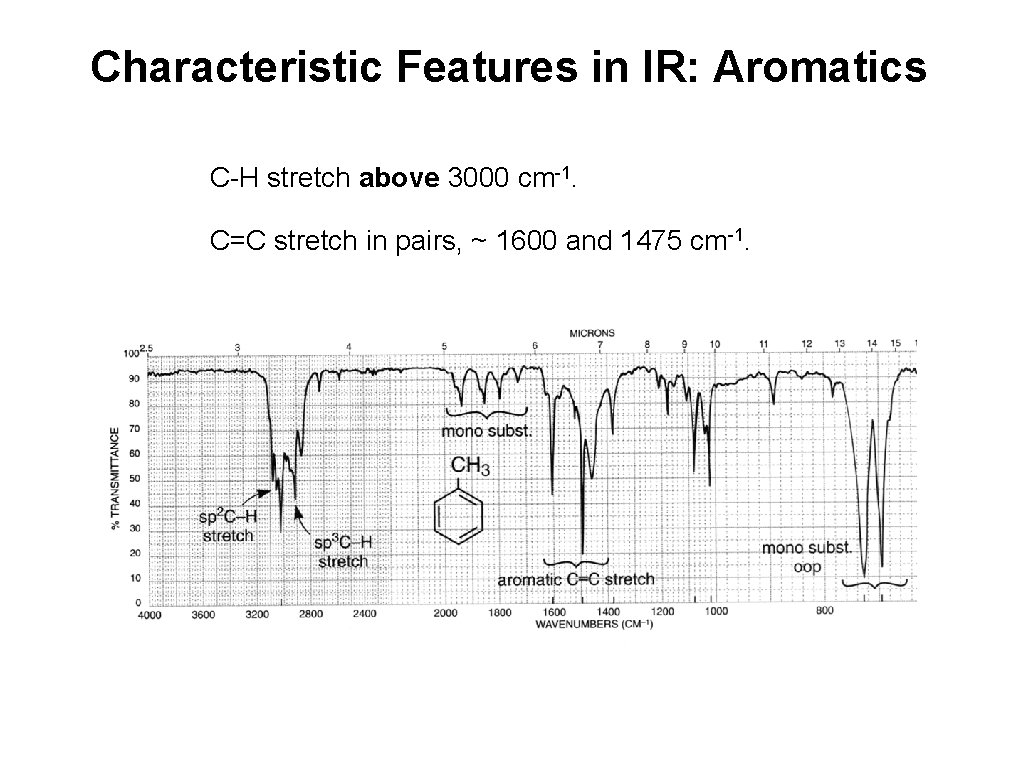
Characteristic Features in IR: Aromatics C-H stretch above 3000 cm-1. C=C stretch in pairs, ~ 1600 and 1475 cm-1.

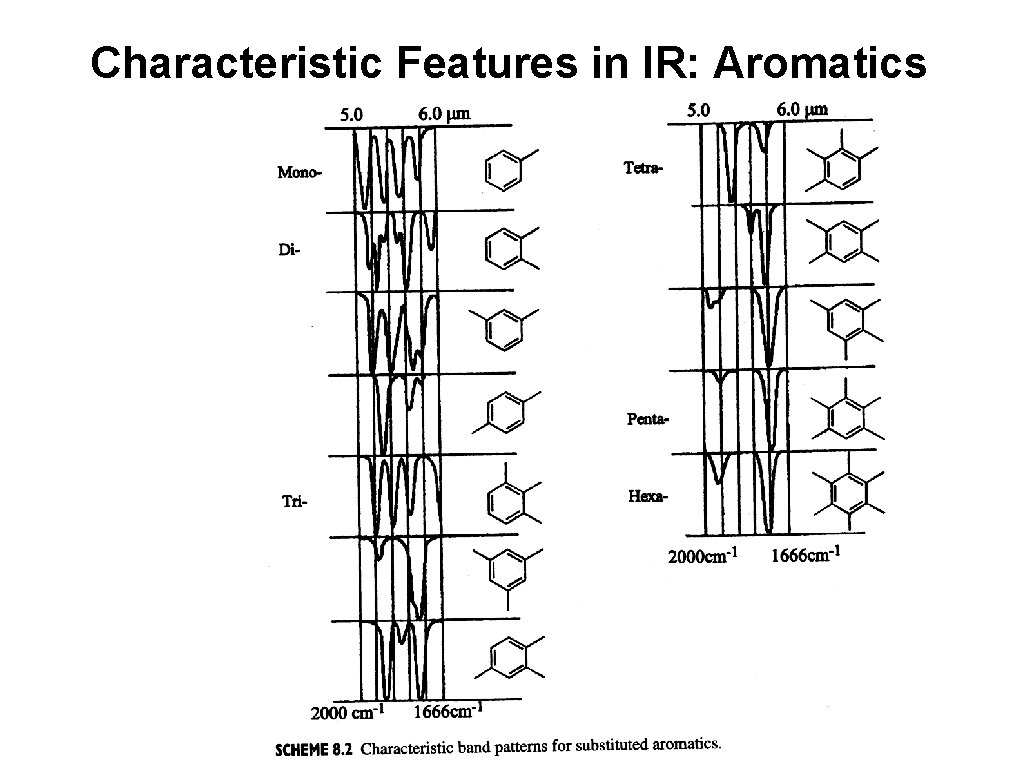
Characteristic Features in IR: Aromatics

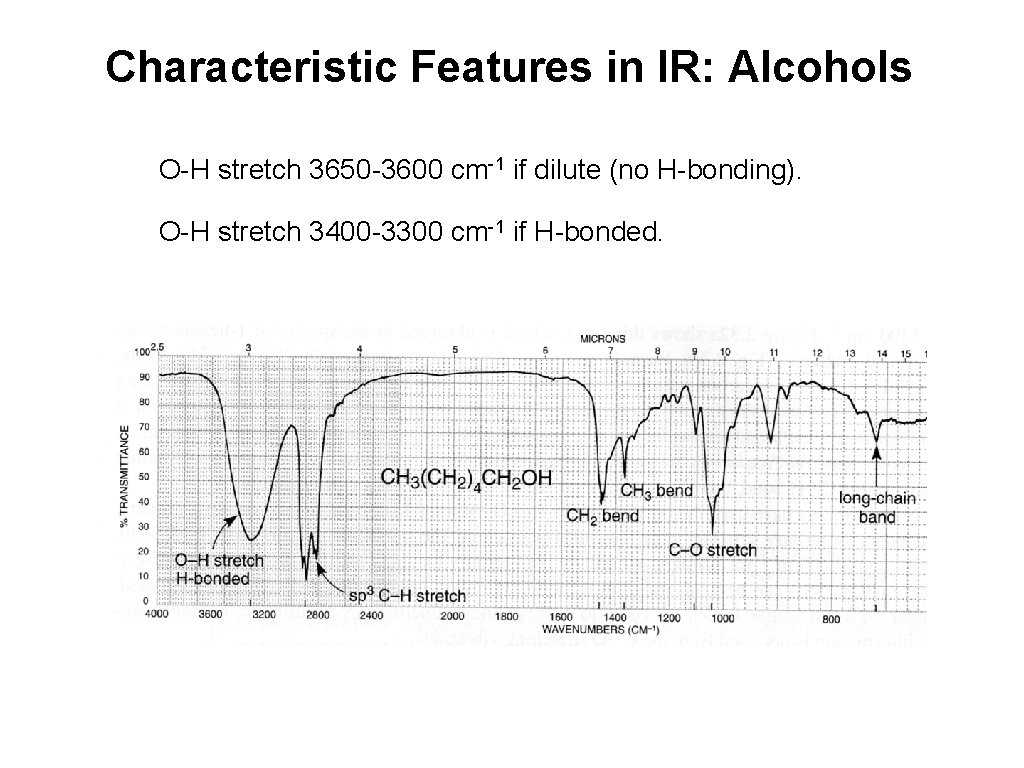
Characteristic Features in IR: Alcohols O-H stretch 3650 -3600 cm-1 if dilute (no H-bonding). O-H stretch 3400 -3300 cm-1 if H-bonded.

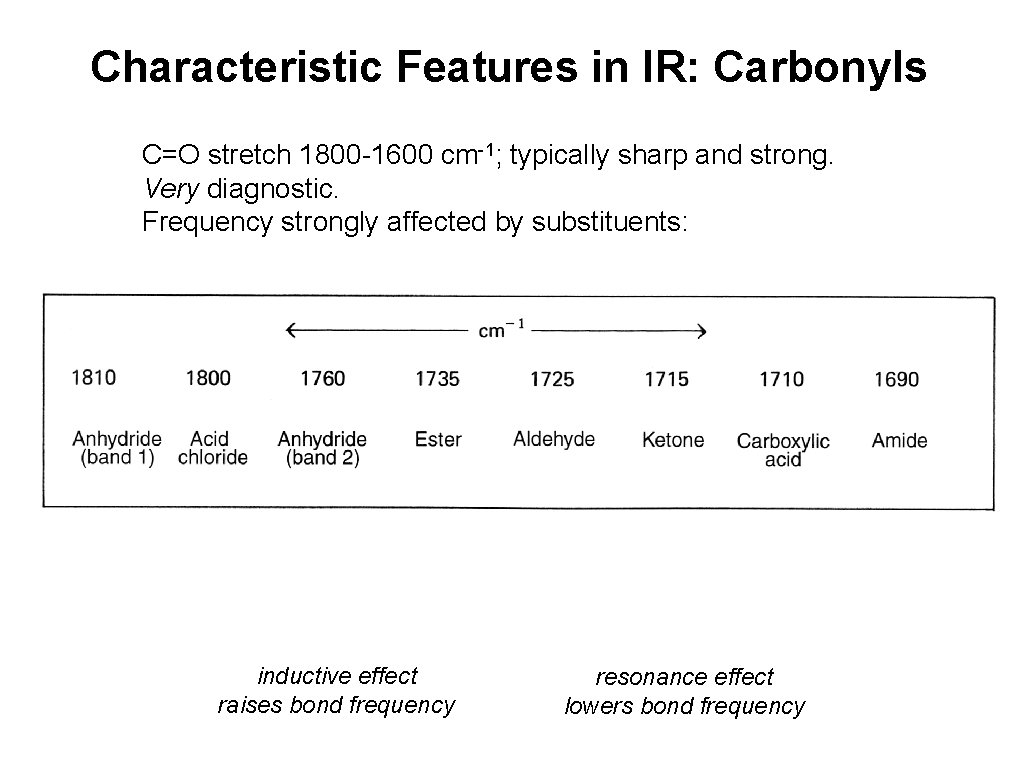
Characteristic Features in IR: Carbonyls C=O stretch 1800 -1600 cm-1; typically sharp and strong. Very diagnostic. Frequency strongly affected by substituents: inductive effect raises bond frequency resonance effect lowers bond frequency

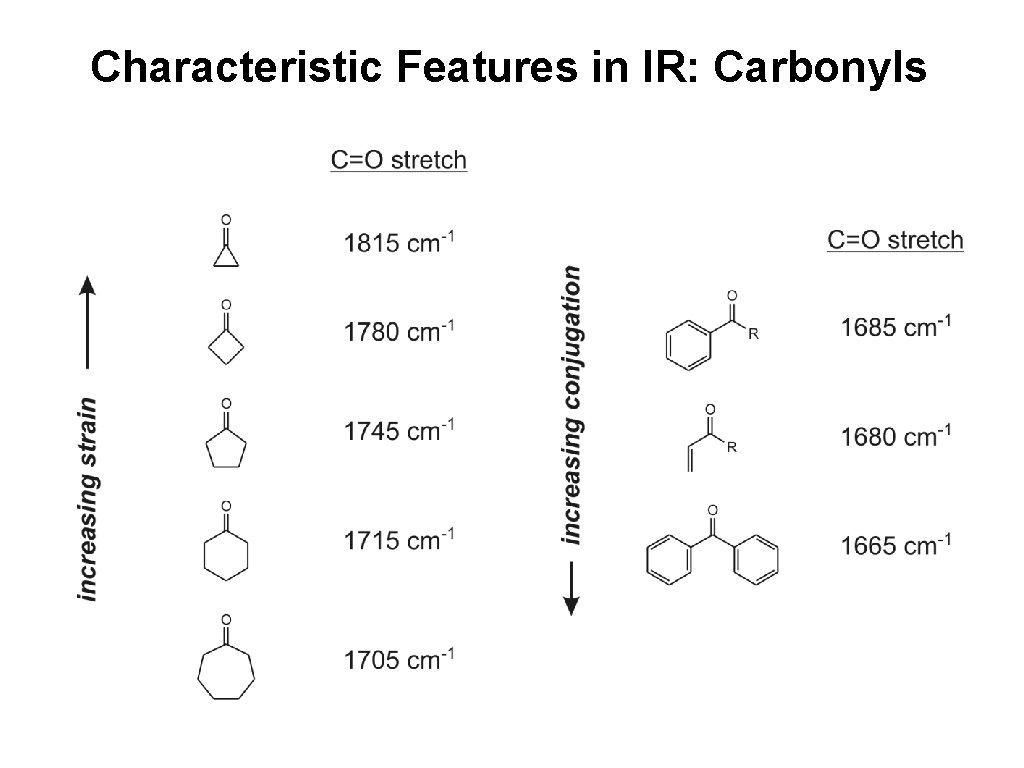
Characteristic Features in IR: Carbonyls

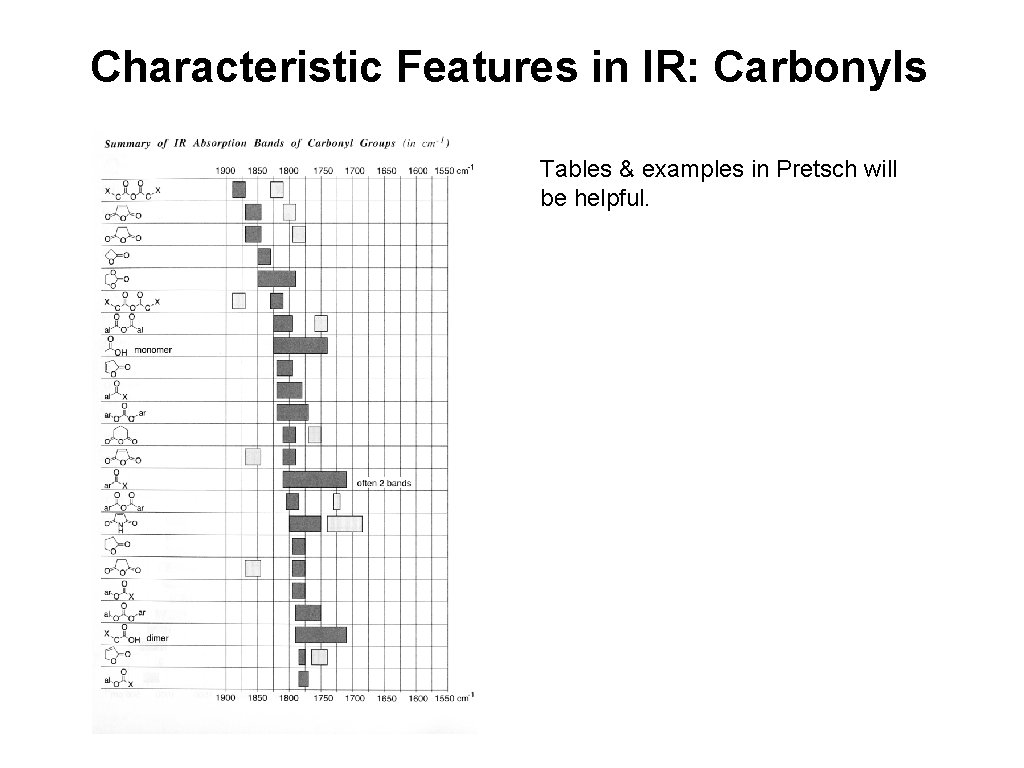
Characteristic Features in IR: Carbonyls Tables & examples in Pretsch will be helpful.

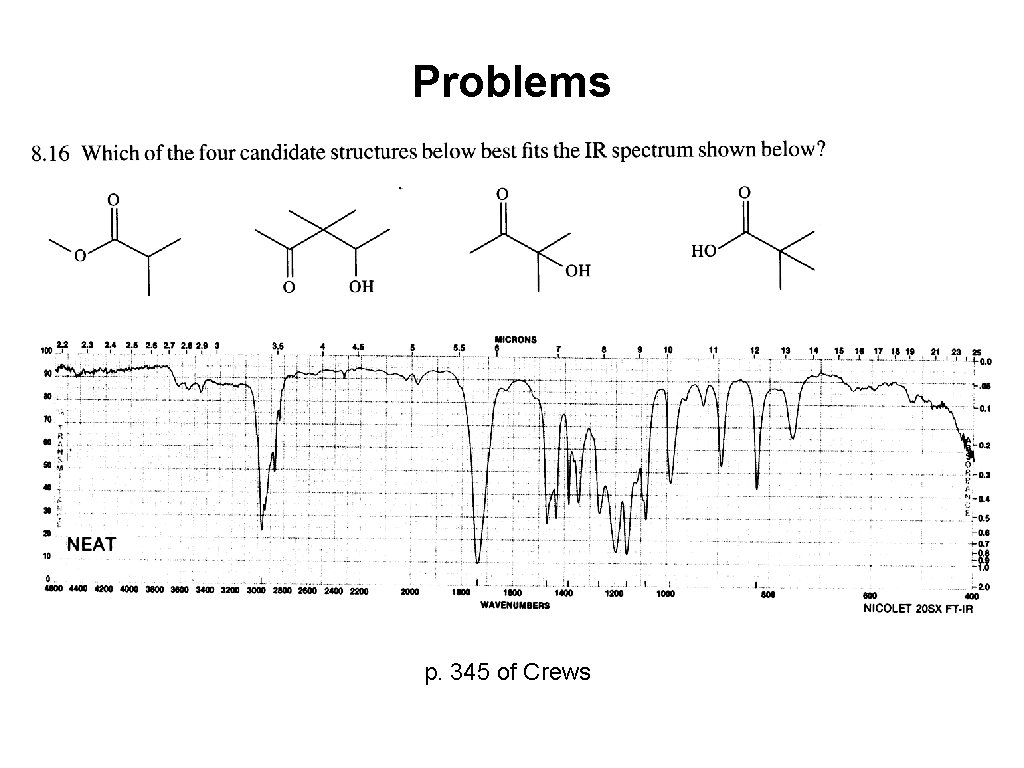
Problems 1880 cm-1 p. 345 of Crews

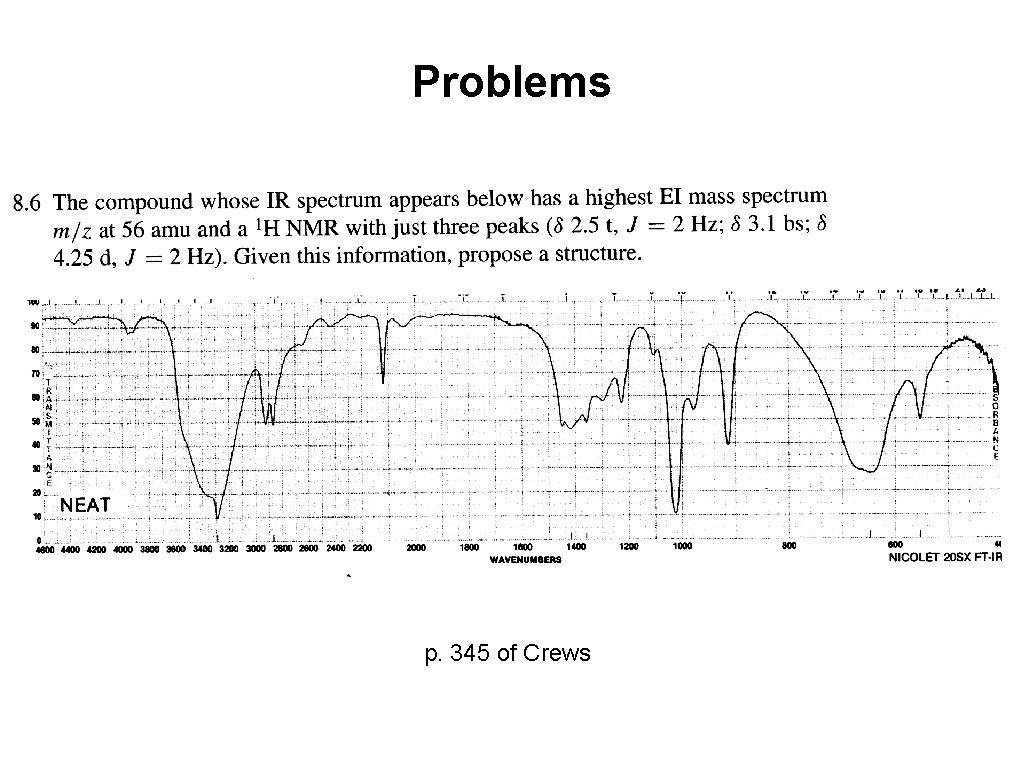
Problems p. 345 of Crews

Problems p. 345 of Crews

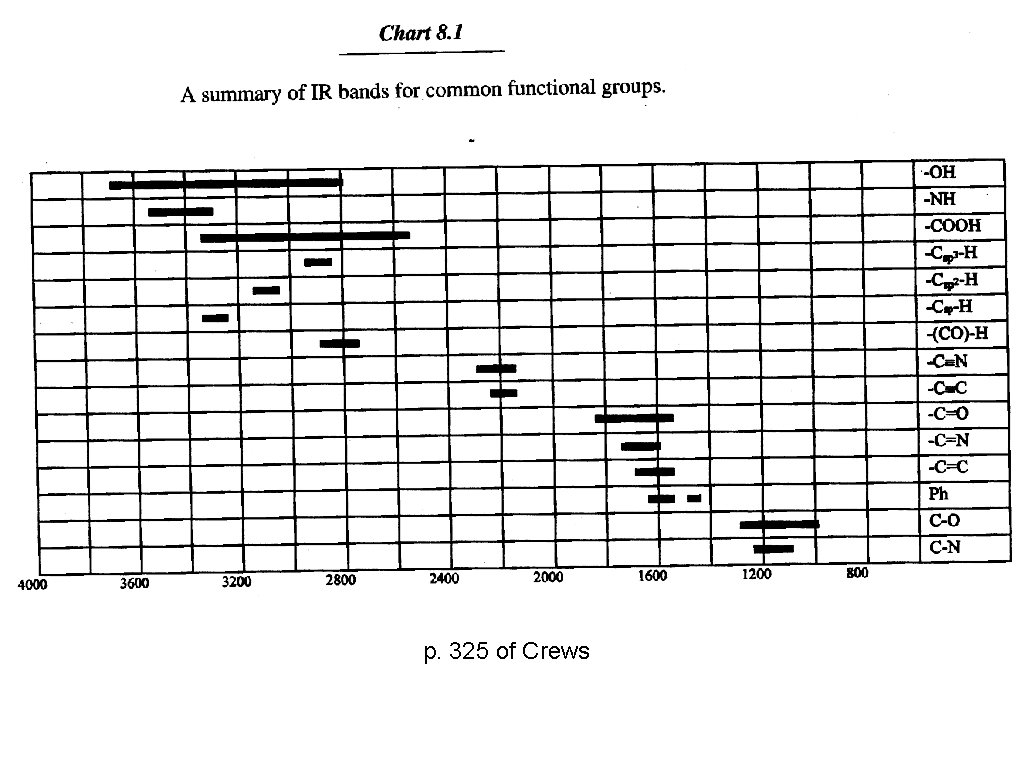
p. 325 of Crews

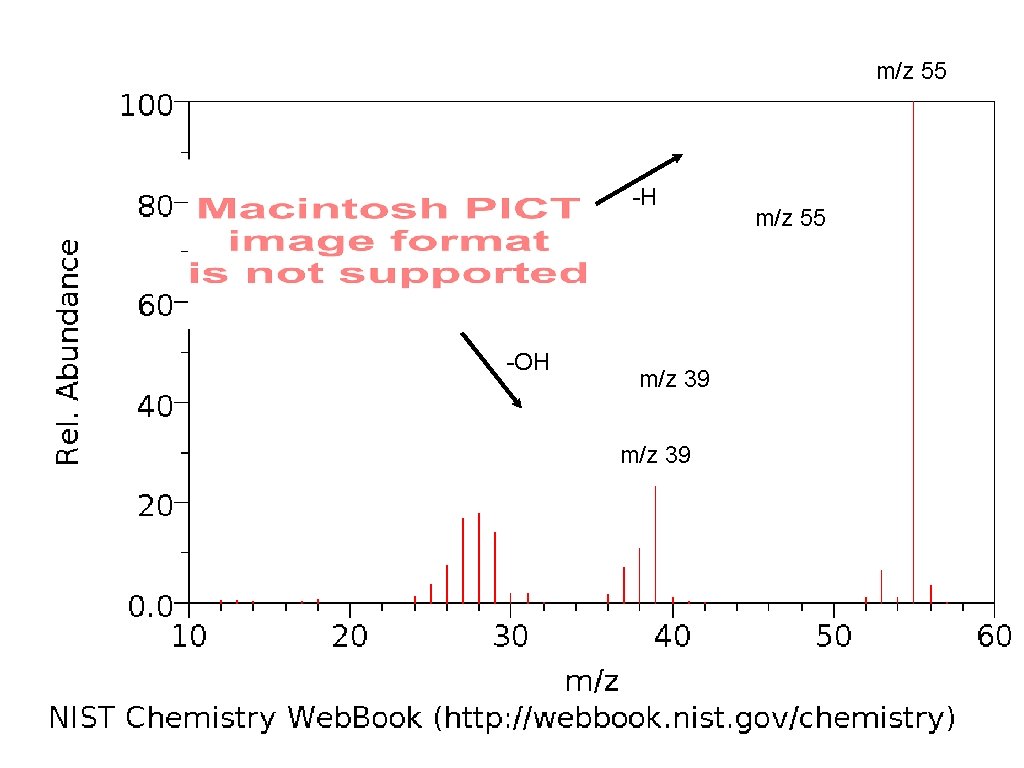
m/z 55 -H -OH m/z 39 m/z 55